Abstract
Background
Contemporary rates of oral anticoagulant (OAC) therapy and associated outcomes among patients undergoing percutaneous coronary intervention (PCI) have been poorly described.
Methods and Results
Using data from an integrated health care system from 2009 to 2014, we identified patients on OACs within 30 days of PCI. Outcomes included in‐hospital bleeding and mortality. Of 9566 PCIs, 837 patients (8.8%) were on OACs, and of these, 7.9% used non–vitamin K antagonist agents. OAC use remained stable during the study (8.1% in 2009, 9.0% in 2014; P=0.11), whereas use of non–vitamin K antagonist agents in those on OACs increased (0% in 2009, 16% in 2014; P<0.01). Following PCI, OAC‐treated patients had higher crude rates of major bleeding (11% versus 6.5%; P<0.01), access‐site bleeding (2.3% versus 1.3%; P=0.017), and non–access‐site bleeding (8.2% versus 5.2%; P<0.01) but similar crude rates of in‐hospital stent thrombosis (0.4% versus 0.3%; P=0.85), myocardial infarction (2.5% versus 3.0%; P=0.40), and stroke (0.48% versus 0.52%; P=0.88). In addition, prior to adjustment, OAC‐treated patients had longer hospitalizations (3.9±5.5 versus 2.8±4.6 days; P<0.01), more transfusions (7.2% versus 4.2%; P<0.01), and higher 90‐day readmission rates (22.1% versus 13.1%; P<0.01). In adjusted models, OAC use was associated with increased risks of in‐hospital bleeding (odds ratio 1.50; P<0.01), 90‐day readmission (odds ratio 1.40; P<0.01), and long‐term mortality (hazard ratio 1.36; P<0.01).
Conclusions
Chronic OAC therapy is frequent among contemporary patients undergoing PCI. After adjustment for potential confounders, OAC‐treated patients experienced greater in‐hospital bleeding, more readmissions, and decreased long‐term survival following PCI. Efforts are needed to reduce the occurrence of adverse events in this population.
Keywords: anticoagulant, bleeding, mortality, percutaneous coronary intervention, readmission
Subject Categories: Epidemiology, Risk Factors, Percutaneous Coronary Intervention, Quality and Outcomes, Complications
Introduction
Bleeding complications following percutaneous coronary intervention (PCI) are associated with increased short‐ and long‐term morbidity and mortality.1, 2, 3, 4, 5 Oral anticoagulant (OAC) use prior to coronary stenting is a significant predictor of postprocedure bleeding events.6, 7 Previous studies estimated that the frequency of chronic OAC use among patients undergoing PCI is between 3% and 7%.7, 8, 9, 10, 11 Many of these analyses, however, examined selected patient populations, such as those admitted with acute myocardial infarction (MI) or atrial fibrillation, and preceded the market approval of non–vitamin K antagonist OACs (NOACs).12 As such, the contemporary rate of chronic OAC use among all patients undergoing PCI is presently unknown.
Furthermore, recent use of OAC therapy among ambulatory patients has increased,13, 14 corresponding with the growing use of NOACs, which are now prescribed as frequently as vitamin K antagonists (VKAs).14, 15, 16, 17 Compared with VKAs, NOACs are associated with reduced thromboembolic events and severe bleeding in patients with atrial fibrillation.18 Nevertheless, there is a lack of data assessing management practices and outcomes among patients undergoing coronary stenting on NOAC therapy.19
With this in mind, we sought to determine contemporary practice patterns and temporal trends in use of OAC therapies among all patients undergoing coronary stenting, using data from a large integrated health care system between 2009 and 2014. In addition, we examined the association of chronic OAC therapy with short‐ and long‐term outcomes and whether these relationships were modified by use of NOACs in addition to traditional VKAs.
Methods
Study Population
Partners HealthCare is an integrated health care system that currently includes 8 Massachusetts hospitals, 21 community health centers, and a network of independent ambulatory practices with >500 affiliated primary care doctors. The PCI cohort was derived from the Partners Long‐Term Outcomes Database, an ongoing, institutionally sponsored registry of patients undergoing PCI within the Partners HealthCare system.20 All PCIs in which patients presenting to 1 of 2 Partners HealthCare academic medical centers (Brigham and Women's Hospital and Massachusetts General Hospital) between June 2009 and September 2014 were included. If >1 PCI was performed in the same patient during the same hospitalization, only the first PCI was included in the analysis. There were no other exclusion criteria. The present project was reviewed and approved with a waiver of informed consent from the institutional review board at Partners HealthCare.
Covariates
Clinical and procedural characteristics analyzed for all PCIs were abstracted from institutional registry data, based on the data collection form from the National Cardiovascular Data Registry's (NCDR) CathPCI Registry. A full description of the data elements of the CathPCI registry are available online (http://cvquality.acc.org/~/media/QII/NCDR/Data%20Collection%20Forms/CathPCI%20Registry_DataCollectionForm.ashx).21
Exposure of Interest
To determine chronic anticoagulant status, we developed an algorithm that was applied to the entire electronic medical record, including admission notes, consultation notes, nursing notes, outpatient notes, catheterization reports, pharmacy notes, discharge summaries, and medication prescriptions, to identify the presence of OAC use within the 30 days preceding the PCI. The algorithm was capable of searching structured and unstructured data. The search terms included the medications warfarin, Coumadin, dabigatran, Pradaxa, rivaroxaban, Xarelto, apixaban, and Eliquis. Edoxaban was not included because the study preceded its market approval. The algorithm was designed to maximize sensitivity over specificity to provide a screen of the study population for patients taking OAC therapy prior to PCI. To remove false‐positive records, all electronic medical records of identified OAC patients were manually reviewed by 2 physicians (E.A.S. and N.B.) to confirm documented OAC use within 30 days of the admission. Of patients identified as taking OAC therapy, 70.6% were confirmed to be on OAC treatment and composed the exposure group. Exclusion on manual review primarily resulted from the algorithm incorrectly identifying patients for whom phrases reflecting nonuse of OACs were found in unstructured data (eg, “patient not on warfarin” in a clinic visit note).
Additional data for OAC patients were also manually collected and included: type and dose of anticoagulant at admission, type and dose of antiplatelet at admission, aspirin status and dose at admission, indication for anticoagulant, use and duration of bridging agent prior to PCI, type and dose of antiplatelet at discharge, aspirin status and dose at discharge, type and dose of anticoagulant at discharge, discontinuation of anticoagulant after PCI, and reason for discontinuation of anticoagulant after PCI.
Outcome Measures
The primary outcome measures were in‐hospital major bleeding, in‐hospital mortality, and long‐term mortality. In‐hospital major bleeding was based on the NCDR version 4 definition,22 which included any of the following occurring before hospital discharge: arterial access‐site bleeding (defined as external bleeding at the access site or a hematoma >10 cm for femoral access, >5 cm for brachial access, or >2 cm for radial access); retroperitoneal, gastrointestinal, or genitourinary bleeding; intracranial hemorrhage; cardiac tamponade; postprocedure hemoglobin decrease of 3 g/dL in patients with a preprocedure hemoglobin level ≤16 g/dL; or postprocedure non–bypass surgery–related blood transfusion for patients with a preprocedure hemoglobin level ≥8 g/dL. Long‐term mortality was assessed through the National Death Index and subsequent linkage with the institutional registry, as described previously.23 Secondary outcome measures included access‐site bleeding (defined as external bleeding at the access site, hematoma at the access site, or retroperitoneal bleeding), non–access‐site bleeding (defined as gastrointestinal or genitourinary bleeding, intracranial hemorrhage, or cardiac tamponade), red blood cell transfusion, postprocedure MI (new occurrence of a biomarker‐positive MI after PCI), in‐hospital stent thrombosis (defined as a subsequent PCI performed during the same index hospitalization as the related PCI for the indication of stent thrombosis), cerebrovascular accident or stroke (defined as loss of neurological function caused by an ischemic or hemorrhagic event with residual symptoms lasting at least 24 hours after onset or leading to death), and postprocedure length of stay in days. In addition, readmissions to hospitals only within the Partners HealthCare system were identified for all PCI patients within 30 and 90 days of discharge.
Statistical Analysis
Clinical and procedural characteristics with dichotomous and categorical variables were reported as counts and percentages, and continuous variables were reported as means with standard deviations. Between‐group differences were assessed using Fisher exact or chi‐square tests for binary and categorical variables and Student t tests or Wilcoxon rank sum tests for continuous variables. Temporal trends were analyzed using the Cochran‐Armitage trend test. Kaplan–Meier methods were used to estimate the cumulative incidence of long‐term mortality following PCI stratified by use of OACs at admission, and log‐rank tests were used to compare the curves. Predicted risks of in‐hospital bleeding and in‐hospital mortality were calculated for each group using previously validated risk models developed within the NCDR CathPCI Registry.22, 24 Logistic regression models were created to measure the association of OAC status at admission with in‐hospital outcomes. Cox proportional hazards models were created to assess the influence of OAC use at admission on long‐term mortality following PCI. For all outcome models, we adjusted by the propensity score predicting treatment with an OAC. The propensity score was based on the following variables: sociodemographic variables (age, sex, ethnicity, and body mass index), comorbidities (tobacco use, diabetes mellitus, hypertension, dyslipidemia, prior MI, prior coronary artery bypass grafting, prior valve surgery, prior PCI, family history of coronary artery disease, prior heart failure, chronic lung disease, cerebrovascular disease, peripheral vascular disease, renal failure, and anemia), clinical presentation (PCI urgency, acute coronary syndrome, history of angina, history of heart failure symptoms, cardiogenic shock, cardiac arrest, ejection fraction, and need for mechanical support), and procedural characteristics (lesion complexity, lesion location, stent thrombosis, preprocedure thrombolysis in MI flow, postprocedure thrombolysis in MI flow, arterial access site, and multivessel disease). This propensity score was used as a covariate for adjustment and had a C statistic of 0.772. Because 31.8% of patients were missing data on ejection fraction, these data were imputed by stratifying the population based on history of heart failure, prior MI, preprocedure cardiogenic shock, and the presence of ST‐segment elevation MI, as done previously.24 Similar analyses were performed to examine the association between NOAC versus VKA use and outcomes, limited to patients who were receiving OACs. These models were adjusted by the propensity score for receiving treatment with a VKA, based on the variables listed above, which had a C statistic of 0.783.
A value of P<0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 software (SAS Institute).
Results
Temporal Trends and Practice Patterns Among Patients on OAC Therapy
From June 2009 through September 2014, a total of 9566 PCIs met the inclusion criteria. Of these procedures, 837 (8.8%) were performed in patients with OAC use within 30 days of admission. NOACs were used in 66 (7.9%) of these PCIs, with VKAs used in the remainder. During the study period, overall OAC use remained stable (8.1% of PCIs in 2009, 9.0% in 2014; P=0.11 for trend), whereas NOACs comprised an increasing proportion of all OAC use among PCIs (0% in 2009, 17.6% of OACs in 2014; P<0.01 for trend) (Figure 1). Use of triple therapy (OAC plus a P2Y12 inhibitor and aspirin) at discharge after PCI remained constant during the study period (83.2% in 2009, 83.8% in 2014; P=0.66 for trend) (Figure S1).
Figure 1.
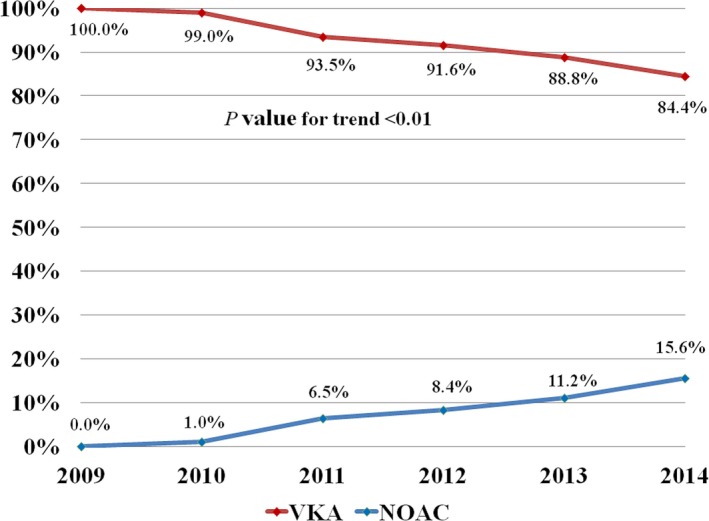
Temporal changes in use of NOACs compared with VKAs among patients on chronic OAC therapy undergoing PCI during the study period. Note the brisk increase in use of NOACs in place of VKAs beginning in 2010, which corresponds with the market approval of the first NOAC. NOAC indicates non‐vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; VKA, vitamin K antagonist.
Among patients on OAC therapy, the main indication for treatment was atrial fibrillation or flutter (78.0%), followed by venous thromboembolism (16.4%) and left ventricular dysfunction, aneurysm, or thrombus (9.1%) (Table 1). Of patients on NOACs, the majority took dabigatran (60.0%), followed by rivaroxaban (30.8%) and apixaban (4.5%). At admission, dabigatran was primarily dosed at 150 mg twice a day (92.3%), rivaroxaban at 20 mg daily (66.7%), and apixaban at 5 mg twice a day (66.7%). Aspirin was concomitantly used by 70.7% of OAC‐treated patients at admission, with the majority taking a dose of 81 mg daily (79.5%); P2Y12 inhibitors were used by 21.3%, of which 97.2% were taking clopidogrel therapy (Table 1, Figure 2). Of the 307 elective PCIs performed in patients taking OACs during the study period, bridging therapy was used in 68 (22.2%).
Table 1.
Patient Characteristics by OAC Use
| Characteristic | OAC Use (n=837) | No OAC Use (n=8729) | P Value |
|---|---|---|---|
| Age, y, mean±SD | 71.7±11.2 | 65.5±12.1 | <0.01 |
| Male | 621 (74.2) | 6308 (72.3) | 0.23 |
| White | 778 (93.0) | 7908 (90.6) | 0.024 |
| BMI, kg/m2, mean±SD | 29.7±15.4 | 29.2±7.3 | 0.16 |
| Current/recent smoking (within 1 year) | 75 (8.96) | 1608 (18.4) | <0.01 |
| Hypertension | 750 (89.6) | 7111 (81.5) | <0.01 |
| Dyslipidemia | 793 (94.7) | 8110 (92.9) | 0.045 |
| Family history of CAD | 132 (15.8) | 2082 (23.9) | <0.01 |
| Renal failure (currently on dialysis or creatinine >2 mg/dL) | 76 (9.08) | 453 (5.19) | <0.01 |
| Cerebrovascular disease | 245 (29.3) | 1177 (13.5) | <0.01 |
| Peripheral artery disease | 195 (23.3) | 1380 (15.8) | <0.01 |
| Chronic lung disease | 172 (20.6) | 1152 (13.2) | <0.01 |
| Diabetes mellitus | 342 (40.9) | 2977 (34.1) | <0.01 |
| Prior heart failure | 344 (41.1) | 1191 (13.6) | <0.01 |
| Prior myocardial infarction | 407 (48.6) | 3023 (34.6) | <0.01 |
| Prior valve surgery | 115 (13.7) | 160 (1.83) | <0.01 |
| Prior PCI | 333 (39.8) | 3329 (38.1) | 0.35 |
| Prior CABG | 278 (33.2) | 1598 (18.3) | <0.01 |
| Indication for oral anticoagulant | |||
| Atrial fibrillation/flutter | 653 (78.0) | — | — |
| Venous thromboembolism | 137 (16.4) | — | — |
| Left ventricular dysfunction, aneurysm, or thrombus | 76 (9.08) | — | — |
| Hypercoagulable syndrome | 49 (5.85) | — | — |
| Valvular heart disease | 39 (4.66) | — | — |
| Cardioembolic stroke | 14 (1.67) | — | — |
| Other | 37 (4.42) | — | — |
| Type of OAC | |||
| Warfarin | 771 (92.2) | — | — |
| Dabigatran | 39 (4.67) | — | — |
| Rivaroxaban | 20 (2.39) | — | — |
| Apixaban | 3 (0.36) | — | — |
| Other | 4 (0.48) | — | — |
| P2Y12 inhibitor at admission | 178 (21.3) | — | — |
| Type of P2Y12 inhibitor | |||
| Clopidogrel | 173 (97.2) | — | — |
| Ticagrelor | 1 (0.56) | — | — |
| Prasugrel | 3 (1.69) | — | — |
| Other | 1 (0.56) | — | — |
| Aspirin at admission | 592 (70.7) | — | — |
| Bridging therapy among elective PCIs | 68/307 (22.1) | — | — |
| OAC among survivors to discharge | 727/816 (89.1) | — | — |
| P2Y12 inhibitor among survivors to discharge | 790/816 (96.8) | 8237/8541 (96.4) | 0.58 |
| Type of P2Y12 inhibitor | |||
| Clopidogrel | 773/790 (97.8) | 7489/8541 (87.7) | <0.01 |
| Ticagrelor | 10/790 (1.27) | 466/8541 (5.46) | |
| Prasugrel | 7/790 (0.89) | 322/8541 (3.77) | |
| Aspirin among survivors at discharge | 800/816 (98.0) | 8340/8541 (97.6) | 0.48 |
| Triple therapy among survivors at discharge | 693/816 (84.9) | — | — |
Data are shown as n (%) except as noted. BMI indicates body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; OAC, oral anticoagulant; PCI, percutaneous coronary intervention.
Figure 2.
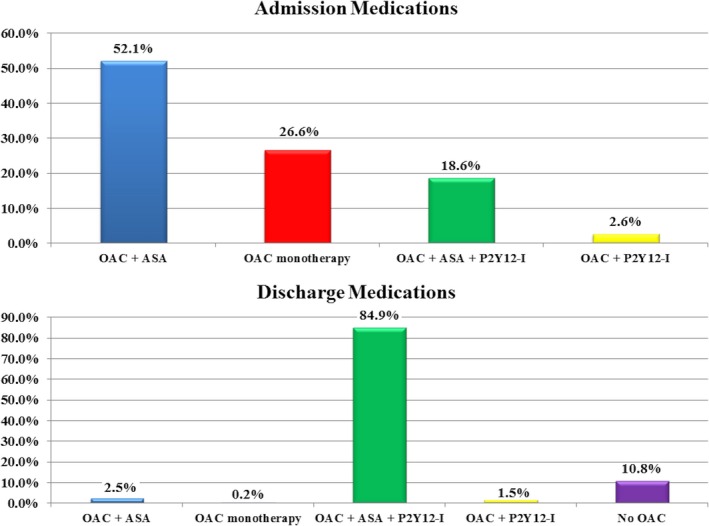
Antithrombotic regimens at admission and discharge among patients on chronic oral anticoagulant therapy undergoing PCI. ASA indicates aspirin; OAC, oral anticoagulant; P2Y12‐I, P2Y12 inhibitor; PCI, percutaneous coronary intervention.
Following PCI, 89.1% of patients admitted on OAC therapy who survived to discharge were on an OAC agent. In addition, 96.8% were discharged on a P2Y12 inhibitor, 98.0% were discharged on aspirin, and 84.9% were discharged on triple therapy (Figure 2). The majority were discharged on an aspirin dose of 81 mg daily (64.1%). Clopidogrel was the preferred P2Y12 inhibitor at discharge among patients on OAC therapy, was primarily dosed at 75 mg daily (97.3%), and was used with greater frequency compared with those not on OAC therapy (97.8% versus 87.7%; P<0.01).
Clinical Characteristics and Outcomes Associated With OAC Therapy
Compared with patients not on OAC therapy, OAC users were older, were more often of white race, and had more cardiovascular risk factors and prior cardiovascular disease (Table 1). In addition, OAC patients presented less often with ST‐segment elevation MI, were less often treated with glycoprotein IIb/IIIa inhibitors or bivalirudin, and less frequently received drug‐eluting stents and had lower risk lesions treated (Table 2). Rates of radial and femoral arterial access were comparable between groups. Patients on OACs had higher predicted risk of post‐PCI bleeding compared with those not on OACs (2.9% versus 2.5%; P<0.01) but similar predicted risk of in‐hospital mortality (2.3% for OAC use versus 2.0% for no OAC; P=0.23).
Table 2.
Procedural Characteristics by OAC Use
| Characteristic | OAC Use (n=837) | No OAC Use (n=8729) | P Value |
|---|---|---|---|
| Presentation type | <0.01 | ||
| STEMI | 46 (5.50) | 1257 (14.4) | — |
| NSTEMI | 171 (20.4) | 2062 (23.6) | — |
| Unstable angina | 236 (28.2) | 2422 (27.8) | — |
| Stable angina | 157 (18.8) | 1780 (20.4) | — |
| Other | 227 (27.1) | 1208 (13.8) | — |
| Cardiogenic shock within 24 hours | 19 (2.27) | 216 (2.47) | 0.72 |
| Cardiac arrest within 24 hours | 18 (2.15) | 214 (2.45) | 0.59 |
| Cardiomyopathy or LV systolic dysfunction | 341 (40.7) | 2157 (24.7) | <0.01 |
| Arterial access | |||
| Femoral | 544 (65.0) | 5475 (62.7) | 0.19 |
| Radial | 271 (32.4) | 3080 (35.3) | 0.092 |
| Procedure anticoagulants | |||
| Unfractionated heparin | 686 (82.0) | 6943 (79.5) | 0.10 |
| Low molecular weight heparin | 14 (1.67) | 124 (1.42) | 0.56 |
| Glycoprotein IIb/IIIa inhibitor | 26 (3.11) | 617 (7.07) | <0.01 |
| Bivalirudin | 292 (34.9) | 3569 (40.9) | <0.01 |
| Highest risk lesion class | |||
| SCAI class II/III vs I | 237 (28.3) | 2671 (30.6) | 0.17 |
| SCAI class IV vs I | 53 (6.33) | 726 (8.32) | 0.044 |
| Highest risk lesion | |||
| pLAD (vs other) | 118 (14.1) | 1488 (17.1) | 0.029 |
| Left main (vs other) | 64 (7.65) | 327 (3.75) | <0.01 |
| DES placed | 341 (40.7) | 5913 (67.7) | <0.01 |
| BMS placed | 427 (51.0) | 2232 (25.6) | <0.01 |
| IABP | 26 (3.11) | 353 (4.04) | 0.18 |
| Other mechanical support | 17 (2.03) | 99 (1.13) | 0.023 |
| Number of diseased vessels | |||
| 1 | 320 (38.2) | 4187 (48.0) | <0.01 |
| 2+ | 498 (59.5) | 4411 (50.5) | |
| Number of lesions, mean±SD | 1.40±0.69 | 1.42±0.73 | 0.33 |
| Total number of stents during lab visit, mean±SD | 1.56±0.87 | 1.60±0.96 | 0.27 |
| Thrombus present | 125 (14.9) | 2265 (26.0) | <0.01 |
| In‐stent restenosis | 115 (13.7) | 975 (11.2) | 0.025 |
| Preprocedure hemoglobin, g/dL, mean±SD | 12.4±2.22 | 13.0±2.05 | <0.01 |
| Postprocedure hemoglobin, g/dL, mean±SD | 11.4±2.04 | 12.0±1.96 | <0.01 |
| Preprocedure creatinine, mg/dL, mean±SD | 1.41±1.23 | 1.24±1.09 | <0.01 |
| Postprocedure creatinine, mg/dL, mean±SD | 1.52±1.44 | 1.29±1.21 | <0.01 |
| Predicted risk of post‐PCI bleeding, mean±SD | 0.029±0.025 | 0.025±0.026 | <0.01 |
| Predicted risk of in‐hospital mortality, mean±SD | 0.023±0.076 | 0.020±0.083 | 0.23 |
Data are shown as n (%) except as noted. BMS indicates bare metal stent; DES, drug‐eluting stent; IABP, intra‐aortic balloon pump; LV, left ventricular; NSTEMI, non–ST‐segment elevation myocardial infarction; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; pLAD, proximal left anterior descending; SCAI, Society for Cardiac Angiography and Interventions; STEMI, ST‐segment elevation myocardial infarction.
Chronic treatment with OACs was associated with higher crude rates of in‐hospital major bleeding (10.5% versus 6.5%; P<0.01), access‐site bleeding (2.3% versus 1.3%; P=0.017), and non–access‐site bleeding (8.2% versus 5.2%; P<0.01) following PCI (Table 3). In addition, prior to adjustment, patients taking OACs used more hospital resources after PCI, including requiring more transfusions (7.2% versus 4.2%; P<0.01), needing longer lengths of postprocedure stays (3.9±5.5 versus 2.8±4.6 days; P<0.01), and undergoing more rehospitalizations within 30 days (10.3% versus 6.9%; P<0.01) and 90 days (22.1% versus 13.1%; P<0.01) after discharge. There were no differences in the crude frequencies of post‐PCI in‐hospital stent thrombosis (0.36% versus 0.32%; P=0.85), MI (2.50% versus 3.02%; P=0.40), or cerebrovascular accident or stroke (0.48% versus 0.52%; P=0.88) between chronic OAC users versus nonusers (Table 3).
Table 3.
Unadjusted Rates of Outcomes by OAC Use
| Outcome | OAC Use (n=837) | No OAC Use (n=8729) | Absolute Risk Difference With OAC Use | Number Needed to Harm | P Value |
|---|---|---|---|---|---|
| Primary | |||||
| In‐hospital major bleeding | 88 (10.5) | 565 (6.47) | +4.04 | 25 | <0.01 |
| In‐hospital mortality | 21 (2.51) | 188 (2.15) | +0.36 | — | 0.50 |
| Secondary | |||||
| Access‐site bleeding | 19 (2.27) | 111 (1.27) | +1.00 | 101 | 0.017 |
| Non–access‐site bleeding | 69 (8.24) | 454 (5.20) | +3.04 | 33 | <0.01 |
| RBC transfusion | 60 (7.17) | 364 (4.17) | +3.00 | 34 | <0.01 |
| In‐hospital stent thrombosis | 3 (0.36) | 28 (0.32) | +0.04 | — | 0.85 |
| Post‐PCI MI | 21 (2.51) | 264 (3.02) | −0.51 | — | 0.40 |
| CVA/stroke | 4 (0.48) | 45 (0.52) | −0.04 | — | 0.88 |
| Post‐PCI length of stay, days, mean±SD | 3.94±5.50 | 2.79±4.56 | — | — | <0.01 |
| 30‐day readmissions within index health care system | 86 (10.3) | 606 (6.94) | +3.33 | 30 | <0.01 |
| 90‐day readmissions within index health care system | 185 (22.1) | 1146 (13.1) | +8.97 | 12 | <0.01 |
Data are shown as n (%) except as noted. CVA indicates cerebrovascular accident; MI, myocardial infarction; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; RBC, red blood cell.
In adjusted models, chronic OAC use remained associated with increased risks of in‐hospital major bleeding (odds ratio 1.50, 95% CI 1.14–1.99; P<0.01), access‐site bleeding (odds ratio 1.82, 95% CI 1.07–3.09; P=0.028), and non–access‐site bleeding (odds ratio 1.69, 95% CI 1.27–2.26; P<0.01) after PCI (Table 4). In addition, the association between OAC use prior to PCI and 90‐day readmission persisted after adjustment (odds ratio 1.40, 95% CI 1.16–1.69; P<0.01). Chronic OAC users and nonusers had similar risk of post‐PCI in‐hospital mortality (2.51% versus 2.15%, respectively [P=0.50]; adjusted odds ratio 1.15, 95% CI 0.66–1.99; P=0.63). Patients on OAC therapy, however, had greater risk of long‐term mortality following PCI, with an unadjusted cumulative incidence of 4‐year survival of 79.9% compared with 89.8% among those not on OACs (log‐rank P<0.01) (Figure 3), and an adjusted 36% increased hazard of long‐term mortality (hazard ratio 1.36, 95% CI 1.11–1.66; P<0.01).
Table 4.
Unadjusted and Adjusted Risks of Outcomes With OAC Use
| Unadjusted | Adjusted by Propensity Score | |||||
|---|---|---|---|---|---|---|
| Risk Ratio | 95% CI | P Value | Risk Ratio | 95% CI | P Value | |
| In‐hospital mortality | 1.17 | 0.74–1.85 | 0.50 | 1.15 | 0.66–1.99 | 0.63 |
| Long‐term mortality | 2.01 | 1.68–2.40 | <0.01 | 1.36 | 1.11–1.66 | <0.01 |
| In‐hospital major bleeding | 1.70 | 1.34–2.15 | <0.01 | 1.50 | 1.14–1.99 | <0.01 |
| Access‐site bleeding | 1.80 | 1.10–2.95 | 0.019 | 1.82 | 1.07–3.09 | 0.028 |
| Non–access‐site bleeding | 1.64 | 1.26–2.13 | <0.01 | 1.69 | 1.27–2.26 | <0.01 |
| 90‐day readmission within index health care system | 1.88 | 1.58–2.24 | <0.01 | 1.40 | 1.16–1.69 | <0.01 |
OAC indicates oral anticoagulant.
Figure 3.
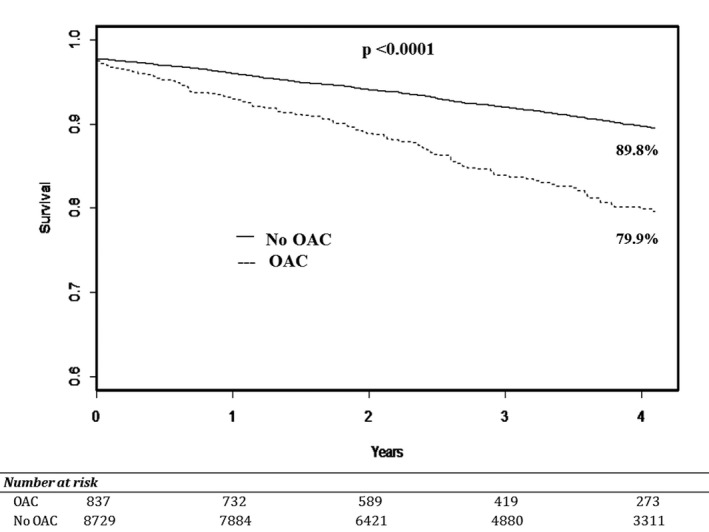
Long‐term mortality following PCI by use of OAC therapy. Kaplan–Meier estimates comparing long‐term mortality among patients undergoing PCI stratified by OAC use. In unadjusted analysis, OAC use was associated with a significant decrease in long‐term survival compared with those not on OAC therapy (no OAC; log‐rank P<0.001). OAC indicates oral anticoagulant; PCI, percutaneous coronary intervention.
Clinical Characteristics and Outcomes Associated With NOAC Therapy
Patients on NOAC therapy, compared with VKAs, had lower rates of cerebrovascular disease, chronic lung disease, and prior valve surgery but otherwise had comparable demographics, burden of cardiovascular risk factors, and prior cardiovascular disease (Table S1). In addition, between OAC treatment strategies, there were similar indications for anticoagulant therapy and use of antiplatelet agents at admission. Among patients on NOACs versus VKAs, there were no significant differences in the type of presentation for PCI, procedural characteristics, or predicted risk of bleeding (Table S2). At discharge, there was comparable use of triple therapy between treatment strategies; however, those on NOACs who were discharged on P2Y12 inhibitors were less often treated with clopidogrel (Table S1).
Following PCI, there were no significant differences in crude rates of in‐hospital major bleeding (10.6% versus 10.5%; P=0.98), access‐site bleeding (3.03% versus 2.20%; P=0.67), and non–access‐site bleeding (7.58% versus 8.30%; P=0.84) among patients on NOAC versus VKA therapy (Table S3). In addition, there were comparable unadjusted rates of post‐PCI in‐hospital stent thrombosis (0% versus 0.39%; P=0.61), MI (3.03% versus 2.46%; P=0.78), and cerebrovascular accident or stroke (0% versus 0.52%; P=0.56) between treatment strategies. Hospital resources were used similarly between patients on NOAC and VKA therapy in unadjusted analyses, with no significant differences in need for transfusion (9.1% versus 7.0%; P=0.53), post‐PCI length of stay (3.1±4.8 days versus 4.0±5.6 days; P=0.10), or 30‐ and 90‐day readmission rates (30 days: 6.1% versus 10.6% [P=0.24]; 90 days: 13.6% versus 22.8% [P=0.08]). In adjusted models, chronic NOAC versus VKA therapy was not associated with differing risks of in‐hospital bleeding, in‐hospital mortality, long‐term mortality, or 90‐day readmission (P>0.05 for all) (Table S4).
Discussion
Among contemporary patients undergoing PCI, 1 in 11 patients was on chronic OAC therapy. From 2009 through 2014, there was no significant change in the number of patients treated with OACs undergoing PCI; however, there was an increase in the use of non‐VKA agents among these patients. In addition, triple therapy after coronary stenting was used with high frequency throughout the study period. Patients on chronic OAC therapy had greater adjusted risks of in‐hospital bleeding, need for readmission, and long‐term death compared with those not on OAC therapy.
Our analysis found that 9% of all patients undergoing coronary stenting are currently treated with chronic OAC therapy, comprising a large proportion of the PCI population. Atrial arrhythmias were the main reason for treatment, yet PCI patients also had a variety of other indications for chronic anticoagulation. Consistent with previous analyses in ambulatory patients,14, 15 we noted a brisk increase in use of NOACs during the study period, starting at the time of market approval of the first NOAC in 2010; however, we did not observe any major change in the overall number of patients being treated with OACs. This finding contrasts with results from a recent analysis of ambulatory patients with atrial fibrillation on OAC therapy that demonstrated a near doubling in patient visits since 2009.14 Our results likely differ due to the expanded use of NOACs in patients at lower risk of stroke,17 many of whom have fewer comorbidities and thus lower likelihood of undergoing PCI.
PCI patients were discharged on triple therapy (OAC plus a P2Y12 inhibitor and aspirin) at a rate of 85%, which remained constant during the study period. This preference for triple therapy persisted among treating clinicians despite presentation of the WOEST (What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing) trial results in August 2012. The WOEST trial randomized stented patients with an indication for OAC to treatment with a VKA plus clopidogrel with or without aspirin and found no difference in outcomes at 1 year between treatment strategies.25 This study was further supported by similar findings in large registry analyses26, 27 and resulted in the inclusion of restricted OAC plus P2Y12 inhibitor use following PCI in the most recent consensus document for the management of atrial fibrillation patients following coronary stenting.19 Nevertheless, it appears from our analysis that these data have yet to influence actual clinician practice patterns within the studied health care system, at least at the time of discharge. We did not assess whether changes in antithrombotic regimens may have occurred later in follow‐up.
Despite continued efforts among coronary interventionalists to improve treatment practices to reduce post‐PCI bleeding,28, 29 1 in 10 patients on chronic OAC therapy had an in‐hospital major bleeding event following coronary stenting. Conversely, chronic OAC use prior to PCI did not reduce rates of postprocedure ischemic events, similar to a prior study among patients with acute MI.7 Bleeding events primarily consisted of non–access‐site bleeds, suggesting that current PCI vascular bleeding avoidance strategies, such as increased use of radial access29, 30 and arterial closure devices,28, 31 may not alone mitigate the bleeding risk associated with OAC use. It is noteworthy that OAC patients had greater predicted risk of bleeding regardless of OAC treatment, suggesting that the bleeding hazard in these patients is not solely related to use of these agents but also due to a greater proportion of clinical characteristics associated with post‐PCI bleeding.
Patients taking OACs at the time of PCI had decreased survival compared with those not on anticoagulants, with a 36% greater adjusted risk of long‐term mortality. Although causality cannot be established from our analysis, this association may be related to the long‐term consequences of increased bleeding events32, 33 or mediated by inherent patient factors, such as a higher prevalence of frailty among those on OAC therapy.34 In addition, use of triple therapy at discharge may be associated with risk of decreased long‐term survival.25 At minimum, chronic treatment with an OAC represents a strong marker of risk for future morbidity and mortality following PCI.
Use of hospital resources was also greater among OAC‐treated patients following PCI, including longer durations of stay and more need for transfusions. In addition, the frequency of readmission was marked, with 22% of chronically anticoagulated patients requiring rehospitalization within our health care system within 90 days of PCI. Of importance, at discharge, the majority of these patients were on triple therapy, which increases the risk of out‐of‐hospital adverse events necessitating medical attention, particularly risk of bleeding.26 Consequently, it is important to recognize that OAC patients have a substantial influence on hospital‐related costs after coronary stenting.
Demographics and cardiovascular risk factors were comparable between those receiving NOACs and VKAs and differ from those of ambulatory patients on NOACs, who are often younger and have fewer comorbidities.17 In addition, patients undergoing PCI on NOACs versus VKAs did not vary in procedural characteristics, including sites of access, types of parenteral anticoagulants administered, and use of triple therapy at discharge. Although the point estimates comparing short‐ and long‐term outcomes following PCI between treatment strategies were not statistically different, the study was underpowered to draw definitive conclusions.
The results of this analysis must be considered in the context of the study design. The analysis used a computerized method of searching electronic health records to identify patients on OAC therapy. Although all patients identified as being on OAC therapy were manually reviewed to confirm treatment, this analysis could have missed patients whose treatment was poorly documented or who had treatment terms not included in the search algorithm. In addition, data were not available for time in the therapeutic range among patients treated with VKAs, including prior to the procedure, and for events occurring after discharge, such as discontinuation of OAC therapy or the occurrence of out‐of‐hospital bleeding events. This study primarily analyzed in‐hospital events that were not adjudicated. As such, rates of events could have been under‐ or overestimated; however, we expected misclassification of events to be similar between OAC and non‐OAC patients, thus biasing the comparison of the treatment strategies to the null. This study was conducted using PCI data from 2 academic tertiary care medical centers from a single integrated health care system and may not be generalizable to other settings. Readmission data were available only for patients returning to a hospital within the Partners HealthCare system. Patients were primarily treated with clopidogrel and received PCI via femoral arterial access, whereas more potent antiplatelets and greater use of transradial arterial access may have differential effects on outcomes. Last, we do not know whether the associations observed between OAC treatment and adverse events following PCI are causal in nature, and unmeasured factors could confound the relationships observed.
Conclusions
Chronic OAC therapy is frequently used among contemporary patients undergoing PCI. Patients on OAC therapy had greater adjusted risks of in‐hospital bleeding, need for readmission, and long‐term mortality following PCI. As such, use of OACs at the time of PCI is an important prognostic marker. Efforts are needed to specifically reduce the occurrence of adverse events following PCI in this population.
Sources of Funding
The work was funded, in part, by a SPARK Award from the Corrigan Minehan Heart Center at the Massachusetts General Hospital.
Disclosures
Dr Yeh serves on an advisory board for Abbott Vascular and Boston Scientific. He has provided expert witness testimony for Merck. Dr Wasfy provides consulting for Gilead, QPID health, and Biotronik. All other authors have nothing to disclose.
Supporting information
Table S1. Patient Characteristics of Percutaneous Coronary Interventions by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S2. Presentation and Procedural Characteristics of Percutaneous Coronary Interventions by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S3. Unadjusted Rates of Outcomes by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S4. Unadjusted and Adjusted Risks of Outcomes With Vitamin K Antagonist Oral Anticoagulant Use
Figure S1. Temporal changes in use of antithrombotic agents at admission and discharge among patients on chronic OAC therapy undergoing percutaneous coronary intervention during the study period. OAC indicates oral anticoagulant therapy; P2Y12‐I, P2Y12 inhibitor.
(J Am Heart Assoc. 2016;5:e004310 doi: 10.1161/JAHA.116.004310)
Results from this study have previously been presented at the 2016 American College of Cardiology Scientific Sessions, April 2–4, 2016, in Chicago, IL.
References
- 1. Segev A, Strauss BH, Tan M, Constance C, Langer A, Goodman SG. Predictors and 1‐year outcome of major bleeding in patients with non‐ST‐elevation acute coronary syndromes: insights from the Canadian Acute Coronary Syndrome Registries. Am Heart J. 2005;150:690–694. [DOI] [PubMed] [Google Scholar]
- 2. Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, Bertrand M, Ohman EM, Parise H, Lansky AJ, Lincoff AM, Stone GW. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient‐level pooled analysis of the REPLACE‐2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–664. [DOI] [PubMed] [Google Scholar]
- 3. Verheugt FW, Steinhubl SR, Hamon M, Darius H, Steg PG, Valgimigli M, Marso SP, Rao SV, Gershlick AH, Lincoff AM, Mehran R, Stone GW. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:191–197. [DOI] [PubMed] [Google Scholar]
- 4. Chhatriwalla AK, Amin AP, Kennedy KF, House JA, Cohen DJ, Rao SV, Messenger JC, Marso SP. Association between bleeding events and in‐hospital mortality after percutaneous coronary intervention. JAMA. 2013;309:1022–1029. [DOI] [PubMed] [Google Scholar]
- 5. Rubboli A, Sciahbasi A, Briguori C, Saia F, Palmieri C, Moroni LA, Calabro P, Leone AM, Franco N, Valgimigli M, Varani E, Santi M, Pasqualini P, Capecchi A, Piccalo G, Margheri M, di Pasquale G, Galvani M, Bolognese L, Gonzini L, Maggioni AP. In‐hospital management and outcome of patients on warfarin undergoing coronary stent implantation: results of the multicenter, prospective WARfarin and coronary STENTing (WAR‐STENT) registry. J Invasive Cardiol. 2013;25:170–176. [PubMed] [Google Scholar]
- 6. Naruse Y, Sato A, Hoshi T, Takeyasu N, Kakefuda Y, Ishibashi M, Misaki M, Abe D, Aonuma K. Triple antithrombotic therapy is the independent predictor for the occurrence of major bleeding complications: analysis of percent time in therapeutic range. Circ Cardiovasc Interv. 2013;6:444–451. [DOI] [PubMed] [Google Scholar]
- 7. Oudot A, Steg PG, Danchin N, Dentan G, Zeller M, Sicard P, Buffet P, Laurent Y, Janin‐Manificat L, L'Huillier I, Beer JC, Makki H, Morel P, Cottin Y. Impact of chronic oral anticoagulation on management and outcomes of patients with acute myocardial infarction: data from the RICO survey. Heart. 2006;92:1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossini R, Musumeci G, Lettieri C, Molfese M, Mihalcsik L, Mantovani P, Sirbu V, Bass TA, Della Rovere F, Gavazzi A, Angiolillo DJ. Long‐term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol. 2008;102:1618–1623. [DOI] [PubMed] [Google Scholar]
- 9. Wang TY, Robinson LA, Ou FS, Roe MT, Ohman EM, Gibler WB, Smith SC Jr, Peterson ED, Becker RC. Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry. Am Heart J. 2008;155:361–368. [DOI] [PubMed] [Google Scholar]
- 10. Rubboli A, Colletta M, Valencia J, Capecchi A, Franco N, Zanolla L, La Vecchia L, Piovaccari G, Di Pasquale G. Periprocedural management and in‐hospital outcome of patients with indication for oral anticoagulation undergoing coronary artery stenting. J Interv Cardiol. 2009;22:390–397. [DOI] [PubMed] [Google Scholar]
- 11. Jackson LR II, Ju C, Zettler M, Messenger JC, Cohen DJ, Stone GW, Baker BA, Effron M, Peterson ED, Wang TY. Outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention receiving an oral anticoagulant and dual antiplatelet therapy: a comparison of clopidogrel versus prasugrel from the TRANSLATE‐ACS Study. JACC Cardiovasc Interv. 2015;8:1880–1889. [DOI] [PubMed] [Google Scholar]
- 12. Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, Berger PB, Iakovou I, Dangas G, Waksman R, Antoniucci D, Sartori S, Krucoff MW, Hermiller JB, Shawl F, Gibson CM, Chieffo A, Alu M, Moliterno DJ, Colombo A, Pocock S. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. [DOI] [PubMed] [Google Scholar]
- 13. Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in Medicare beneficiaries: trends by age, sex, and race, 1992–2010. J Am Heart Assoc. 2014;3:e000756 doi: 10.1161/JAHA.113.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128: 1300–1305.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olesen JB, Sorensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, Kober L, Gislason GH, Torp‐Pedersen C, Fosbol EL. Non‐vitamin K antagonist oral anticoagulation agents in anticoagulant naive atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace. 2015;17:187–193. [DOI] [PubMed] [Google Scholar]
- 17. Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation‐ quality and cost implications. Am J Med. 2014;127:1075–1082.e1. [DOI] [PubMed] [Google Scholar]
- 18. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 19. Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia‐Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014;35:3155–3179. [DOI] [PubMed] [Google Scholar]
- 20. Waldo SW, Secemsky EA, O'Brien C, Kennedy KF, Pomerantsev E, Sundt TM III, McNulty EJ, Scirica BM, Yeh RW. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014;130:2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Cardiovascular Data Registry . Data elements & definitions, technology downloads and risk adjustment. Available at: http://cvquality.acc.org/~/media/QII/NCDR/Data%20Collection%20Forms/CathPCI%20Registry_DataCollectionForm.ashx. Accessed December 10, 2014.
- 22. Rao SV, McCoy LA, Spertus JA, Krone RJ, Singh M, Fitzgerald S, Peterson ED. An updated bleeding model to predict the risk of post‐procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6:897–904. [DOI] [PubMed] [Google Scholar]
- 23. McCabe JM, Kennedy KF, Eisenhauer AC, Waldman HM, Mort EA, Pomerantsev E, Resnic FS, Yeh RW. Reporting trends and outcomes in ST‐segment‐elevation myocardial infarction national hospital quality assessment programs. Circulation. 2014;129:194–202. [DOI] [PubMed] [Google Scholar]
- 24. Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dewilde WJM, Oirbans T, Verheugt FWA, Kelder JC, De Smet BJGL, Herrman J‐P, Adriaenssens T, Vrolix M, Heestermans AACM, Vis MM, Tijsen JGP, van‘t Hof AW, ten Berg JM. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 26. Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, Mikkelsen A, Christensen CB, Lip GY, Kober L, Torp‐Pedersen C, Hansen ML. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013;62:981–989. [DOI] [PubMed] [Google Scholar]
- 27. Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, Kober L, Torp‐Pedersen C, Gislason GH, Hansen ML. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126:1185–1193. [DOI] [PubMed] [Google Scholar]
- 28. Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. [DOI] [PubMed] [Google Scholar]
- 29. Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. [DOI] [PubMed] [Google Scholar]
- 30. Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, Summaria F, Patrizi R, Borghi A, Di Russo C, Moretti C, Agostoni P, Loschiavo P, Lioy E, Sheiban I, Sangiorgi G. Radial versus femoral randomized investigation in ST‐segment elevation acute coronary syndrome: the RIFLE‐STEACS (Radial Versus Femoral Randomized Investigation in ST‐Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60:2481–2489. [DOI] [PubMed] [Google Scholar]
- 31. Wimmer NJ, Secemsky EA, Mauri L, Roe MT, Saha‐Chaudhuri P, Dai D, McCabe JM, Resnic FS, Gurm HS, Yeh RW. Effectiveness of arterial closure devices for preventing complications with percutaneous coronary intervention: an instrumental variable analysis. Circ Cardiovasc Interv. 2016;9:e003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long‐term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65:1411–1420. [DOI] [PubMed] [Google Scholar]
- 33. Lopes RD, Subherwal S, Holmes DJN, Thomas L, Wang TY, Rao SV, Magnus Ohman E, Roe MT, Peterson ED, Alexander KP. The association of in‐hospital major bleeding with short‐, intermediate‐, and long‐term mortality among older patients with non‐ST‐segment elevation myocardial infarction. Eur Heart J. 2012;33:2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics of Percutaneous Coronary Interventions by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S2. Presentation and Procedural Characteristics of Percutaneous Coronary Interventions by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S3. Unadjusted Rates of Outcomes by Non–Vitamin K Antagonist Oral Anticoagulant Versus Vitamin K Antagonist Use
Table S4. Unadjusted and Adjusted Risks of Outcomes With Vitamin K Antagonist Oral Anticoagulant Use
Figure S1. Temporal changes in use of antithrombotic agents at admission and discharge among patients on chronic OAC therapy undergoing percutaneous coronary intervention during the study period. OAC indicates oral anticoagulant therapy; P2Y12‐I, P2Y12 inhibitor.


