Abstract
Objective
To determine if serum levels of endothelial adhesion molecules were associated with the development of multiple organ failure (MOF) and in-hospital mortality in adult patients with severe sepsis.
Design
This study was a secondary data analysis of a prospective cohort study.
Setting
Patients were admitted to two tertiary intensive care units in San Antonio, TX, between 2007 and 2012.
Patients
Patients with severe sepsis at the time of intensive care unit (ICU) admission were enrolled. Inclusion criteria were consistent with previously published criteria for severe sepsis or septic shock in adults. Exclusion criteria included immunosuppressive medications or conditions.
Interventions
None.
Measurements
Baseline serum levels of the following endothelial cell adhesion molecules were measured within the first 72 hours of ICU admission: Intracellular Adhesion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule-1 (VCAM-1), and Vascular Endothelial Growth Factor (VEGF). The primary and secondary outcomes were development of MOF (≥2 organ dysfunction) and in-hospital mortality, respectively.
Main results
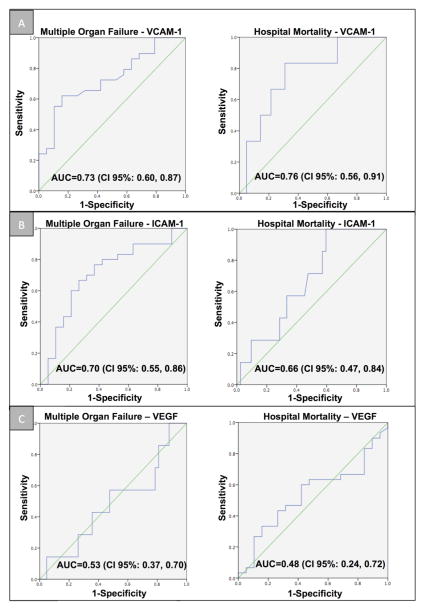
Forty-eight patients were enrolled in this study, of which 29 (60%) developed MOF. Patients that developed MOF had higher levels of VCAM-1 (p=0.01) and ICAM-1 (p=0.01), but not VEGF (p=0.70) compared with patients without MOF (single organ failure only). The area under the curve (AUC) to predict MOF according to VCAM-1, ICAM-1 and VEGF was 0.71, 0.73, and 0.54, respectively. Only increased VCAM-1 levels were associated with in-hospital mortality (p=0.03). These associations were maintained even after adjusting for APACHE and SOFA scores using logistic regression.
Conclusions
High levels of serum ICAM-1 was associated with the development of MOF. High levels of VCAM-1 was associated with both MOF and in-hospital mortality.
Keywords: Biomarkers, Sepsis, Shock, Mortality, Multiple Organ Failure, Intracellular Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and Vascular Endothelial Growth Factor
INTRODUCTION
Mortality rates for patients with severe sepsis and septic shock range from 40–60%, costing the United States health care system approximately $17 billion annually [1]. To expedite the initiation of effective treatments and thereby reduce mortality and associated costs, better methods to identify patients with severe sepsis are needed Hemodynamic parameters and laboratory tests, including lactate levels are currently used to predict multiple organ failure (MOF) in patients with sepsis; however information regarding how to detect early organ failure is limited [2, 3]. Clinical deterioration and death result from a complex interaction between inflammation and coagulation that leads to organ dysfunction [4]. Vascular endothelial damage precedes organ dysfunction and plays an important role by increasing vascular permeability, promoting activation of the coagulation cascade and compromising regional perfusion in vital organs (e.g. kidneys, liver, gut, etc.) [5]. Different biomarkers have been proposed to assess vascular endothelial damage in patients with sepsis and the development of MOF and mortality [6]. Cell adhesion molecules (CAMs) have emerged as potential biomarkers that may be used to detect early endothelial injury in septic patients [7].
Vascular Endothelial Growth Factor (VEGF), Intracellular Adhesion Molecule 1 (ICAM-1), and Vascular Cell Adhesion Molecule-1 (VCAM-1) are a group of transmembrane CAM proteins that are responsible for the cell adhesion process. These CAMs allow cells to interact with the extracellular matrix, the cytoskeleton, and other cells within the vascular endothelium, as well as other cells within the circulation [8]. ICAM-1 and VCAM-1 are present in the cell membrane of both leukocytes and the cells lining the vascular endothelium, allowing inflammatory cells to transmigrate into nearby tissues [8]. These adhesion molecules are expressed in very large quantities in patients with an uncontrolled inflammatory state such as sepsis [9]. As a result, some of these CAMs also leak into circulation, and especially after the vascular endothelial injury that occurs during sepsis, making them measurable. The value of VEGF for prediction of clinical outcomes in patients with sepsis is at present still controversial[10, 11], and limited data are available in adult patients with sepsis regarding ICAM-1 and VCAM-1 levels [12]. Therefore, more studies are needed to evaluate the role of CAMs in predicting the outcomes of patients with sepsis.
Our hypothesis was that higher levels of CAMs are related to a higher incidence of MOF and in-hospital mortality. Therefore, the aim of this study was to determine the association of levels of CAMs with the development of MOF and in-hospital mortality in adult patients with severe sepsis or septic shock.
MATERIAL AND METHODS
Study Design
This study was a secondary analysis of the data derived from a cohort of patients admitted to the intensive care unit (ICU) with severe sepsis or septic shock at two hospitals (South Texas Veterans Health Care System and University Hospital, San Antonio, TX), between 2007 and 2012, as previously descried [13]. This study was approved by the local institutional review board (HSC20070713H), and is posted on www.clinicaltrials.gov (NCT00708799). All participants signed a consent form before entry into the study.
Inclusion criteria for enrollment included age >18 years, written informed consent obtained from the patient/patient’s legal representative, and criteria for severe sepsis or septic shock [14]. Diagnosis of severe sepsis was established according to the International Sepsis Definition in which patients with Systemic Inflammatory Response Syndrome (SIRS) with suspected or proven source of infection develop at least one organ dysfunction [15]. Septic shock was defined as the need for vasopressor support in septic patients with hypotension non-responsive to fluid resuscitation [16]. Patients who developed MOF before blood collection were excluded. Immunosuppressed patients were excluded if they fulfilled the following definitions: 1) Chemotherapy within the last one month, 2) Leukemia/lymphoma not in remission, 3) Solid organ or bone marrow stem cell transplant, 4) HIV infection with CD4+ T lymphocyte count <200 cells/mm3, or 5) Chronic steroid use, defined as > 10 mg/day of prednisone.
Enrollment and Follow-Up
All patients were screened for eligibility at the time of admission to the ICU and followed daily until hospital discharge. Both the ‘Acute Physiology and Chronic Health Evaluation (APACHE) II’ and the ‘Sepsis-related Organ Failure Assessment (SOFA) scores were obtained for each patient during the first 24-hours of admission to the ICU [17].
Clinical Outcomes
The primary outcome of this study was development of MOF and the secondary outcome was in-hospital mortality. Multiple organ failure was defined as two or more organ failures during the ICU hospitalization among patients with severe sepsis or septic shock. Organ failure criteria are shown in Table 1. All the patients had their blood collected before the onset of MOF, and within 96 hours post ICU admission.
Table 1.
Organ failure criteria
| Organ/system | Criteria |
|---|---|
| Cardiovascular | Mean arterial pressure <65 mmHg or requirement for vasopressors or inotropes after appropriate volume resuscitation (30 mL/Kg) |
| Respiratory | PaO2/FiO2 <300 or requirement for mechanical ventilation or non-invasive ventilation |
| Renal | Patients who receive renal replacement therapy or serum creatinine ≥354 umol/L (4 mg/dL) or urine output <0.3 mL/kg/h |
| Hematologic | Prothrombin time and partial thromboplastin time >1.5 – 3 times the normal range or platelet count <100.000/mm3 |
| Hepatic | Alanine aminotransferase and aspartate aminotransferase > 100 Units/L or total plasma bilirubin >1 mg/dL (0.17 mmol/L) |
| Central nervous system | Glasgow coma scale score <12 points in absence of sedation |
Biomarkers and Assays
Venous blood was drawn from patients within the first 72 hours of ICU admission. Serum ICAM-1, VCAM-1 and VEGF were measured using a commercially available Human Inflammation Panel kit from Luminex Technology that was analyzed at Myriad Rules Based Medicine Inc. (Austin, Texas).
Statistical Analysis
Categorical variables were compared between groups using Fisher’s exact test. Continuous variables were evaluated using non-parametric analysis with the Mann-Whitney U Test. Values are expressed as median (IQR). Statistical significance was defined as p-value ≤ 0.05. A receiver operating characteristic (ROC) curve was developed to assess the accuracy of ICAM-1, VCAM-1 and VEGF to predict outcomes. Multivariate analysis was performed using multiple logistic regressions to evaluate the relation of serum levels of ICAM-1, VCAM-1 and VEGF with the proposed outcomes after adjusting the analysis with the APACHE II and SOFA scores. All statistical analyses were performed with IBM SPSS, Statistics for Macintosh, version 23.0 (Armonk, NY: IBM Corp).
RESULTS
Patient characteristics
Forty-eight patients were included in the study cohort, of which 29 (60%) developed MOF. There were no significant differences in baseline characteristics among severe septic patients who developed MOF compared with those that did not develop MOF (Table 2). Severe septic patients with MOF had higher median APACHE II scores on admission compared with those without MOF (21[IQR 15,28] vs. 19[14,21]; p=0.04).
Table 2.
Baseline characteristics of patients with severe sepsis stratified according to the presence of multiple organ failure (MOF) during ICU hospitalization
| Characteristic | No MOF (n=19) | MOF (n=29) | p Value |
|---|---|---|---|
| Demographic | |||
| Male | 19 (100) | 27 (93) | 0.60 |
| Age, mean (IQR*) | 59 (51, 65) | 58 (45, 79) | 0.60 |
| Comorbid conditions, n (%) | |||
| Obesity | 7 (37) | 12 (41) | 0.50 |
| Active cancer | 2 (10) | 0 (0) | 0.20 |
| Prior cancer | 3 (16) | 5 (17) | 0.60 |
| Cardiovascular disease | 7 (37) | 5 (17) | 0.10 |
| Chronic heart failure | 3 (16) | 1 (3) | 0.20 |
| COPD | 3 (16) | 3 (10) | 0.40 |
| Chronic kidney disease | 3 (16) | 2 (7) | 0.30 |
| Depression | 2 (10) | 7 (24) | 0.20 |
| Diabetes mellitus | 10 (53) | 13 (45) | 0.40 |
| HIV infection | 1 (5) | 0 (0) | 0.70 |
| Hyperlipidemia | 4 (17) | 1 (3) | 0.60 |
| Leukemia | 1 (5) | 0 (0) | 0.40 |
| Liver disease | 1 (5) | 2 (7) | 0.60 |
| Tobacco use | 6 (32) | 8 (28) | 0.50 |
| Alcohol use | 4 (21) | 7 (24) | 0.50 |
| Asthma | 1 (5) | 3 (10) | 0.50 |
| Source of infection, n (%) | |||
| Pulmonary | 7 (37) | 8 (28) | 0.50 |
| Urinary tract | 6 (32) | 10 (34) | 0.30 |
| Gastrointestinal | 2 (10) | 4 (14) | 0.40 |
| Skin | 3 (16) | 5 (17) | 0.30 |
| Endocarditis | 0 (0) | 1 (3) | 0.90 |
IQR, interquartile range
Outcomes
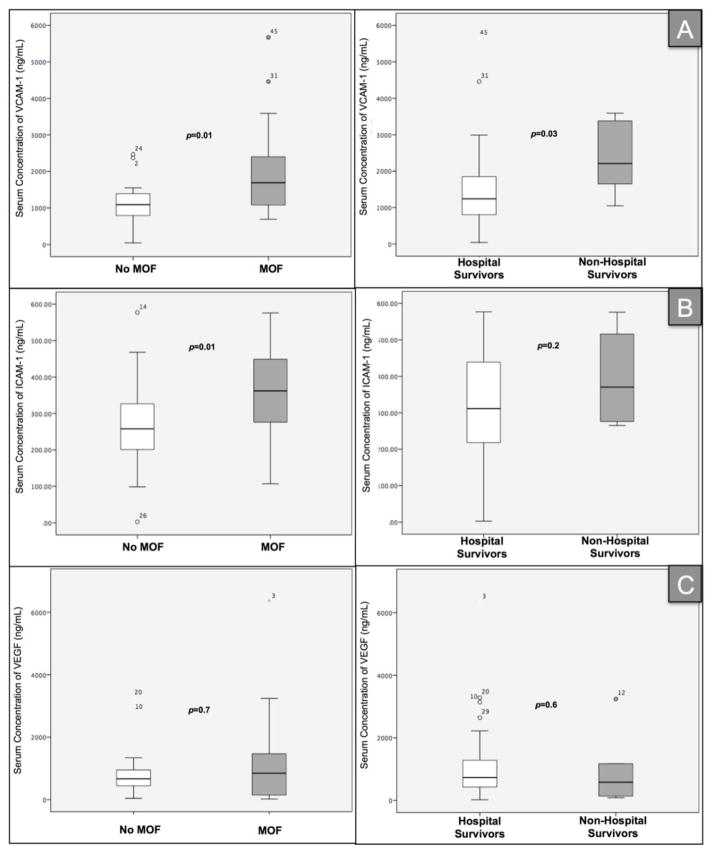
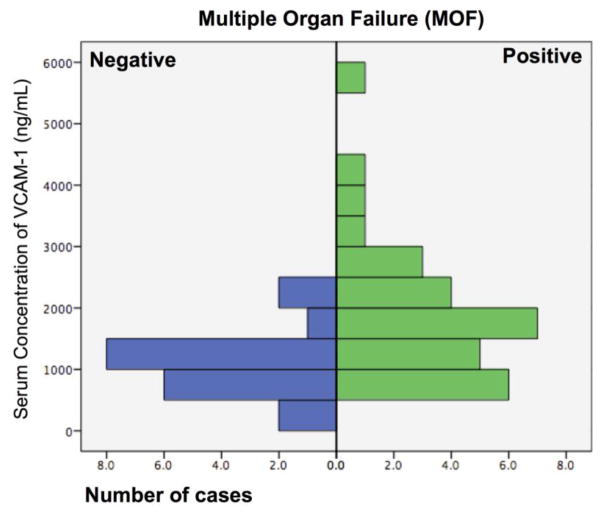
Patients with severe sepsis who developed MOF had higher levels of VCAM-1 (median 1,690 ng/mL [IQR 1,065, 3,590] vs. 1,090 ng/mL [789, 1,410]; p=0.01) and ICAM-1 (median 362 ng/mL [IQR 270, 449] vs. median 258 ng/mL [IQR199, 341]; p=0.01), compared to patients with severe sepsis alone respectively (Figure 1). This association remained significant even after controlling for APACHE II and SOFA scores in the logistic regression analysis (odds ratio (OR)=1.2, 95%CI: 1.1–1.3, p=0.01). No significant association between VEGF and the development of MOF was found (p=0.70). Figure 2 shows the number of cases of MOF and its relation with serum levels of VCAM-1, ICAM-1 and VEGF. The area under the ROC curve (AUC) for MOF was 0.73 for VCAM-1, 0.70 for ICAM-1 and 0.53 for VEGF (Figure 3).
Figure 1.
Boxplots representing serum levels of VCAM-1 (Panel A), ICAM-1 (Panel B) and VEGF (Panel C) according to multiple organ failure (MOF) and in-hospital mortality
Figure 2.
Distribution of VCAM-1, ICAM-1 and VEGF levels by the number of subjects according to the presence or absence of multiple organ failure (MOF).
Figure 3.
ROC curves of VCAM-1 (Panel A), ICAM-1 (Panel B) and VEGF (Panel C) predict multiple organ failure (MOF) and in-hospital mortality.
Only higher VCAM-1 levels showed a statistically significant association with in-hospital mortality (median 2,210 ng/mL [IQR 1,500, 3,432] vs. 1,240 ng/mL [806, 1,882]; p=0.03). This association was maintained even after controlling for APACHE II and SOFA scores in the logistic regression analysis (OR=1.06, 95%CI: 1.02 –1.11, p=0.02). The AUC for in-hospital mortality for CAMs are shown in Figure 3.
DISCUSSION
The main finding of our study was uncovering the major role of endothelial adhesion molecules in the clinical evolution of critically ill patients. Whilst ICAM-1 played a role in the development of MOF alone, VCAM-1 showed a significant association not only with MOF but also with the clinical outcome of hospital mortality.
Previous studies have shown that VCAM-1 is usually expressed at low levels in the membranes of leukocytes, macrophages, and vascular endothelial cells [5]. Infection increases transcription of VCAM-1, which is expressed on the surface of vascular endothelial cells [5]. Leukocytes activated by inflammatory mediators bind to the VCAM-1 endothelial surface receptors and translocate into local tissues to combat infection [5]. During states of diffuse and uncontrolled inflammation, as in sepsis, VCAM-1 is expressed in large quantities. The ensuing leukocyte adhesion/transmigration and the associated inflammatory cascade have been linked to vascular endothelial damage, capillary leakage, and organ dysfunction [5]. Several in vitro studies have demonstrated this phenomenon, but few clinical studies have evaluated the significance of elevated VCAM-1 levels during sepsis. One study in neonates demonstrated that higher levels of VCAM-1 were associated with more severe forms of sepsis and MOF [18]. These findings are consistent with our experimental results and the notion that elevated serum levels of VCAM-1 at the onset of organ dysfunction predicts the development of MOF in adult patients with severe sepsis and septic shock. However, there is a paucity of data evaluating the association of VCAM-1 levels with the clinical outcome of mortality. Our study is among the first to assess in-hospital mortality, finding that in-hospital mortality was in fact associated with higher VCAM-1 levels.
During inflammatory states, ICAM-1 has the same role as VCAM-1, a vascular endothelial surface receptor that allows for leukocytes and other inflammatory cells to bind and translocate into local tissues [5]. Thus, ICAM-1 plays a similar role to VCAM-1 in capillary leakage and organ dysfunction when the normally localized inflammatory cascade becomes wider spread [5]. Considering these similarities to VCAM-1, previous studies have shown that higher serum levels of ICAM-1 are also associated with severe sepsis and MOF in neonates [12]. Furthermore, multiple mouse models have shown that ICAM-1 knockout mice with severe forms of sepsis have lower mortality rates [5, 19]. Our finding that higher serum levels of ICAM-1 predict development of MOF in patients with severe sepsis and septic shock is a novel finding in the adult population.
In contrast to studies with mouse models, ICAM-1 levels were not associated with increased in-hospital mortality in our sample of adult patients with severe sepsis and septic shock. A non-significant statistical association may have been due to the small sample size of our study. Alternatively, the pathological role of VCAM-1 may be more pronounced during sepsis compared to ICAM-1. This may explain why VCAM-1 levels were found to correlate more highly with vascular endothelial damage and poor clinical outcomes. However, this speculation requires further testing to confirm.
VEGF also plays an integral role in the integrity of the vascular endothelium. VEGF has been shown to induce the expression of both VCAM-1 and ICAM-1, and to contribute to vascular endothelial damage and capillary leakage due to up-regulation during sepsis[20]. Unlike VCAM-1 and ICAM-1, VEGF has a multitude of other roles. In adults with sepsis, high VEGF levels have been shown to increase vascular permeability by binding to the tyrosine-protein kinase receptor-1 (FLT-1), altering the configuration of endothelial actin filaments, and increasing fenestrations in the endothelium [21]. Consequently, the vascular endothelium is predisposed to damage, ultimately resulting in organ dysfunction [21]. FLT-1 receptor antagonists/anti-VEGF receptor antibodies have been shown to decrease mortality in septic mice[21]. VEGF also has a procoagulant effect, causing microthrombi in the peripheral vasculature [21]. Yet despite these diverse functions that are active during sepsis, an association for high VEGF levels on mortality in human clinical studies have yielded inconsistent results [10, 11]. Along such lines, our study demonstrated that VEGF levels were not associated with MOF or mortality. This was consistent with the study by Karlsson at al [11] that involved 215 patients with severe sepsis and septic shock. Of note, Van Der Flier et al. in 2005 showed a positive association of VEGF levels and mortality in a sample size of only 18 septic adult patients [10]. Baseline patient characteristics and differences in sample size are potential explanations for the discrepancy in these findings, and as such further investigation is required. Also of note, Jiang et al [22] found that the VEGF-to-platelet ratio was predictive of 28-day mortality in patients with sepsis in China, illustrating the need for further investigation.
Our results are novel and supported by a recent systematic review that summarize the data on multiple endothelial-derived molecules including the ones reported in our study [23]. The key difference between our study and those cited in this review were the comparison/control groups which in fact strengthens the clinical utility of our results. Among the 19 studies that reported data on sICAM-1 the comparison groups included mainly healthy controls, patients with trauma, postoperative patients, patients with other forms of shock, and non-septic ICU patients [23]. Similarly, in the 12 studies that evaluated sVCAM-1 in sepsis, they evaluated patients in the emergency department, postoperative patients, critically ill trauma patients and patients with sepsis not necessarily with organ dysfunction [23]. Moreover, the four studies that examined sVEGF in septic patients compared non-septic critically ill patients to healthy controls [23]. Our study compared the levels of ICAM-1, VCAM-1 and VEGF in septic patients with one organ dysfunction to those with MOF. It is clear from previous studies that levels of these markers are elevated in comparison to healthy controls or non-septic patients, but what is not clear is whether they are elevated in comparison to those with single organ dysfunction. This is clinically useful because our study illustrates that ICAM-1 and VCAM-1 are even more sensitive of a predictor (and more specific) for MOF and mortality than previously thought. They are not only significantly elevated in septic patients compared to health controls, but even significantly elevated when comparing to patients with MOF compared to those with single organ damage, with threshold values determined in this study to be significantly predictive of in-hospital and 30-day mortality. Said in another way, the results from our study predict further organ dysfunction even when the process has already started.
Furthermore, of the 12 studies that evaluated sVCAM-1, only 1 found that it was statistically predictive of in-hospital mortality [23]. Our study was the second study to do this. The methodology preferred to address organ dysfunction is the use of the SOFA score, which is usually assessed at the time of presentation and at 24 hours after enrollment [24]. Skibsted S et al showed a weak correlation of sICAM-1 (r=0.15) and sVCAM-1 (r=0.35) with the SOFA score, which although they reported a statistically significant p-value for this correlation, is not likely clinically significant. Considering this, there are still much unanswered questions regarding sICAM-1 and sVCAM-1 when it comes to their correlation with SOFA score and ultimately mortality. Although this study is far from definitive and needs further study and validation, our study serves to add evidence that VCAM-1 levels are indeed able to predict mortality. The only biomarker that showed a significant association with the SOFA score was soluble fms-like tyrosine kinase (sFIT-1; r=0.58). Similar results were reported by Shapiro NI et al [25] in which only sFIT-1 had the strongest association with SOFA score, but not sICAM-1 and sVCAM-1. In addition, only one study addressed the correlation of sVCAM-1 and showed a modest correlation with SOFA and APACHE II scores [25]. Therefore, a progression from one to two or more organ dysfunction may be determined by the use of sICAM-1 and sVCAM-1 as we suggest in our study. To put this in prospective, in our study, of those patients that had single organ dysfunction (the inclusion criteria to be included in our study in the first place), 60% went on to develop MOF, and our results indicate that sICAM-1 and sVCAM-1 could predict this. Furthermore, of those 29 (60%) that developed MOF, sVCAM-1 levels could predict in-hospital.
Our study has potential limitations including the small sample size, however these patients with severe sepsis and septic shock were well characterized. Other limitations included the absence of a control group and that serial follow-up of the different biomarkers were not performed. Generalizations based on these results require further exploration in larger cohorts. Several other mechanisms may also be associated with the development of MOF beyond the endothelial adhesion molecules. Finally, it is important to clarify that all the patients had a blood collection before the MOF developed and within 96 hours of ICU admission, leading to a gap of 72 hours were potential therapeutic strategies could be used.
CONCLUSIONS
High serum levels of VCAM-1 and ICAM-1 at the onset of acute organ dysfunction are associated with the development of MOF, with high VCAM-1 levels also being associated with higher in-hospital mortality. These biomarkers have the potential to assist in early recognition and initiation of appropriate therapies to ultimately improve clinical outcomes. Further studies are needed to investigate the role of VCAM-1, ICAM-1, and VEGF as prognostic markers in patients with sepsis.
Highlights.
VEGF, ICAM-1, and VCAM-1 are a group of key vascular endothelial proteins
Elevated ICAM-1 levels predict multi-organ failure (MOF) during sepsis
Elevated VCAM-1 levels predict MOF and in-hospital mortality during sepsis
Footnotes
Conflict of interest: All authors have no conflicts of interest. Dr. Restrepo’s time is partially protected by Award Number K23HL096054 from the National Heart, Lung, and Blood Institute. Dr. Rodriguez’s time is protected by grant M-BAE 15/00063 from Carlos III Health Institute from Spain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health or the Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–6. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–9. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Chen G, Cao Y, Xue J, Li J, Wu Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Crit Care. 2015;30(2):271–5. doi: 10.1016/j.jcrc.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 4.de Montmollin E, Annane D. Year in review 2010: Critical Care--Multiple organ dysfunction and sepsis. Crit Care. 2011;15(6):236. doi: 10.1186/cc10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Griensven M, Probst C, Muller K, Hoevel P, Pape HC. Leukocyte-endothelial interactions via ICAM-1 are detrimental in polymicrobial sepsis. Shock. 2006;25(3):254–9. doi: 10.1097/01.shk.0000196497.49683.13. [DOI] [PubMed] [Google Scholar]
- 6.De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5(1):73–9. doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000;22(2–3):299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- 8.Kerr JR. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol Pathol. 1999;52(4):220–30. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parent C, Eichacker PQ. Neutrophil and endothelial cell interactions in sepsis. The role of adhesion molecules. Infect Dis Clin North Am. 1999;13(2):427–47. x. doi: 10.1016/s0891-5520(05)70084-2. [DOI] [PubMed] [Google Scholar]
- 10.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23(1):35–8. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson S, Pettila V, Tenhunen J, Lund V, Hovilehto S, Ruokonen E G. Finnsepsis Study. Vascular endothelial growth factor in severe sepsis and septic shock. Anesth Analg. 2008;106(6):1820–6. doi: 10.1213/ane.0b013e31816a643f. [DOI] [PubMed] [Google Scholar]
- 12.Figueras-Aloy J, Gomez-Lopez L, Rodriguez-Miguelez JM, Salvia-Roiges MD, Jordan-Garcia I, Ferrer-Codina I, Carbonell-Estrany X, Jimenez-Gonzalez R. Serum soluble ICAM-1, VCAM-1, L-selectin, and P-selectin levels as markers of infection and their relation to clinical severity in neonatal sepsis. Am J Perinatol. 2007;24(6):331–8. doi: 10.1055/s-2007-981851. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CH, Reyes LF, Orihuela CJ, Buitrago R, Anzueto A, Soni NJ, Levine S, Peters J, Hinojosa CA, Aliberti S, Sibila O, Rodriguez A, Chalmers JD, Martin-Loeches I, Bordon J, Blanquer J, Sanz F, Marcos PJ, Rello J, Sole-Violan J, Restrepo MI. Chromogranin A levels and mortality in patients with severe sepsis. Biomarkers. 2015;20(3):171–6. doi: 10.3109/1354750X.2015.1046932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Pediatric, Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. Sccm/Esicm/Accp/Ats/Sis, 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 18.Whalen MJ, Doughty LA, Carlos TM, Wisniewski SR, Kochanek PM, Carcillo JA. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med. 2000;28(7):2600–7. doi: 10.1097/00003246-200007000-00070. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand F, Pape HC, Harwood P, Muller K, Hoevel P, Putz C, Siemann A, Krettek C, van Griensven M. Role of adhesion molecule ICAM in the pathogenesis of polymicrobial sepsis. Exp Toxicol Pathol. 2005;56(4–5):281–90. doi: 10.1016/j.etp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276(10):7614–20. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 21.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203(6):1447–58. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang W, Ouyang W, Chen C, Zhu G, Huang L, Zeng H. Significance of the ratio of plasma vascular endothelial growth factor level to platelet count in the prognosis of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(7):484–8. doi: 10.3760/cma.j.issn.2095-4352.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis--a systematic review. Crit Care. 2012;16(1):R7. doi: 10.1186/cc11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, Shapiro NI. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39(5):427–32. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro NI, Schuetz P, Yano K, Sorasaki M, Parikh SM, Jones AE, Trzeciak S, Ngo L, Aird WC. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care. 2010;14(5):R182. doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]