Abstract
Background:
The relationships between adiponectin and clinical outcomes in hemodialysis (HD) patients remain highly controversial. Meanwhile, the association between adiponectin and the peripheral artery disease (PAD) has not been well studied in HD patients without diabetic mellitus.
Materials and Methods:
The ankle-brachial index was measured in HD patients. Adiponectin levels in 105 HD patients were measured by Enzyme-Linked Immunosorbant Assay.
Results:
105 HD patients were enrolled; 14 (13%) patients had PAD. Using receiver-operating-characteristic (ROC) curve analysis for PAD, adiponectin (area under the curve [AUC] 0.935, 95% confidence interval [CI]: 0.848–0.981, P < 0.001) showed significantly positive predictive value. During follow-up (mean 63 ± 30 months), 34 deaths (32%) occurred. Kaplan–Meier analysis found those patients lower median adiponectin had a significantly poor outcome (P < 0.05), and Cox analysis further confirmed that adiponectin was an independent predictor of overall mortality (hazard ratio [HR], 0.832, 95% CI: 0.696–0.995, P < 0.05). The ROC curve of overall mortality showed that the AUC of adiponectin was 0.719 (95% CI: 0.586–0.813, P < 0.05). In HD patients with PAD, the univariate analysis showed that adiponectin (HR, 0.649, 95% CI: 0.527–0.800, P < 0.001) was also associated with overall mortality.
Conclusion:
Decreasing levels of adiponectin were associated with a significant increase in the risk of PAD in HD patients without diabetic mellitus. Furthermore, as the results of our observation period (maximum of 7 years) showed, adiponectin was a predictor of all-cause mortality in HD patients.
Keywords: Adiponectin, ankle-brachial index, atherosclerosis, follow-up studies
INTRODUCTION
Approximately, 50% of deaths among the patients with end-stage renal disease (ESRD) originate from cardiovascular disease (CVD).[1] Peripheral arterial disease (PAD) could be seen as a marker for systemic atherosclerotic disease, leading to fatal (such as death) or nonfatal events (such as CV events).[2] Moreover, PAD is also common among ESRD patients.[1] Some experts have begun to pay attention to the importance of PAD and its biomarkers.[3] Recent data suggest that PAD is a strong predictor for overall mortality in hemodialysis (HD) patients.[4,5]
Adiponectin is an adipocytokine with anti-inflammatory and antiatherogenic effects, secreted especially by the adipose tissue, which is considered to be an active endocrine organ.[6] The levels of adiponectin are low in obesity, type 2 diabetes mellitus, insulin resistance, dyslipidemia, coronary artery disease, and PAD.[7,8,9] Meanwhile, there is an inverse association adiponectin levels and CV morbidity.[10,11,12] But the impact of adiponectin on the CV health and survival of HD patients remains unclear. Epidemiological evidence has shown conflicting results regarding the association between levels of adiponectin and occurrence of adverse outcomes. Some studies suggested that higher adiponectin levels are associated with decreased CV and all-cause mortality risk in the HD population,[13,14] while others indicated that elevated circulating adiponectin levels are associated with adverse outcomes in nondialysis-dependent patients with chronic kidney disease and HD patients.[15,16]
The aim of this study was therefore to evaluate the association between adiponectin and PAD and investigate the relationship between adiponectin and adverse outcomes in HD patients without diabetic mellitus.
MATERIALS AND METHODS
Study population
The study population was comprised of HD patients from the dialysis center of Renji Hospital, Shanghai, China, between May 2008 and July 2008. Inclusion criteria were stable HD for at least 3 months at the time of evaluation and ability and willingness to provide informed consent. All patients were dialyzed on synthetic (polysulfone) membranes with bicarbonate dialysate. Exclusion criteria consisted of (1) severe medical conditions (e.g., cancer, sepsis, advanced liver or lung disease, and severe heart failure), (2) diabetic mellitus, active tuberculosis, systemic lupus erythematosus, systemic vasculitis, or rheumatoid arthritis, and (3) peritoneal dialysis or kidney transplantation patients who converted to HD.
Definition
PAD was defined based on clinical symptoms (intermittent claudication, which evolves to pain at rest and a risk of tissue necrosis, and even to amputation), ankle-brachial index (ABI) of <0.9 in either leg,[17] previous history of lower extremity revascularization procedure, and/or angiography findings). The ABI was measured in all HD patients using an ABI device (MICROLITE 84M/0289, Sony) by a professional technician; the device measures arm and ankle (brachial and posterior tibial arteries, respectively) blood pressure by an ultrasonic technique. The measurement was obtained after completion of the dialysis treatment and after allowing patients to rest in a supine position at least for 5 min. ABI was calculated by the ratio of the ankle systolic pressure divided by the arm systolic pressure. The systolic pressure of the arm without dialysis access and the lower value of the ankle pressure were used for the calculation.
CVD defined as previous angiogram showing significant occlusive disease, a history of a myocardial infarction, or a history of coronary artery bypass surgery or angioplasty, a history of cerebrovascular accident including cerebral bleeding and infarction.
Adiponectin measurement
Blood was drawn in the morning after an overnight fast of at least 12 h before a dialysis session. The samples were separated via centrifugation (4000 rpm, 10 min) and immediately stored at − 80°C for subsequent assays. The serum concentrations of adiponectin were evaluated using an Enzyme-Linked Immunosorbant Assay kit (USCNLIFE, USA).
Outcome ascertainment
Clinical outcome in this study included actual patient survival. The observation period ended on May 31, 2015. At the end of the follow-up, the status of all patients assessed and data on mortality were obtained for the entire cohort.
Statistical methods
Data are reported as a mean ± standard deviation (SD) or as median and interquartile range, as appropriate. Comparison between groups was performed using an unpaired t-test for mean (SD) data, nonparametric Wilcoxon rank sum test for median (interquartile range) data, and the Chi-square test for categorical variables. Correlations were reported as the Pearson correlation coefficient. Sensitivity, specificity, and cutoff level for adiponectin as predictors of the presence of PAD and mortality were analyzed using receiver-operating-characteristic (ROC) curves. Survival curves were generated using the Kaplan–Meier technique. In addition, variables predictive of all-cause mortality and CV mortality were determined using Cox regression models. Any selected variable for which there was a significant difference for prediction of mortality in univariate Cox models was considered as a risk factor and entering into the multivariate Cox model. All P - values were two-tailed, and values of < 0.05 were considered to indicate statistical significance. All confidence intervals (CIs) were calculated at the 95% level. All calculations were performed using a standard statistical package (SPSS version 20.0 for Windows, SPSS Inc., Chicago, IL, USA).
RESULTS
Incidence of peripheral arterial disease in hemodialysis patients
105 HD patients (55 males, mean age 57.38 ± 14.36 years) were enrolled. Diagnosis of renal failure was as follows: Glomerulonephritis in 41.6%, hypertension in 9.4%, obstructive uropathy in 3.8%, tubulointerstitial nephropathy in 2.8%, and 42.4% of the patients already have uremia at the first visit, so the causes of renal failure were unknown.
The incidence of PAD was 14/105 (13%). In PAD patients, five cases presented with claudication, one with claudication and rest pain, and three with angiography findings, who underwent limb vascularization procedures.
Clinical and biochemical parameters at enrollment
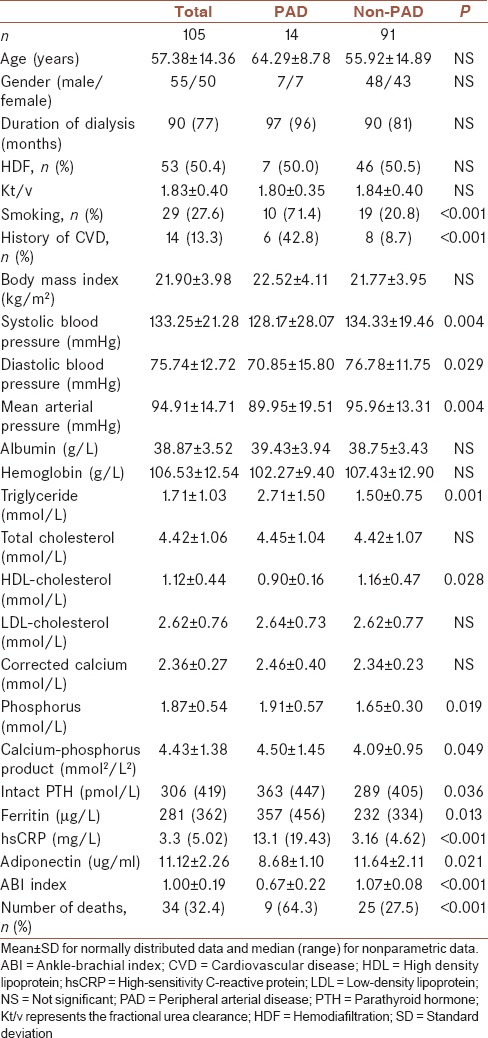
Baseline characteristics of the HD patients are shown in Table 1. The mean age of the HD patients was 57.38 ± 14.36 years. Among the 105 patients, 14 subjects fulfilled the clinical diagnosis of PAD. The PAD and non-PAD groups were matched for age, gender, body mass index (BMI), and several established CV risk factors. The patients in the PAD group had higher prevalences of preexisting CV events than subjects without the PAD. The PAD group also had significantly higher blood pressure, high-sensitivity C-reactive protein (hsCRP) levels, serum triglyceride (TG), high-density lipoprotein (HDL), phosphorus, intact parathyroid hormone (PTH), and ferritin.
Table 1.
Baseline demographic at the start of study
In the HD patients, the concentration of serum adiponectin in the PAD group was lower than that of the non-PAD subjects (8.68bj. 10 ug/ml vs. 11.64lm. 11ug/ml P = 0.021) [Table 1].
The correlations between adiponectin and other factors
The univariate analysis showed a positive correlations in these patients between adiponectin levels and ABI values (r = 0.576, P < 0.001), hsCRP (r = −0.312, P < 0.001), TG levels (r = −0.175, P = 0.004), and total cholesterol (TC) levels (r = −0.146, P = 0.018). After adjustment for age, BMI, hsCRP, TG, TC the association between adiponectin levels and ABI values still remained (r = 0.499, P < 0.001). On the other hand, there was no significant association between adiponectin and blood pressure, serum albumin, HDL, LDL, and PTH.
Receiver-operating-characteristic analysis of peripheral arterial disease
The ROC curve of PAD showed that the area under the curve (AUC) of adiponectin was 0.935 (95% CI: 0.848–0.981, P < 0.001). With the cutoff value for adiponectin of 9.81 ug/ml, the diagnostic sensitivity and specificity for PAD were 84% and 85.7%, respectively [Figure 1].
Figure 1.
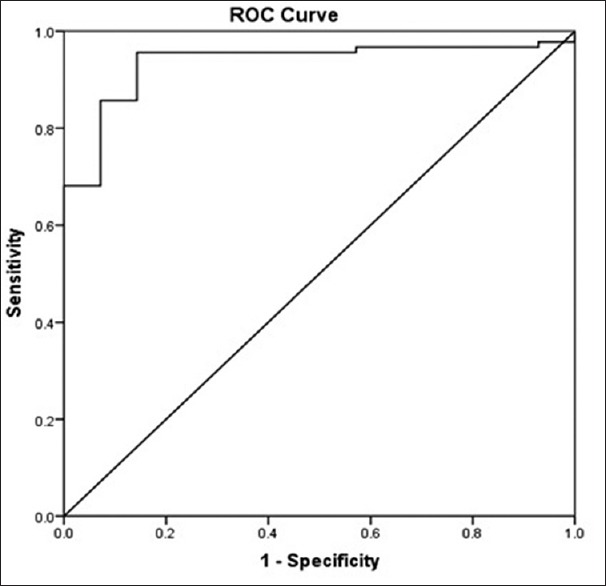
Receiver-operating-characteristic curve for adiponectin to peripheral artery disease
Peripheral arterial disease predicts all-cause mortality in hemodialysis patients
During the follow-up period (mean 63 ± 30 months), 34 deaths occurred. The causes of death were cerebrovascular disease in 12 patients (35.3%), infection with sepsis in 10 patients (29.4%), CV disease in 4 patients (11.8%), malnutrition in 4 patients (11.8%), malignancies in 2 patients (5.9%), arterial aneurysm in 1 patient (2.9%), and pulmonary embolism in 1 patients (2.9%). The overall CV cause (cerebrovascular disease and CVD) of death was 47%.
In the univariate regression analysis, the hazard ratio (HR) of patients with PAD was 5.538 (P < 0.001). Adiponectin, hsCRP, ferritin, age, blood pressure, serum lipids, albumin, smoking history, diabetes history, hypertension history, and medication history were introduced into the equation. Table 2 showed a Cox proportional hazards regression analysis for overall mortality. The multivariate Cox analysis identified PAD as independent predictors of all-cause mortality (HR, 3.073; P = 0.001). The results from the Kaplan–Meier analysis for overall mortality were shown in Figure 2. Patients with an ABI of less than 0.9 had a worse overall survival compared with those with a normal ABI (P = 0.001).
Table 2.
Predictors of overall mortality using Cox proportional hazards model in hemodialysis patients
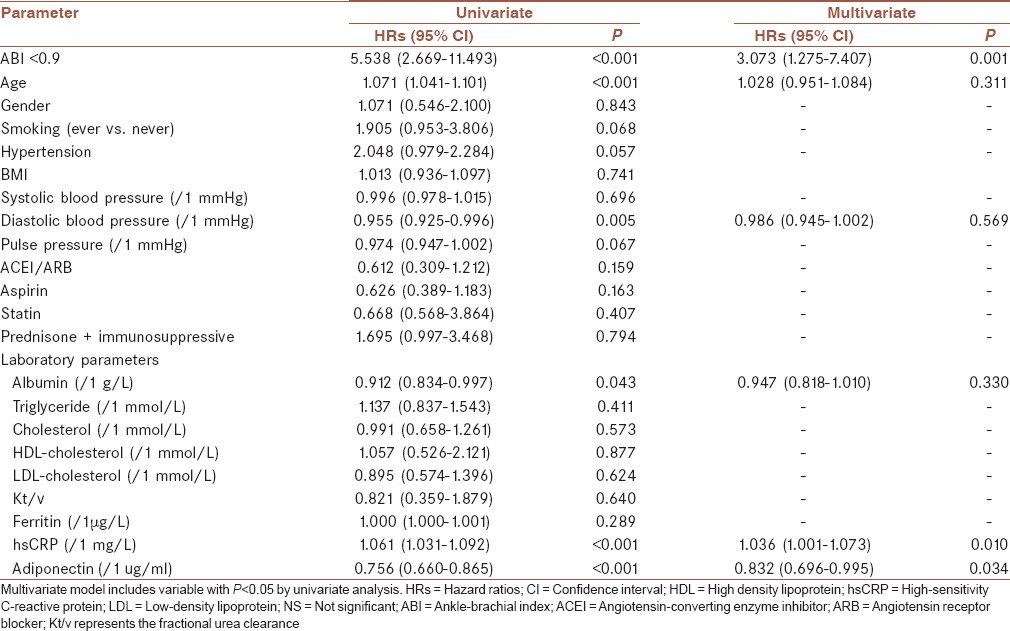
Figure 2.
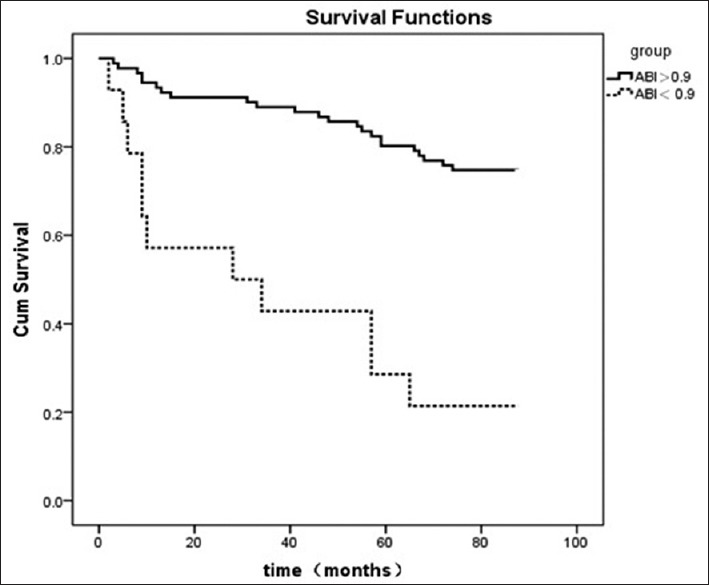
Kaplan–Meier plot of ankle-brachial index for overall mortality
About 16 CV deaths were documented during the follow-up period. We also analyzed the ABI values in predicting CV mortality and found that an ABI of less than 0.9 (HR, 3.283, 95% CI: 2.027–7.582; P < 0.001) was a strong predictor for CV mortality. Figure 3 showed the Kaplan–Meier analysis for CV mortality. Patients with an ABI of less than 0.9 had a worse CV survival compared with those with a normal ABI (P < 0.001).
Figure 3.
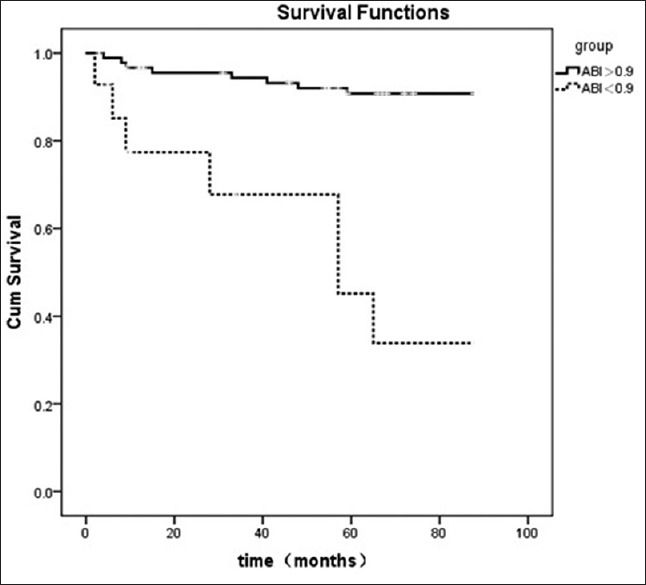
Kaplan–Meier plot of ankle-brachial index for cardiovascular mortality
Adiponectin as a predictor of all-cause mortality in hemodialysis patients
The univariate regression shows the HR of adiponectin for overall mortality was 0.649 (P < 0.001) [Table 2]. In the multivariate analysis, adiponectin (HR, 0.832, P = 0.034) was associate with overall mortality [Table 2]. Figure 4 showed the results from the Kaplan–Meier of adiponectin for overall mortality. We found that patients who had lower median adiponectin had a worse outcome (P < 0.05).
Figure 4.
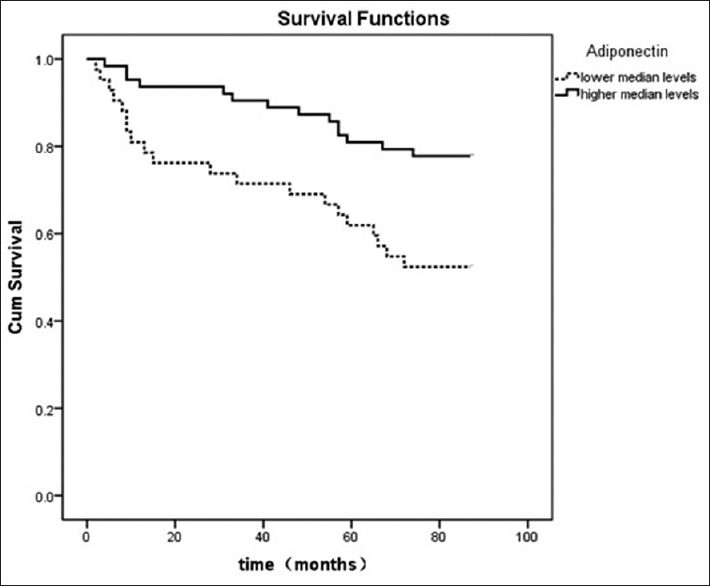
Kaplan–Meier plot of adiponectin for overall mortality
The ROC curve of overall mortality showed that the AUC of adiponectin was 0.719 (95% CI: 0.586–0.813, P < 0.05). With the cutoff value for adiponectin of 11.01 ug/ml, the diagnostic sensitivity and specificity for all-cause mortality were 66.2% and 67.6%, respectively [Figure 5].
Figure 5.
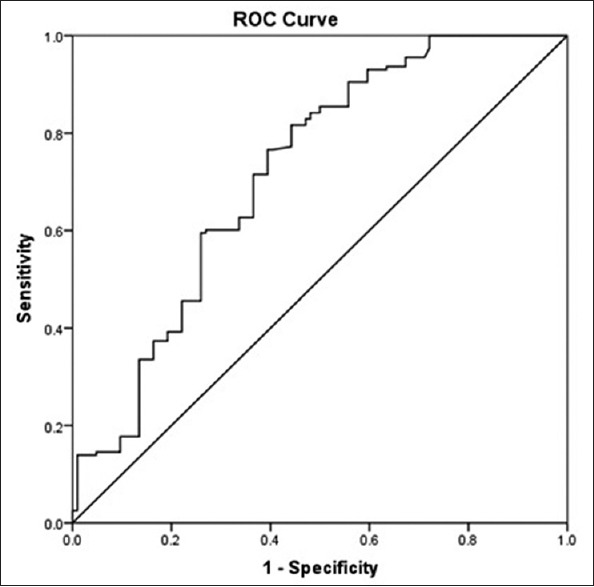
Receiver-operating-characteristic curve for adiponectin to overall mortality
Furthermore, in HD patients with PAD, the univariate regression shows the HR of adiponectin for overall mortality was 0.649 (95% CI: 0.527–0.800, P < 0.001). HD patients with PAD who had lower median adiponectin levels had a worse outcome in follow-up period (P < 0.01) [Figure 6].
Figure 6.
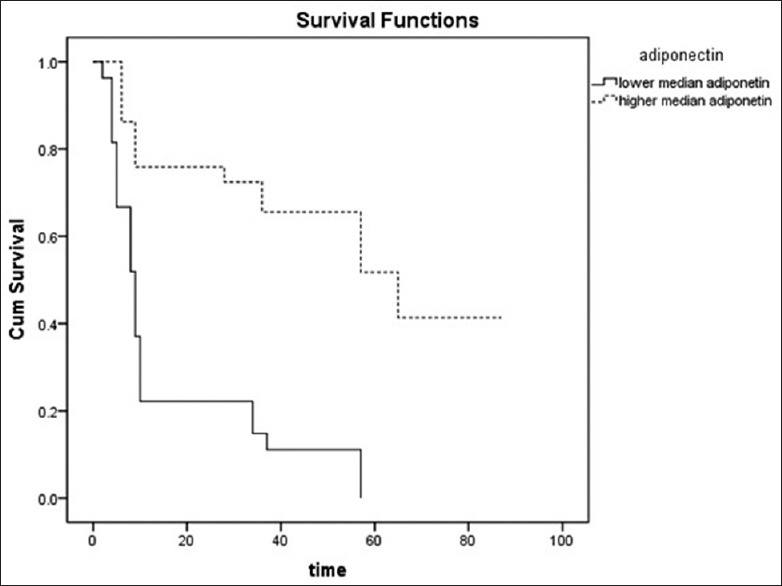
Kaplan–Meier plot of adiponectin for overall mortality in hemodialysis patients with peripheral artery disease
However, there was no significant correlation between CV mortality and adiponectin.
DISCUSSION
In our 7-year prospective study of 105 HD patients, we evaluated the predictors for overall and CV mortality. We found that PAD predicted all-cause mortality and CV mortality. Moreover, adiponectin might be a predictor of all-cause mortality in HD patients. Another observation of this study was that decreasing levels of adiponectin were associated with a significant increase in the risk of PAD in HD patients without diabetic mellitus.
Our study showed that the incidence of PAD in HD patients without diabetic mellitus was 13%. Epidemiological studies have reported the prevalence of PAD in the general population to be 1–5%.[18,19] The Chronic Renal Insufficiency Cohort Study data showed PAD in 7% of adult chronic kidney disease patients.[20] In HD patients, the incident of PAD is higher than that of the general population. Based on the United States Renal Data System 2013 database, the prevalence of PAD was 557.9/1000 patients years in chronic HD patients.[1] In Japan, Ono et al. showed that the incidence of PAD was 16.5% in 1,010 chronic HD patients using the ABI method.[21] In Taiwan, Chen et al. reported that the incidence was 15.6% in 125 HD patients, using the same method.[22] The incidence of PAD was 13% in our study, close to that in Japan and Taiwan.
CVD is the main reason of death in HD patients.[23,24] Therefore, vascular disease prevention in these patients is important to reduce the incident of CV events and the high morbidity and mortality.[24] Several recent studies have shown an association between an abnormal ABI and overall and CV mortality.[4,5,25] As the results of our observation period (maximum of 7 years) showed, PAD was an independent risk factor for all-cause and CV mortality.
Adiponectin has known complex associations with multiple biological pathways, including those that favorably affect atherogenic risk.[26] In addition to improving the insulin sensitivity of hepatic and skeletal muscle;[27] suppressing and increasing expression of proinflammatory and anti-inflammatory cytokines, respectively;[28] and attenuating platelet aggregation and thrombus formation,[26] adiponectin is also associated with a favorable serum lipid profile.[29] Similar to patterns observed in prior studies of the general population and dialysis patients, we found that adiponectin levels were associated inversely with TG and TC levels and positively with hsCRP.
One of the observations of the present study was the significant relationship between serum adiponectin and PAD in the HD patients. Even after adjusting for several confounding factors such as age, BMI, hsCRP, TG, TC, and serum adiponectin, concentration was still significantly associated with ABI. The ROC curve of PAD also showed that the AUC of adiponectin was 0.935. With the cutoff value for adiponectin of 9.81 ug/ml, the diagnostic sensitivity and specificity for PAD were 84% and 85.7%, respectively. These results are consistent with Lim et al.[29] These observations suggest a close link may exist between adiponectin and PAD in HD patients. Adiponectin may have a potential anti-atherogenic property. On the other hand, we found negative correlations between adiponectin levels and hsCRP. Therefore, low concentrations of adiponectin may cause an excessive inflammatory response and accelerate the development of the atherosclerotic process.[29]
There have been inconsistent findings of the adiponectin-mortality association in HD patients. In a study of 227 HD patients by Zoccali et al.,[30] 1 ug/mL higher adiponectin concentration was associated with a 3% lower CV event risk, although an association with mortality was not observed. Subsequently, Rao et al.[31] observed that higher base-line and time-dependent adiponectin concentrations were associated with decreased mortality among 182 HD patients from the HEMO (HD) study and two Boston dialysis centers, and these findings have been corroborated in other studies of HD patients. In contrast, Drechsler et al.[15] found that higher adiponectin concentrations were associated with increased risk of sudden cardiac death among 1,255 HD patients with diabetes type 2 from 4D (Die Deutsche Diabetes Dialyze Studie), although estimates were no longer significant after adjustment for BMI, serum lipid levels (TGs and HDL cholesterol), and other covariates. One recent study by Rhee et al.[16] show that higher adiponectin level is associated with a 3-fold higher death risk in HD patients independent of body composition and lipid levels. Despite intensive past study, the impact of adiponectin on the CV health and survival of HD patients remains unclear. In our study, the multivariate analysis showed adiponectin was associated with overall mortality. The Kaplan–Meier of adiponectin for overall mortality demonstrated that patients who had lower median adiponectin had worse outcome. Furthermore, in HD patients with PAD, the univariate analysis showed adiponectin was associated with overall mortality. Our study showed that adiponectin was a predictor of all-cause mortality in HD patients.
The strengths of our study include a stable HD study cohort with long-term follow-up and detailed demographic, clinical, and treatment characteristics representative of the HD population. Furthermore, our study is among the first to explore the prognostic value of adiponectin with PAD and investigate the relationship between adiponectin and adverse outcomes in ESRD patients with PAD. We acknowledge several limitations of this study. First, it was a single-center trial with a small population carried out and the number of the patients with PAD in the study was relatively low. It is suggested that an open-labeled, prospective, multicentered, and controlled research is necessary to estimate the relationship of relationship of adiponectin and patients’ outcomes and reduce the bias in ours. Second, it is an observational study which cannot fully explain why there is a relationship between adiponectin and overall mortality in HD patients. It provides a clue for us to further investigate the mechanism of this phenomenon.
In summary, we showed that lower levels of adiponectin were found in HD patients with PAD. Second, we showed that decreasing levels of adiponectin were associated with a significant increase in the risk of PAD in HD patients without diabetic mellitus. Furthermore, as the results of our observation period (maximum of 7 years) showed, PAD was an independent risk factor for all-cause and CV mortality. Adiponectin was a predictor of all-cause mortality in HD patients.
CONCLUSION
We showed that lower levels of adiponectin were found in HD patients with PAD. Second, we showed that decreasing levels of adiponectin were associated with a significant increase in the risk of PAD in HD patients without diabetic mellitus. Furthermore, as the results of our observation period (maximum of 7 years) showed, PAD was an independent risk factor for all-cause and CV mortality. Adiponectin was a predictor of all-cause mortality in HD patients.
Financial support and sponsorship
This study was supported in part by the National Key Technology Research and Development Program (No. 2011BAI10B08). The study was also sponsored by the National Natural Science Foundation of China (81370794).
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
YZ and ZN contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
JZ and WZ contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
We thank all the patients for the cooperation during the research. The study was also supported by grants from the National Key Technology Research and Development Program (No. 2011BAI10B08) and the National Natural Science Foundation of China (81370794).
REFERENCES
- 1.U.S. Renal Data System. USRDS 2013 Annual Data Report (ADR) Atlas. Atlas of End-Stage Renal Disease. Ch. 4. Cardiovascular Disease. 2013 [Google Scholar]
- 2.Aboyans V, Desormais I, Lacroix P, Salazar J, Criqui MH, Laskar M. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. J Am Coll Cardiol. 2010;55:898–903. doi: 10.1016/j.jacc.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 3.Wilson AM, Kimura E, Harada RK, Nair N, Narasimhan B, Meng XY, et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation. 2007;116:1396–403. doi: 10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 4.Otsubo S, Kitamura M, Wakaume T, Yajima A, Ishihara M, Takasaki M, et al. Association of peripheral artery disease and long-term mortality in hemodialysis patients. Int Urol Nephrol. 2012;44:569–73. doi: 10.1007/s11255-010-9883-8. [DOI] [PubMed] [Google Scholar]
- 5.Al Thani H, El-Menyar A, Hussein A, Sadek A, Sharaf A, Singh R, et al. Prevalence, predictors, and impact of peripheral arterial disease in hemodialysis patients: A cohort study with a 3-year follow-up. Angiology. 2013;64:98–104. doi: 10.1177/0003319711436078. [DOI] [PubMed] [Google Scholar]
- 6.Knudson JD, Dick GM, Tune JD. Adipokines and coronary vasomotor dysfunction. Exp Biol Med (Maywood) 2007;232:727–36. [PubMed] [Google Scholar]
- 7.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 8.Antoniades C, Antonopoulos AS, Tousoulis D, Ste-fanadis C. Adiponectin: From obesity to cardiovascular disease: Etiology and Pathophysiology. Obes Rev. 2009;10:269–79. doi: 10.1111/j.1467-789X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 9.Dieplinger B, Poelz W, Haltmayer M, Mueller T. Hypoadiponectinemia is associated with symptomatic atherosclerotic peripheral arterial disease. Clin Chem Lab Med. 2006;44:830–3. doi: 10.1515/CCLM.2006.145. [DOI] [PubMed] [Google Scholar]
- 10.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–53. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 11.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 13.Díez JJ, Estrada P, Bajo MA, Fernández-Reyes MJ, Grande C, del Peso G, et al. High stable serum adiponectin levels are associated with a better outcome in prevalent dialysis patients. Am J Nephrol. 2009;30:244–52. doi: 10.1159/000221147. [DOI] [PubMed] [Google Scholar]
- 14.Tsigalou C, Chalikias G, Kantartzi K, Tziakas D, Kampouromiti G, Vargemezis V, et al. Differential effect of baseline adiponectin on all-cause mortality in hemodialysis patients depending on initial body mass index. Long-term follow-up data of 4.5 years. J Ren Nutr. 2013;23:45–56. doi: 10.1053/j.jrn.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 2009;76:567–75. doi: 10.1038/ki.2009.200. [DOI] [PubMed] [Google Scholar]
- 16.Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, et al. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66:313–21. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 18.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, et al. Critical issues in peripheral arterial disease detection and management: A call to action. Arch Intern Med. 2003;163:884–92. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 19.Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 20.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic renal insufficiency cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–11. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14:1591–8. doi: 10.1097/01.asn.0000065547.98258.3d. [DOI] [PubMed] [Google Scholar]
- 22.Chen SC, Su HM, Mai HC, Chen JH, Chen CY, Chang JM, et al. Associated risk factors for abnormal ankle-brachial index in hemodialysis patients in a hospital. Kaohsiung J Med Sci. 2008;24:473–80. doi: 10.1016/S1607-551X(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 23.Kanbay M, Afsar B, Gusbeth-Tatomir P, Covic A. Arterial stiffness in dialysis patients: Where are we now? Int Urol Nephrol. 2010;42:741–52. doi: 10.1007/s11255-009-9675-1. [DOI] [PubMed] [Google Scholar]
- 24.Paraskevas KI, Kotsikoris I, Koupidis SA, Tzovaras AA, Mikhailidis DP. Cardiovascular events in chronic dialysis patients: Emphasizing the importance of vascular disease prevention. Int Urol Nephrol. 2010;42:999–1006. doi: 10.1007/s11255-010-9795-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu JH, Chen JY, Lin SY, Lin HH, Ting IW, Liang CC, et al. Comparing Survival between peritoneal dialysis and hemodialysis patients with subclinical peripheral artery disease: A 6-year follow-up. Int J Med Sci. 2013;10:434–40. doi: 10.7150/ijms.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013;33:2–13. doi: 10.1016/j.semnephrol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 28.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–86. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim PS, Hu CY, Wu MY, Wu TK, Chang HC. Plasma adiponectin is associated with ankle-brachial index in patients on haemodialysis. Nephrology (Carlton) 2007;12:546–52. doi: 10.1111/j.1440-1797.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 30.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–41. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 31.Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS HEMO Study Group. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrol Dial Transplant. 2008;23:2619–28. doi: 10.1093/ndt/gfn070. [DOI] [PMC free article] [PubMed] [Google Scholar]


