Abstract
Background:
While the most nontuberculous mycobacteria (NTMs) species are considered as opportunistic pathogens, some of them are related to several human infections. It is believed that environment is the main source for these infections. Distribution and scattering pattern of NTMs has not been well studied in Iran and a few studies about this subject have been done, so the aim of this study was to determine prevalence of NTMs in environmental samples from Iran.
Materials and Methods:
Data about prevalence of NTMs in environmental samples from Iran were obtained by searching databases. The studies presenting cross-sectional or cohort and the papers with sample size ≥30 were included. Then, the meta-analysis was performed using Comprehensive Meta-Analysis software and Cochran's Q and I2 tests. The strategy search was based PRISMA protocol is available online (PRISMA, http://www.prisma-statement.org).
Results:
The results of this meta-analysis showed that overall combined prevalence of NTMs in environmental samples from Iran was 38.3%. The frequency of NTM was higher in the north of Iran (73.2%). The most prevalent rapid-growing mycobacterium was Mycobacterium fortuitum (19.8%), and the most dominant slow-growing mycobacterium was Mycobacterium flavescens (16.8%).
Conclusion:
In regard to increasing incidence of disease in immunocompromised patients and existence of different types of mycobacteria species in environmental samples, efforts should be focused on measures that will specifically remove NTMs from habitats where susceptible individuals are exposed.
Keywords: Meta-analysis, nontuberculous mycobacteria, prevalence
INTRODUCTION
Environmental opportunistic mycobacteria are found in natural and human and can cause disease in humans and animals. The atypical and nontuberculous mycobacteria (NTMs) names are used for these organisms.[1] In relation to Runyon's classification, based on growth rates and pigment production, NTMs are classified to Groups I to IV in which Groups I to III are slow-growing NTMs and Group IV are rapid-growing NTMs.[2] While most NTM species are considered opportunistic pathogens, but some of them are related powerfully with several human diseases, for example, pulmonary infection, nosocomial infections, hypersensitivity, pneumonitis, asthma, gang ionic infection, and infection of skin/soft tissue.[3] Infections are present in immunocompetent and immunocompromised humans, more often HIV-positive patients all over the world.[4,5,6] Because of increasing rates of NTMs infections, there is interest to identify the source of the NTMs transmission.[7] The possible ways for infect the human beings are: Ingestion or inhalation and may have a 2-fold effect.[8]
NTMs are isolated from natural waters, water distribution systems, drinking waters, soil, food, biofilms, aerosols, and dust.[9] A study conducted in 1999[10] showed that the ideal condition for more growth of NTM species was aquatic environments, low pH values with the presence of high organic matter concentrations, with presence of low numbers of heterotrophic bacteria, in versus Bland et al.[10] presented that the ideal conditions were presence of large numbers of heterotrophic bacteria, low temperature, alkaline characteristic, and high conductivity of water.
NTM species are very resistant to conventional disinfection techniques, particularly chlorination.[11] The most important NTM studied is Mycobacterium avium because can grow in conditions with temperatures from 0 to 50°C and pH values between 3 and 8.[12] Geographic distribution in prevalence of NTM species is different. For example, Mycobacterium malmoense is most prevalent in Europe than the USA. A few species of rapidly growing mycobacteria such as Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum are opportunistic pathogens and not saprophytes and can produce various diseases in human. Like their slowly growing relatives, members of these species are very resistant to antibiotics and conventional disinfectants and they are normal residents of water and animal reservoirs.[9] Due to the increasing immunocompromised diseases in the world and high rate of NTMs in various geographical regions, understanding the NTMs species distribution and prevalence in environment is necessary. Distribution and scattering pattern of NTM has not been well studied in Iran and a few studies about this subject have been done,[13] so the aim of this meta-analysis was to determine prevalence of NTMs in environmental samples from Iran. The results of our meta-analysis can give comprehensive data about distribution, prevalence, and diversity of NTMs in environmental samples.
MATERIALS AND METHODS
Search strategies
The searching process (PRISMA, http://www.prisma-statement.org) was conducted for prevalence of environmental-NTMs in Iran from papers that were published by the end of 2014 using Web of Science, PubMed, Scopus, MEDLINE, Cochrane Library, ScienceDirect, Google Scholar, and the Scientific Information Database. The original articles published in English and Persian were included in our research. The keywords such as environmental nontuberculous mycobacteria, atypical mycobacteria, NTM infection or NTM diseases, distribution, prevalence, incidence, and Iran were used for searching process. Likewise, the searching was done with similar strategies and related Persian keywords among Iranian databases. We searched Scientific Information Database (www.sid.ir), Iranmedex (www.iranmedex.com), magiran (www.Magiran.com), and Irandoc (www.irandoc.ac.ir). Furthermore, we checked references from retrieved papers in both English and Persian for additional data. Two reviewers independently searched the databases with the similar way and they reviewed the titles, abstracts, and full texts to determine if they met eligibility criteria for inclusion. To identify further papers, references in these papers were reviewed. The last search was done by the end of May 2015.
Criteria for inclusion and exclusion
The studies presenting cross-sectional or cohort on the prevalence of environmental-NTMs were included in our meta-analysis. The papers with sample size ≥30 which report the prevalence of NTMs in environmental samples by the end of 2014 were selected. We excluded review articles, congress and meeting abstracts, papers reported in languages other than English or Persian, meta-analysis or systematic reviews, abstract forms of papers, case report papers, duplicate publication of the same paper. To reduce the risk of errors, two reviewers independently completed this process.
Extraction of data
A data abstraction form for reviewers was designed. The following data were included in these forms: The first author's name, time of the study, year of publication, location of study, and the prevalence of NTMs.
Statistical analysis
Analysis of data was performed by Comprehensive Meta-Analysis Software Version 3.3.070 (Bio stat Company). Prevalence was reported by 95% confidence intervals (CIs). Random effects model was used for meta-analysis as well as to take into account the possibility of heterogeneity between studies which was tested with the Cochrane Q and I2 tests. To evaluate possible publication bias, Egger weighted regression method was used (P < 0.05 was considered as indicative of a statistically significant publication bias). For check of publication bias, the funnel plot was used; further, for likely asymmetrical distribution of studies, the Egger's linear regression test was applied. Subgroups analysis was performed for NTMs prevalence and distribution of different species of NTMs in environmental samples. Finally, sensitivity meta-analysis was assessed.
RESULTS
Characteristics of selected studies
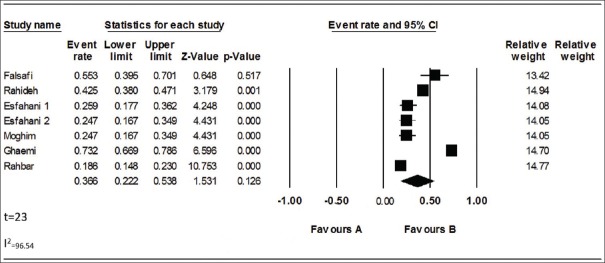
The study inclusion process is shown in Figure 1. In brief, initially using multiple databases, 83 relevant papers were recognized. Forty papers were excluded because of duplication, and due to the irrelevant titles, 19 papers were excluded. Then, 15 full texts papers were evaluated, nine papers were excluded (due to topic of four papers were irrelevant, four papers were case report, and one were review article). Eight papers were deleted after full-text evaluation because they did not report NTM prevalence. Finally, seven papers were included in this meta-analysis. The features of included papers in this meta-analysis are summarized in Table 1. Majority of studies were directed in Center of Iran [Table 1]; among total seven studies from three geographical locations of Iran, five cases (71.4%) were reported from Center of Iran (two cases from Tehran province and three from Isfahan province), from both West (West Azarbaijan) and North (Golestan) provinces was reported one case(14.2%). In included studies, the prevalence of NTMs varied from 18.6% to 73.2% [Table 1 and Figure 2]. The primary detection carried out based on phenotypic tests such as smear microscopy and culture on Lowenstein-Jensen medium, and identification was done by morphology, biochemical tests, pigment production, and growth rate. From total studies, 2 (28.5%) of those have used from molecular methods and the other studies have used only from phenotypic tests for identification.
Figure 1.
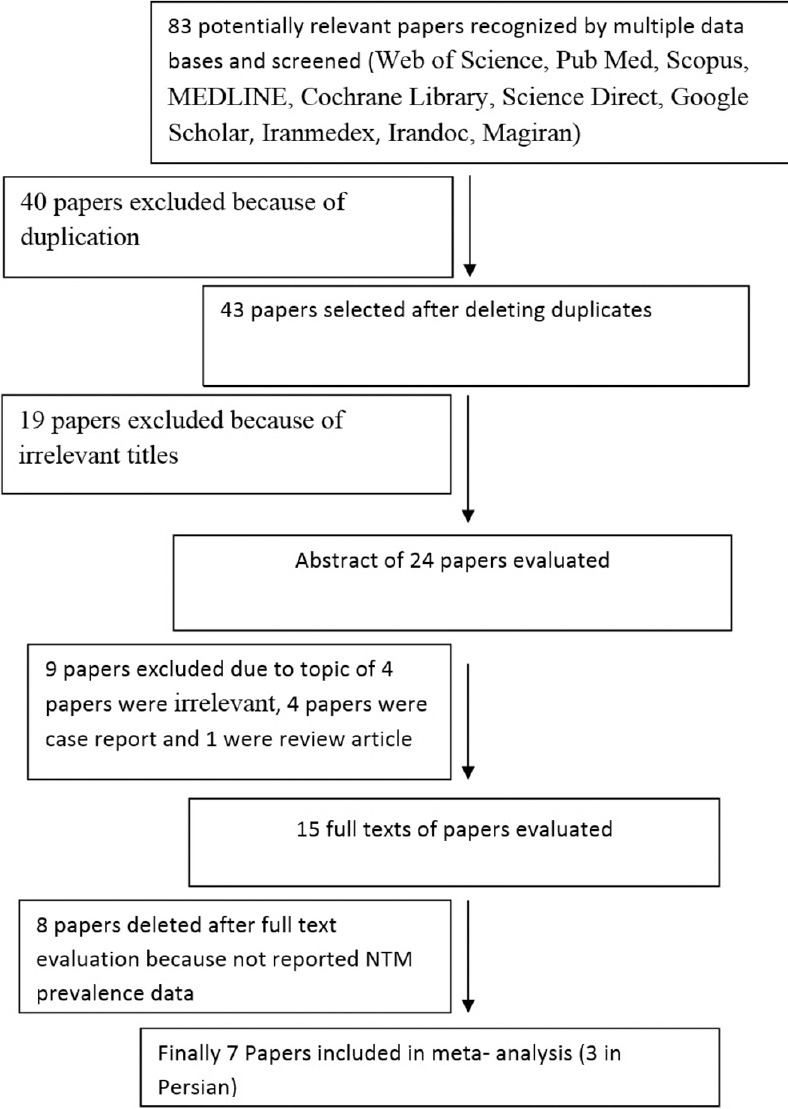
A study inclusion process for meta-analysis
Table 1.
Characteristics of included studies for meta-analysis
Figure 2.
Forest plot of the meta-analysis on prevalence of nontuberculous mycobacteria in environmental samples from Iran
Overall effects
From the total papers selected for our meta-analysis, in accordance with heterogeneity test, there were heterogeneities between studies (Q2 = 173.623, I2 = 96.54, t = 23, P < 0.001); therefore, we used random model to combine the prevalence of NTMs in environmental samples. In regarding overall meta-analysis, the combined prevalence of NTMs was 38.3% (95% CI [35.7–40.9%]) [Table 2]. Funnel plot was used for check of publication bias [Figure 3]. In regarding likely asymmetrical distribution of studies, the Egger's linear regression test was used; the result of this test did not confirm the publication bias in our meta-analysis (P > 0.05).
Table 2.
Subgroups analysis for nontuberculous mycobacteria prevalence in environmental samples
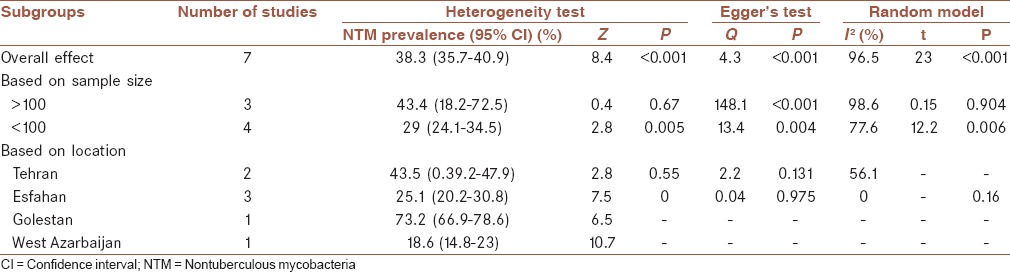
Figure 3.
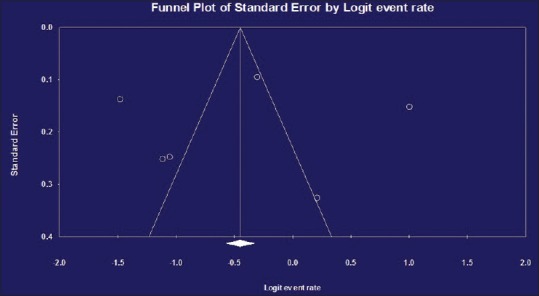
Funnel plot for meta-analysis on prevalence of nontuberculous mycobacteria in environmental samples from Iran
Subgroups analysis for nontuberculous mycobacteria prevalence in environmental samples from Iran
Subgroups analysis presented that the prevalence of NTMs in environmental samples was higher in studies with sample size ≥ 100 in contrast with studies with sample size ≤ 100 ([43.4%, 95% CI (18.2, 72.5%)] vs. [29%, 95% CI (24.1, 34.5%)]). This meta-analysis also showed that the frequency of NTMs in environmental samples was varied in different geographical areas, and the frequency of NTM was higher in the north of Iran (73.2%, 95% CI [66.9, 78.6%]) in comparison with the other geographical areas in Iran [Table 2 and Figure 3].
Subgroups analysis for distribution of different species of nontuberculous mycobacteria in environmental samples
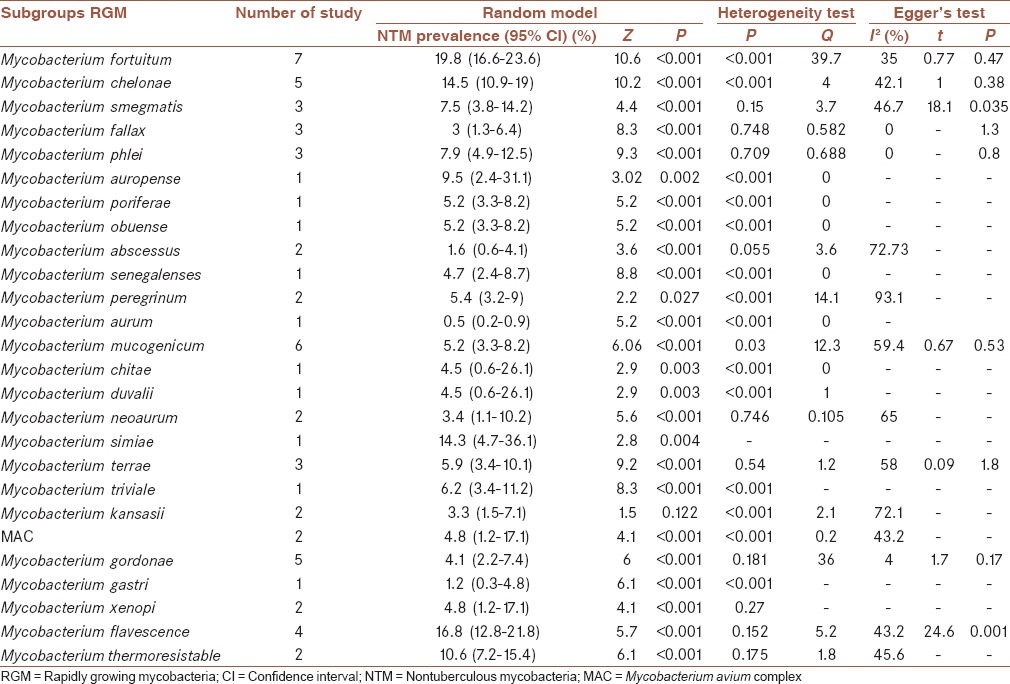
Table 3 outlines the subgroups analysis for distribution of different species of NTMs in environmental samples. The most prevalent rapid-growing mycobacterium was M. fortuitum (19.8%, 95% CI [16.6, 23.6%]) and the most dominant slow-growing mycobacterium was Mycobacterium flavescens (16.8%, 95% CI [12.8, 21.8%]). The frequency of M. fortuitum as highest prevalent NTM species in environmental samples from all geographical locations of Iran is shown in Table 3. The heterogeneity test for M. fortuitum was (P ≤ 0.001, I2 = 35) in contrast with heterogeneity test for M. flavescens (P = 0.152, I2 =43.2). In this study, the second and third highest frequencies of NTM species in the environmental samples were M. chelonae and Mycobacterium simiae, with prevalence ([14.5%, 95% CI (10.9, 19%)] and [14.3%, 95% CI (4.7, 36.1%)]) [Table 3].
Table 3.
Subgroups meta-analysis for rapidly growing mycobacteria and slow-growing mycobacteria species distribution among selected studies
Assessment for sensitivity analysis
By exclusion of the studies that had the biggest[15] or smallest sample size[14] or the study with highest rate of NTMs,[15] the sensitivity of meta-analysis was assessed; the assessment revealed that the meta-analysis estimates was not changed.
DISCUSSION
This study is an extensive meta-analysis on prevalence of NTMs in environmental samples from Iran. The result of our study provides secondary (synthesized) epidemiological information on prevalence of NTMs based on results of research studies in different regions of a climatically heterogeneous of our country. Furthermore, it prepares the baseline information to design a more effective strategy for the national control programs. This study focused on published papers by the end of 2014 to guess a more exact estimation of prevalence of NTMs in environmental samples. Over the recent decades, the ratio of isolation of tubercle bacilli has dropped in the developed countries whereas the occurrence of infection with NTMs has risen.[21]
In general, this meta-analysis showed that prevalence of NTMs in environmental samples from Iran was 38.3% (35.7, 40.9%), but this result cannot reflect the real prevalence of environmental-NTMs in different regions of Iran because these data are from several provinces. The number of studies about NTMs in Iran is few, for that cannot generalize these results to whole our country. The reasons for no attention to the NTMs are: Transmission of NTM between people is said to be really rare and NTM disease was not reported and treatment is not obligatory.[22] However, the high prevalence of NTMs in our country may be due to the neighborhood of Iran with countries such as Afghanistan, Pakistan, and Iraq with high load of NTMs.[23] This high prevalence of NTM in soil and water samples can have subsequent consequences; for example, it can affect the efficacy of BCG vaccine and prior sensitivization with environmental mycobacteria inhibit induction of BCG-mediated immune response and reduce the protection against Mycobacterium tuberculosis[24,25] and presence of environmental mycobacteria in a region cause the immune system of humans have contact with these NTMs and then in immunocompromised humans cause disease.[4] Furthermore, existence of NTMs in water sources, particularly hemodialysis water and potable water, causes contamination of hemodialysis devices and equipment and subsequently NTMs are transmitted and cause disease in patients who are in hemodialysis units[26] and also in individuals with postsurgical wounds as a result of polluted equipment or water.[27] Utilization of contaminated water in the reconstitution of antibiotics/steroids or other surgical methods, for example, liposuction punch biopsies, may possibly produce cutaneous or subcutaneous infections, such as furunculosis which is caused by M. fortuitum in immunocompromised persons.[28] According to subgroups analysis, we noticed that the combined prevalence of NTMs was higher when sample size was ≥100; the reasons for this are unknown, and probably due to the larger sample size, the normal distribution was seen in the prevalence. The prevalence of NTMs in environmental samples from the north (Golestan province) was higher than other regions of Iran; the reasons for this may because neighborhood of this region with countries around the Caspian Sea that have similar patterns in prevalence of NTMs and likely both of those have similarity in climate condition and composition of plant flora.[29] Prevalence of NTMs in environmental samples in various regions of Iran was varied (18.6–73.2%); this is probably due to the difference in geographical locations, climate condition, and use of different decontamination processes.[20] Previously studies have shown that the most of NTMs isolated from soil are rapid growers though some slow-growing species have been accounted;[30] the our meta-analysis showed the similar pattern as the most NTMs isolated from environmental samples were rapid-growing species, and the most common NTM species among rapid-growing mycobacteria were M. fortuitum, followed by M. chelonae and the highest frequent NTM species among slow-growing mycobacteria were belonged to the M. flavescens and M. simiae. M. flavescens, was considered as a nonpathogenic bacterium to humans. Its recovery has constantly been connected to either contamination in the laboratory, but there are some case reports about M. flavescens which can cause pneumonia related to constitutional symptoms, disseminated infections, and glottal abscess.[31] Numerous evidences suggest that there is a direct relationship between the prevalence of environmental mycobacteria isolated from clinical samples in an area with an abundance of NTMs in the environment,[9] as well as the clinical manifestations of NTMs are often mixed with infections result from M. tuberculosis that is a problematic subject in diagnosis of NTM; the NTMs can cause false positive in detection by direct sputum smear microscopy.[32] Different studies conducted on clinical samples from Iran showed that the most commonly isolated NTMs are M. fortuitum and M. simiae[33,34,35] which were comparable with our meta-analysis and confirmed that probably the source of clinical isolates was from environment and person-to-person transmission was not proved.[36] For quick and accurate identification of NTM species directly from clinical samples, molecular methods are essential tools in clinical microbiology and medical laboratories.[37] However, unfortunately, the lack of standard molecular techniques to detect NTMs with high sensitivity, overlaps of infections caused by NTMs with tuberculosis, the lack of facilities for access to environmental samples, and lack of cooperation from companies and organizations for obtaining samples have encountered current and future researches in this field. Contamination is a critical issue in health-care centers, and NTMs monitoring assessment should be evaluated by infection prevention and control professionals.[3] Public and environmental efforts should have focused on measures that will specifically remove NTMs from habitats where predisposed people are exposed.[38] NTMs distribution in Environment is characterized by existence of many immunocompromised individuals in communities and high prevalence of NTMs in environment which can help clinicians to take proper treatment measures and strategies. This study has some strength; we accomplished a comprehensive study for articles by search in multiple databases and also articles selecting was completed independently by two investigators. In some cases, differences among investigators were resolved with debate. Meta-analysis was performed in consistent with available published guidelines and for decreasing the heterogeneity, subgroups analysis was done. However, there are some limitations. We did not contact the authors of the Original selected studies to achieve further information in cases which needed to elucidation, so meta-analysis was performed only based on available information in the selected papers as well as we were not aware of studies that have been carried out, but still unpublished, so they were not included in present study.
CONCLUSION
In regard to increasing incidence of disease in immunocompromised patients and existence of different types of mycobacteria species in environmental samples, efforts should be focused on measures that will specifically remove NTMs from habitats where susceptible individuals are exposed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
This work was conducted in collaboration between all authors. AKh, AB, and DE designed the study and wrote the protocol, the first draft of the manuscript, and KGh revised the final draft of the manuscript. DM and AKh contributed to search and collect data. AT performed the statistical analysis.
The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
REFERENCES
- 1.Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, et al. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal. 2008;22:415–20. doi: 10.1002/jcla.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamrick HJ, Maddux DW, Lowry EK, Taylor LL, rd, Henderson FW, Pillsbury HC. Mycobacterium chelonei facial abscess: Case presentation and review of cutaneous infection due to Runyon Group IV organisms. Pediatr Infect Dis. 1984;3:335–40. [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 4.Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45:421–7. doi: 10.1086/520030. [DOI] [PubMed] [Google Scholar]
- 5.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:390–6. doi: 10.3201/eid1403.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson RM NTM Working Group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–83. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Rodal AI, Mazari-Hiriart M, Lloret-Sánchez LT, Sachman-Ruiz B, Vinuesa P, López-Vidal Y. Potentially pathogenic nontuberculous mycobacteria found in aquatic systems. Analysis from a reclaimed water and water distribution system in Mexico City. Eur J Clin Microbiol Infect Dis. 2012;31:683–94. doi: 10.1007/s10096-011-1359-y. [DOI] [PubMed] [Google Scholar]
- 8.Stanford JL, Shield MJ, Rook GA. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle. 1981;62:55–62. doi: 10.1016/0041-3879(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 9.Falkinham JO., 3rd Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland CS, Ireland JM, Lozano E, Alvarez ME, Primm TP. Mycobacterial ecology of the Rio Grande. Appl Environ Microbiol. 2005;71:5719–27. doi: 10.1128/AEM.71.10.5719-5727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Dantec C, Duguet JP, Montiel A, Dumoutier N, Dubrou S, Vincent V. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl Environ Microbiol. 2002;68:1025–32. doi: 10.1128/AEM.68.3.1025-1032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adabi M, Talebi Taher M, Arbabi L, Afshar M, Fathizadeh S, Minaeian S, et al. Determination of antibiotic resistance pattern of Pseudomonas aeruginosa strains isolated from patients with burn wounds. J Ardabil Univ Med Sci. 2015;15:66–74. [Google Scholar]
- 13.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Rezadehbashi M, Zamani S. Prevalence of drug-resistant tuberculosis in Iran: Systematic review and meta-analysis. Am J Infect Control. 2014;42:1212–8. doi: 10.1016/j.ajic.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Falsafi S, Abad ZBS, Abadi FM. Comparison and optimization of isolation techniques for non-tuberculous mycobacteria from surface waters. New Cell Mol Biotechnol J. 2014;4 In Persian. [Google Scholar]
- 15.Rahideh S, Farnia P, Darboovi M. Identification and isolation of RGM from soil and water samples of robat karim by using PCR-RFLP technique. J Health Hygiene. 2013;4 In Persian. [Google Scholar]
- 16.Nasr-Esfahani B, Sarikhani E, Moghim S, Faghri J, Fazeli H, Hoseini N. Molecular characterization of environmental non-tuberculous mycobacteria using PCR-RFLP analysis of 441 Bp heat shock protein 65 fragments. Iran J Public Health. 2012;41:108. [PMC free article] [PubMed] [Google Scholar]
- 17.Bahram NE, Ensieh S, Shrareh M, Jamshid F, Hossein F, Ghasemian SH, et al. Isolation and phenotypic identification of non-tuberculous mycobacteria existing in Isfahan different water samples. Adv Biomed Res. 2012;1:18. doi: 10.4103/2277-9175.98115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghim S, Esfahani BN, Sadat Hosseini N, Sarikhani A. Identification of Non tuberculous mycobacteria isolated from Isfahan different water sources using phenotypic characterization tests. J Water Waste. 2011;2 In Persian. [Google Scholar]
- 19.Ghaemi E, Ghazisaidi K, Koohsari H, Mansoorian A. Environmental mycobacteria in areas of high and low tuberculosis prevalence in the Islamic Republic of Iran. East Mediterr Health J. 2006;2:280–5. [PubMed] [Google Scholar]
- 20.Rahbar M, Lamei A, Babazadeh H, Yavari SA. Isolation of rapid growing mycobacteria from soil and water in Iran. Afr J Biotechnol. 2010;9:3618–21. [Google Scholar]
- 21.Debrunner M, Salfinger M, Brändli O, von Graevenitz A. Epidemiology and clinical significance of nontuberculous mycobacteria in patients negative for human immunodeficiency virus in Switzerland. Clin Infect Dis. 1992;15:330–45. doi: 10.1093/clinids/15.2.330. [DOI] [PubMed] [Google Scholar]
- 22.Peoples M. Canadian Tuberculosis Standards. Canada: Public Health Agency; 2014. [Google Scholar]
- 23.Velayati AA, Farnia P, Mozafari M, Mirsaeidi M. Nontuberculous mycobacteria isolation from clinical and environmental samples in Iran: Twenty years of surveillance. Biomed Res Int 2015. 2015:254285. doi: 10.1155/2015/254285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demangel C, Garnier T, Rosenkrands I, Cole ST. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun. 2005;73:2190–6. doi: 10.1128/IAI.73.4.2190-2196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen P, Doherty TM. The success and failure of BCG – Implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 26.Wallace R, Silcox V, Tsukamura M, Brown B, Kilburn J, Butler W, et al. Clinical significance, biochemical features, and susceptibility patterns of sporadic isolates of the Mycobacterium chelonae-like organism. J Clin Microbiol. 1993;31:3231–9. doi: 10.1128/jcm.31.12.3231-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa NE, Cataño JC, Mejía GI, Realpe T, Orozco B, Estrada S, et al. Outbreak of mesotherapy-associated cutaneous infections caused by Mycobacterium chelonae in Colombia. Jpn J Infect Dis. 2010;63:143–5. [PubMed] [Google Scholar]
- 28.Simmon KE, Pounder JI, Greene JN, Walsh F, Anderson CM, Cohen S, et al. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J Clin Microbiol. 2007;45:1978–80. doi: 10.1128/JCM.00563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaemi E, Ghazisaidi K, Koohsari H, Khodabakhshi B, Mansoorian A. Environmental mycobacteria in areas of high and low tuberculosis prevalence in the Islamic Republic of Iran. East Mediterr Health J. 2006;12:280–5. [PubMed] [Google Scholar]
- 30.Wang Q, Wang S. Soil organic matter under different forest types in Southern China. Geoderma. 2007;142:349–56. [Google Scholar]
- 31.Moreno Guillen S, Sanz Hospital J, Gomez Mampaso E, Guerrero Espejo A, Ezpeleta Baquedano C, Ortega Calderon A. Gluteal abscess caused by Mycobacterium flavescens. Tubercle. 1986;67:151–3. doi: 10.1016/0041-3879(86)90010-3. [DOI] [PubMed] [Google Scholar]
- 32.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Hashemi Shahraki A. Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: Systematic review and meta-analysis. PLoS One. 2015;10:e0129073. doi: 10.1371/journal.pone.0129073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashemi-Shahraki A, Bostanabad SZ, Heidarieh P, Titov LP, Khosravi AD, Sheikhi N, et al. Species spectrum of nontuberculous mycobacteria isolated from suspected tuberculosis patients, identification by multi locus sequence analysis. Infect Genet Evol. 2013;20:312–24. doi: 10.1016/j.meegid.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Bahrmand AR, Madani H, Samar G, Khalilzadeh L, Bakayev VV, Yaghli M, et al. Detection and identification of non-tuberculous mycobacterial infections in 6,472 tuberculosis suspected patients. Scand J Infect Dis. 1996;28:275–8. doi: 10.3109/00365549609027172. [DOI] [PubMed] [Google Scholar]
- 35.Shafipour M, Ghane M, Alang SR, Livani S, Javid N, Shakeri F, et al. Non tuberculosis mycobacteria isolated from tuberculosis patients in Golestan province, North of Iran. Ann Biol Res. 2013;4:133–7. [Google Scholar]
- 36.Falkinham JO., 3rd Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17:419–24. doi: 10.3201/eid1703.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watterson SA, Drobniewski FA. Modern laboratory diagnosis of mycobacterial infections. J Clin Pathol. 2000;53:727–32. doi: 10.1136/jcp.53.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson RM, Carter R, Tolson C, Coulter C, Huygens F, Hargreaves M. Factors associated with the isolation of nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiol. 2013;13:89. doi: 10.1186/1471-2180-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]