Abstract
Background:
Ventilator-associated pneumonia (VAP) is a type of lung infection that typically affects critically ill patients undergoing mechanical ventilation (MV) in the intensive care unit (ICU). Patients with type 2 diabetes mellitus (T2DM) are considered to be more susceptible to several types of infections including community-acquired pneumonia. However, it is not clear whether T2DM is a risk factor for the development of VAP. The purpose of this study was to determine the risk of VAP for diabetic and nondiabetic mechanically ventilated trauma patients.
Materials and Methods:
This study is a secondary analysis of a prospective observational study of the history of T2DM in the ICU over a period of 1 year at Imam Khomeini Hospital in Iran. A total of 186 critically ill trauma patients who required at least 48 h of MV were monitored for the occurrence of VAP by their clinical pulmonary infection score (CPIS) until ICU discharge, VAP diagnosis, or death.
Results:
Forty-one of the 186 patients developed VAP. The median time from hospitalization to VAP was 29.09 days (95% CI: 26.27–31.9). The overall incidence of VAP was 18.82 cases per 1,000 days of intubation (95% CI: 13.86-25.57). Risk of VAP in diabetic patients was greater than nondiabetic patients after adjustments for other potential factors [hazard ratio (HR): 10.12 [95% confidence interval (CI): 5.1–20.2); P < 0.0001)].
Conclusion:
The findings show that T2DM is associated with a significant increase in the occurrence of VAP in mechanically ventilated adult trauma patients.
Keywords: Diabetes mellitus, intensive care unit (ICU), trauma, ventilator-associated pneumonia (VAP)
INTRODUCTION
Ventilator-associated pneumonia (VAP) is a dangerous complication in patients who need mechanical ventilation (MV).[1] VAP is the most common infection among patients undergoing MV and has been associated with an increased mortality rate (approaching 50%), an increased morbidity rate, the length of intensive care unit (ICU) stay, and the duration of MV. Despite advances in preventive strategies, such as the implementation of the VAP bundle and the Centers for Disease Control and Prevention's (CDC) recommendations for critical care, the mortality and morbidity rates still require substantial improvement.[2]
Type 2 diabetes mellitus (T2DM) is a condition that has been linked to alterations of immune response and is often found in critical care patients.[3] Patients with T2DM have infections more often than those without DM.[3] However, the impact of T2DM as a risk factor for VAP in critically ill patients has not been sufficiently studied. There are limited data concerning selected populations of critically ill patients, which indicate that diabetic patients have a higher probability of developing ICU-acquired infections compared to nondiabetic subjects[4,5] while some studies involving critically ill patients in the ICU found no association between T2DM and the development of VAP infection.[6,7] The purpose of this study was to determine the risk of VAP for diabetic and nondiabetic patients with normal glycemic control who underwent MV for at least 48 h.
MATERIALS AND METHODS
Study design
This is a secondary analysis from a prospective, observational, cohort survey conducted in three general ICUs in the 1,000-bed Imam Khomeini Medical Center located in Mazandaran, Iran. Approval of the institutional review board was obtained for the study. Also, informed consent in the case of conscious patients was obtained from the patients themselves and in unconscious patients, from the proper surrogate decision-maker. We assured all of them that their information would be kept confidential. Informed consent for the present study was obtained regardless of the consent obtained for the main study. This prospective study was conducted between September 22, 2012 and September 23, 2013 to determine the incidence, risk factors, and outcome of VAP in the ICUs.
Patients and follow-up
All traumatic patients aged >18 years without pneumonia at ICU admission and who then required at least 48 h of MV were included in this study. Patients who were undergoing MV before admission to the ICU or those who died within 48 h of starting MV were excluded. A group of attending physicians and nurses prospectively collected data on all patients who underwent MV. They noted relevant data from medical documents, such as bedside flow sheets. Moreover, the clinical review included the clinical pulmonary infection score (CPIS) records for diagnosis of VAP. Patients were assessed every day (in the morning; once every 24 h) during the entire length of the study. Patients were followed until ICU discharge, VAP diagnosis, or death. Our study's primary focus was the rate of diagnosis of VAP. Only the first episode of VAP was evaluated. All the patients undergoing MV routinely received stress ulcer prophylaxis [ranitidine, 50 mg via intravenous (IV) three times a day] and intravenous antibiotic prophylaxis for 24-36 h. Administered antibiotic prophylaxis was based on hospital routine, including cefalotin (1 g, divided into four doses a day) for mechanically ventilated adult trauma patients.
In all patients, the blood glucose was controlled with insulin therapy (infusion or subcutaneous) within a range of 80–180 mg/dL for the duration of ICU stay.[8] In diabetic and nondiabetic patients, blood glucose was checked every 6 h and 24 h, respectively. If it rose up to 200 mg/dL, 2 units of insulin subcutaneously was prescribed for per 20 mg/dL blood glucose, higher than 200 mg/dL. Infusion insulin therapy with rate of 0.5–2 units was commenced in diabetic patients with blood glucose up to 350 mg/dL according to blood glucose per 1 h.[9]
Data collection and baseline data
The baseline data collected included demographic data [sex, age, body mass index (BMI), date of admission to the ICU], primary diagnosis, underlying illness, type of tracheal intubation (elective/emergency), history of hypertension, chronic obstructive pulmonary disease, and T2DM, limitation in positional changes, enteral nutrition (gavage feeding), ICU stay and length of hospital stay, duration of intubation, Glasgow Coma Scale (GCS) with ventilatory support and with or without sedation, and duration of MV.
Ventilator-associated pneumonia definition
Diagnosis of VAP was according to original CPIS after at least 48 h of MV. CPIS was developed in 1991 and it is including a new chest x-ray infiltrate persistent for 48 h or more, a body temperature of more than 38.58°C or less than 35.08°C, changes in white blood cell count (WBC) as a leukocyte count of more than 10,000/lL or less than 3,000/lL, worsening hypoxia (arterial oxygenation, PaO2/fraction of inspired oxygen, FiO2 ratio ≤240 without acute respiratory distress syndrome (ARDS), and ARDS), purulent tracheal secretions, and microorganisms isolated from at least one of the following samples: Bronchoalveolar lavage (BAL) ≥10,000 CFU/mL), endotracheal aspirate (ETA) ≥100,000 CFU/mL, or sputum.[10,11] Semi-quantitative ETA or BAL samples of suspected cases of VAP were collected from ICU patients in this study.[12] The CPIS ranges from zero to 12 and scores higher than 6 indicate VAP. Validity and reliability of the Persian version of the CPIS is confirmed, and it is used widely in research studies to appraise suspected VAP.[13,14]
Data analysis
A cumulative survival curve for each patient was calculated using the Kaplan–Meier method and was compared by use of log-rank tests. All statistically marginally significant prognostic factors identified by univariate analysis (P < 0.2) (sex, age, limitation in positional changes state, GCS score) were entered into a Cox proportional hazard (PH) model with forward stepwise (likelihood ratio) to identify independent predictors of VAP event. Only variables with statistically significant effect were kept in the final model. The Cox PH model assumes that the hazard ratio (HR) for any two specifications of predictors is constant over time. We evaluated this assumption with the Schoenfeld Residuals method.
For all analyses, P values were two-sided and P < 0.05 was considered to be statistically significant. All statistical analyses and graphics were performed using the Statistical Package for the Social Sciences (SPSS) software package (version 16.0, SPSS Inc., Chicago, IL, USA) and STATA statistical package (version 10, STATA, College Station, TX).
RESULTS
Basic and clinical characteristics of diabetic patients are shown in Table 1.
Table 1.
Basic and clinical characteristics of patients according to diabetic state
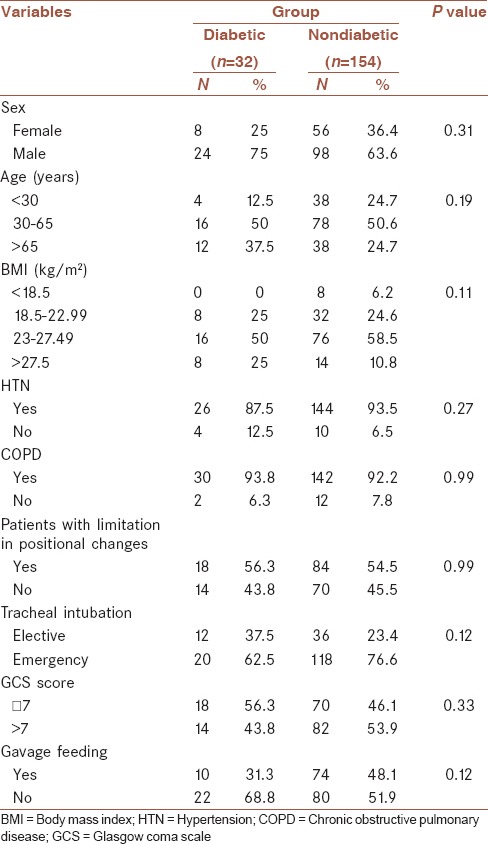
Table 1 shows that there was no significant difference in the sex, age, BMI, hypertension (HTN), chronic obstructive pulmonary disease (COPD), limitation in positional changes, tracheal intubation, GCS, and gavage feeding between diabetic and nondiabetic patients.
Results show that the mean age was 47.81 years (±21.7); the mean duration of admission, ICU stay, and intubation were 17.16 days (±13.34), 16.2 days (±13.19), and 11.71 days (±7.56), respectively. The vast majority of the patients were males of 30–65 years of age and overweight [Table 2].
Table 2.
Median (95% CI) time to the progression of VAP (Kaplan–Meier method) in ICU patients according to the possible prognostic factors
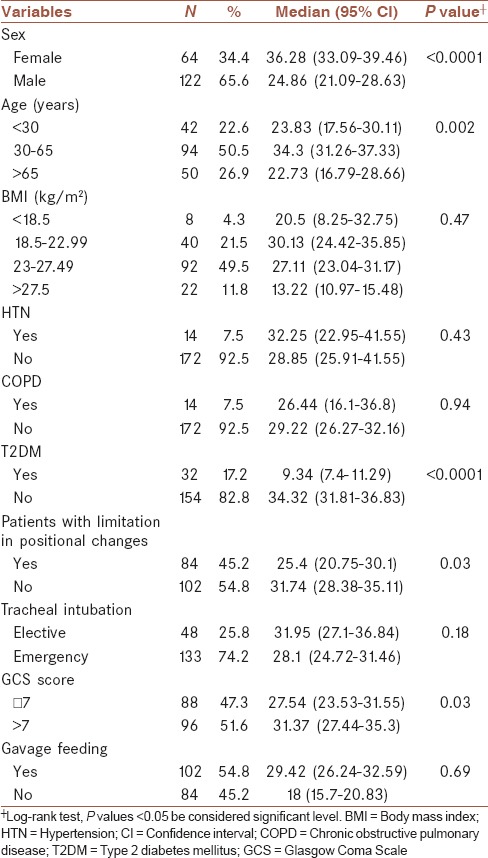
During the study period, 186 patients required MV for more than 48 h out of whom, 41 developed VAP (17 cases (11%) in nondiabetic patients and 24 cases (75%) in diabetic patients), corresponding to an average of 18.82 (95% CI: 13.86–25.57) VAP cases per 1,000 days of intubation [50 (95% CI: 33.51–74.6) and 10.01 (95% CI: 6.22–16.1)] VAP cases per 1,000 days of intubation in diabetic and nondiabetic patients, respectively). The median time from hospitalization to VAP was 29.09 days (95% CI: 26.27–31.9) and this in relation to patient characteristics is shown in Table 2.
As shown in Table 2, median time from hospitalization to VAP occurrence was higher in females, middle-aged patients, nondiabetics, patients with no limitation in positional changes, and patients with higher GCS. The Cox PH model revealed that after adjusting other variables, diabetic patients have a higher hazard of progression to VAP than nondiabetic patients (HR: 10.12; 95% CI: 5.1–20.2, P < 0.0001) [Table 3]. Table 3 shows that aging patients versus patients less than 30 years of age (3.12; 95% CI: 1.56–6.25; P = 0.001), and thin (5.1; 95% CI: 1.1–24.57; P = 0.04) and overweight (2.75; 95% CI: 1.41–5.37; P = 0.003) patients versus patients of normal weight have a higher hazard of progression to VAP. This assumption of the Cox PH model was evaluated with the Schoenfeld Residuals method and we saw that the plot of the residuals were horizontal and close to 0.
Table 3.
Association between prognostic factors and progression to VAP in Cox proportional hazard regression multivariate analysis
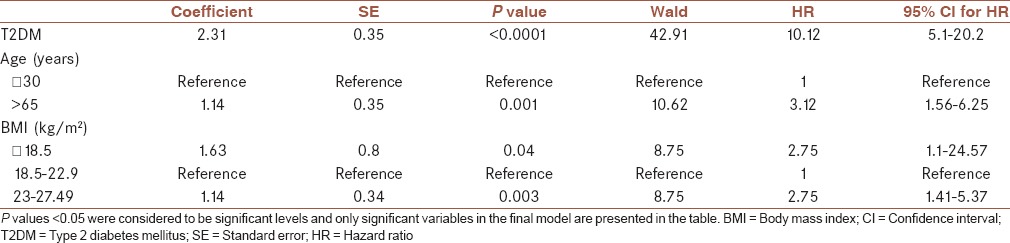
ICU mortality rates in diabetic and nondiabetic patients in our study were 37.5% and 28.6%, respectively (P = 0.32). ICU mortality increased by 66.6% (P < 0.0001) when diabetic patients developed VAP, remaining an independent predictor of mortality [hazard ratio (HR) 18.18; 95% CI: 4.76–100] [Table 3].
A survival curve of the 186 patients, some with and some without T2DM, is shown in Figure 1.
Figure 1.
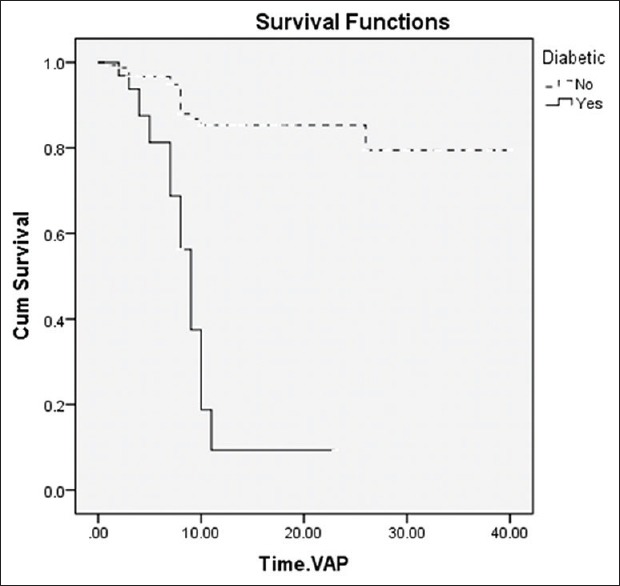
Survival curves of 186 patients with different baseline characteristics of T2DM
As shown in Figure 1, the median time from hospitalization to VAP occurrence in diabetic patients was lower than in nondiabetic patients [9.34 (95% CI: 7.39–11.29) and 34.32 (95% CI: 31.81–36.83) days, respectively; P < 0.0001].
DISCUSSION
We conducted a study to determine the relationship between T2DM and suspected VAP in mechanically ventilated adult trauma patients admitted to ICUs. A major finding of this secondary analysis of the cohort study that is contrary to the findings of previous studies was that the critically ill trauma patients with T2DM had a significantly higher incidence of VAP compared to patients who did not have T2DM. Hence, T2DM is probably associated with an increased risk of VAP in intubated critically ill trauma patients undergoing MV. This study also demonstrated that limitation in positional changes, age, and being overweight were also risk factors for the development of VAP.
There are a few studies that evaluate the association between personal history of T2DM and the occurrence of VAP in critically ill patients. A prospective observational study on patients admitted to the general ICU showed that the overall ICU incidence of VAP was 26%, and T2DM and HbA1c were not associated with increased risk of VAP.[6] Similarly, a review study that surveyed 84 studies sought to identify the risk factors for hospital-acquired pneumonia (HAP). Cohort, case-control, and observational studies were included in this work, which revealed that T2DM was not a risk factor for the development of HAP.[7] In relation to lung infections in diabetic patients, a few studies propose associations between T2DM and the susceptibility to pneumonia.[15,16] Akbar et al. compared the risk of bacterial pneumonia between diabetics and nondiabetics. Of the 354 patients with a positive sputum culture, 125 patients (35.5%) were diabetics. Results of this study revealed that the diabetic patients were generally older with a male predominance compared to nondiabetics.[15] It is possible that the older age of these diabetic patients is one of the reasons for the increased rate of pneumonia in this population. Tamayo et al. conducted a prospective cohort study that included 1,610 postoperative cardiac surgery patients after cardiopulmonary bypass (CPB). It observed that 124 patients (7.7%) developed VAP. After performing the Cox multivariate analysis adjustment, T2DM was identified as one of the important independent mortality risk factors (HR: 1.90).[17] In another prospective study by Falguera et al., data from 106 patients with T2DM were evaluated during a 5-year period. Results of this study showed that patients with T2DM were significantly associated with the evidence of pleural effusion and higher mortality. Diabetics were often older and also had significantly more concomitant comorbid conditions.[16] Advanced age is associated with immune changes that increase the risk of pneumonia.[15] Also, T2DM has been associated with many alterations of the immune system.[16] We speculate that T2DM might affect the risk of VAP through immune dysfunction and age effects in intubated critically ill patients. The observed predisposition of diabetic critically ill patients to VAP could be partly due to significant changes within their immune system, particularly changes related to pulmonary dysfunction, and alterations of pulmonary host defenses.[15,18] This predisposition can be intensified in critically ill trauma patients.[19,20] Although the rate of T2DM in critically ill patients is high,[4] the impact of T2DM as a risk factor for VAP in these patients has not been adequately evaluated.[6] Our findings demonstrate that T2DM in critically ill trauma patients is associated with the increased risk of VAP in the ICU. Well-designed prospective cohort or case-control studies in diabetic critically ill patients could provide more information about the correlation between T2DM and VAP in the ICU.
In agreement with several previous studies, this study showed that the limitation of positional changes,[21] increase in age,[22] and also being overweight[23] are risk factors of developing VAP.
This study, however, has several limitations. Although the data in this analysis were prospectively collected, this study was a secondary analysis and therefore, may have had some limitations consistent with a retrospective approach. Because of these limitations, our results need confirmation, and future cohort studies investigating the relationship between T2DM and VAP in mechanically ventilated adult trauma patients in the ICU are needed. This study was a secondary analysis and thus, was not specifically designed for the given research question. Patients with formerly undiagnosed T2DM and subjects with fasting blood sugar or impaired glucose tolerance may have been neglected, because identifying patients with T2DM was based on a previous history. Also, no data were presented for all diabetic patients concerning the duration of T2DM and the severity of organ damage in these patients. Moreover, some variables including ICU scoring such as the Simplified Acute Physiology Score (SAPS) II score and the Sequential Organ Failure Assessment (SOFA) score were not recorded for analysis. Likewise, mortality in diabetic patients with VAP was not compared to nondiabetic subjects. In our study, we used semi-quantitative ETA more than BAL because of its cost and ease. Some studies have showed that there was a total agreement in bacteriology between ETA and BAL in sensitivity for VAP diagnosis.[11,12] Unfortunately, because of the inadequate sample size of patients, we also did not test the association between early- and late-onset VAP and T2DM in this study. Thus, this study lacks baseline data that will need to be addressed in the next generation of studies.
CONCLUSION
In conclusion, it seems that a diabetic condition in critically ill patients is an important baseline characteristic that can be associated with various aspects of critical illness such as infection. Our results indicate that T2DM is significantly associated with increased VAP risk during ICU stay for mechanically ventilated adult trauma patients. The results suggest that greater care in the prevention of VAP should be considered for this important population.
Financial support and sponsorship
This study was funded by the Research Deputy of Islamic Azad University, Sari Branch, Iran.
Conflicts of interest
All authors declare no conflict of interest; no conflict of interest exists for any of the authors associated with the manuscript, and the entire study was performed without external funding. The funding organization had no role in the design and conduct of the study, or in the collection, analysis, and interpretation of the data.
AUTHOR'S CONTRIBUTIONS
Study concept and design: HDKH, AEZ, MON, and AF. Statistical analysis and interpretation of data: AA. Drafting of the manuscript: HDKH, AF, AEZ, and MON. Critical revision of the manuscript for important intellectual content: HDKH, AEZ, MON, AF, and AA.
Acknowledgements
The authors wish to thank the staff of the ICUs Center in Mazandaran, Sari, Iran, and the patients’ families at the Imam Khomeini Hospital, Division of General ICUs, for their generous and efficient collaboration (include Research Project Number 1091). We are grateful to the physicians and nurses for their aid with the clinical review and data collection in this study.
REFERENCES
- 1.Mortensen BL, Skaar EP. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol. 2013;3:95. doi: 10.3389/fcimb.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah GS, Dutta AK, Shah D, Mishra OP. Role of zinc in severe pneumonia: A randomized double bind placebo controlled study. Ital J Pediatr. 2012;38:36. doi: 10.1186/1824-7288-38-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 4.Michalia M, Kompoti M, Koutsikou A, Paridou A, Giannopoulou P, Trikka-Graphakos E, et al. Diabetes mellitus is an independent risk factor for ICU-acquired bloodstream infections. Intensive Care Med. 2009;35:448–54. doi: 10.1007/s00134-008-1288-0. [DOI] [PubMed] [Google Scholar]
- 5.Ryan T, Mc Carthy JF, Rady MY, Serkey J, Gordon S, Starr NJ, et al. Early bloodstream infection after cardiopulmonary bypass: Frequency rate, risk factors, and implications. Crit Care Med. 1997;25:2009–14. doi: 10.1097/00003246-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Tsakiridou E, Makris D, Chatzipantazi V, Vlachos O, Xidopoulos G, Charalampidou O, et al. Diabetes and hemoglobin A1C as risk factors for nosocomial infections in critically ill patients. Crit Care Res Pract 2013. 2013 doi: 10.1155/2013/279479. 279479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardakas KZ, Siempos II, Falagas ME. Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality. Diabet Med. 2007;24:1168–71. doi: 10.1111/j.1464-5491.2007.02234.x. [DOI] [PubMed] [Google Scholar]
- 8.Egi M, Finfer S, Bellomo R. Glycemic control in the ICU. Chest. 2011;140:212–20. doi: 10.1378/chest.10-1478. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CW. Glycemic control in critically ill patients. World J Crit Care Med. 2012;1:31–9. doi: 10.5492/wjccm.v1.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugin J. Clinical signs and scores for the diagnosis of ventilator-associated pneumonia. Minerva Anestesiol. 2002;68:261–5. [PubMed] [Google Scholar]
- 11.Chastre J, Fagon JY. Diagnosis of ventilator-associated pneumonia. N Engl J Med. 2007;356:1469–71. doi: 10.1056/NEJMc076017. [DOI] [PubMed] [Google Scholar]
- 12.Ranjan N, Chaudhary U, Chaudhry D, Ranjan KP. Ventilator-associated pneumonia in a tertiary care intensive care unit: Analysis of incidence, risk factors and mortality. Indian J Crit Care Med. 2014;18:200–4. doi: 10.4103/0972-5229.130570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalifehzadeh A, Parizade A, Hosseini A, Yousefi H. The effects of an oral care practice on incidence of pneumonia among ventilator patients in ICUs of selected hospitals in Isfahan, 2010. Iran J Nurs Midwifery Res. 2012;17:216–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Fahimi F, Ghafari S, Jamaati H, Baniasadi S, Tabarsi P, Najafi A, et al. Continuous versus intermittent administration of piperacillin-tazobactam in intensive care unit patients with ventilator-associated pneumonia. Indian J Crit Care Med. 2012;16:141–7. doi: 10.4103/0972-5229.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbar DH. Bacterial pneumonia: Comparison between diabetics and non-diabetics. Acta Diabetol. 2001;38:77–82. doi: 10.1007/s005920170017. [DOI] [PubMed] [Google Scholar]
- 16.Falguera M, Pifarre R, Martin A, Sheikh A, Moreno A. Etiology and outcome of community-acquired pneumonia in patients with diabetes mellitus. Chest. 2005;128:3233–9. doi: 10.1378/chest.128.5.3233. [DOI] [PubMed] [Google Scholar]
- 17.Tamayo E, Álvarez FJ, Martínez-Rafael B, Bustamante J, Bermejo-Martin JF, Fierro I, et al. Valladolid Sepsis Study Group. Ventilator-associated pneumonia is an important risk factor for mortality after major cardiac surgery. J Crit Care. 2012;27:18–25. doi: 10.1016/j.jcrc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 19.Winkelman C. The role of inflammation in ICU-acquired weakness. Crit Care. 2010;14:186. doi: 10.1186/cc9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek JH, Kim MS, Lee JC, Lee JH. Systemic inflammation response syndrome score predicts the mortality in multiple trauma patients. Korean J Thorac Cardiovasc Surg. 2014;47:523–8. doi: 10.5090/kjtcs.2014.47.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Yuan Q, Wang L, Sun X, Deng L. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Libr. 2012;CD009946:1–8. doi: 10.1002/14651858.CD009946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SA, Cho SS, Kwak GJ. Factors influencing ventilator-associated pneumonia in cancer patients. Asian Pac J Cancer Prev. 2014;15:5787–91. doi: 10.7314/apjcp.2014.15.14.5787. [DOI] [PubMed] [Google Scholar]
- 23.Anzueto A, Frutos-Vivar F, Esteban A, Bensalami N, Marks D, Raymondos K, et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66:66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]


