Abstract
Background:
Vitamin D deficiency is common in pregnancy, leading to increase in the frequency of preeclampsia, cesarean delivery, neonatal bacterial vaginosis, and gestational diabetes. The current study was designed and implemented to investigate the effect of vitamin D during the first and second trimesters of pregnancy in reducing the risk of gestational diabetes mellitus (GDM) in women who are at high risk [history of GDM, birth macrosomia, family history, and high body mass index (BMI)].
Materials and Methods:
In a randomized, double-blind, and placebo-controlled trial, 90 pregnant women who had at least one risk factor for GDM were randomized into intervention (46 participants) and control (44 participants) groups. Participants in the intervention group took 5000 units of vitamin D daily and the control group took placebo until the 26th week of pregnancy. Then the glucose challenge test (GCT) and the glucose tolerance test (GTT) were performed to evaluate GDM.
Results:
Mean ± standard deviation (SD) age was 31.28 ± 6.38 years and 29 ± 6.24 years for the intervention group and the placebo group, respectively, (P > 0.05). In addition, there were no significant differences between two groups in terms of vitamin D levels and GCT (P > 0.05), and the difference was not significant. The incidence of diabetes in the intervention groups was statistically lower than in control group (11.4% vs 34.8; P < 0.01). The results showed that abnormal GCT in the placebo group was statistically higher than in intervention group (35.9% vs 10.9 P < 0.005).
Conclusion:
The results of the current study showed that the prescription of vitamin D supplementation in the first and second trimesters of pregnancy was effective in reducing GDM and controlling GTT and GTC.
Keywords: Diabetes, first trimester, gestational diabetes, pregnancy, Sanandaj, vitamin D
INTRODUCTION
Vitamin D is one of the fat-soluble vitamins, which has many functions in addition to its vital role in bone health, including prevention of cell proliferation in colorectal, prostate, and breast cancers and its role in prevention of autoimmune diseases.[1] The need for vitamin D is higher in some stages of life, which include the rapid growth of the fetus in the embryonic stage, infancy, the early stage of childhood, puberty, and pregnancy.[2,3] Studies have shown that vitamin D deficiency is common in pregnancy[4,5] and is associated with increased incidence of preeclampsia,[6] cesarean section (C/S),[7] bacterial vaginosis,[8] and gestational diabetes mellitus (GDM).[9]
In addition, the reverse relationship between vitamin D concentration in serum and risk of diabetes type-II has been proved. Vitamin D has a direct effect on pancreatic beta cells and glucose metabolism, thus its level has a reverse relationship with glycosylated hemoglobin (HbA1C) levels.[9] Vitamin D deficiency is known to be related to blood glucose and insulin concentration alteration and target tissue sensitivity to insulin.[10] GDM is characterized by glucose intolerance in pregnancy and its pathophysiology is similar to type 2 diabetes mellitus, that is insulin tolerance and insulin insufficiency. On the other hand, GDM is associated with maternal (premature birth, infectious complications, hydramnios, hypertensive complications) and fetal (dead birth, altered fetus growth, metabolic disturbances, respiratory distress syndrome, obesity in childhood, and diabetes) complications.[11] Vitamin D deficiency is common in pregnancy and evidence shows that vitamin D causes increased sensitivity to insulin and glucose tolerance, and many researchers have shown the relationship between vitamin D deficiency and GDM, but little is known about the effect of prescribing vitamin D supplementation in preventing gestational diabetes.[12] Alzhime et al. in their study concluded that there is a relationship between vitamin D deficiency and gestational diabetes. They suggested that in order to investigate vitamin D prescription effects on gestational diabetes incidence, there is a need for intervention and prescribing vitamin D supplementation.[13] Other studies have shown that vitamin D deficiency causes insufficient secretion of insulin and other pancreatic hormones and glucose intolerance in animal and human models.[9]
Vitamin D deficiency and its effect on gestational diabetes have been investigated in various studies, however, the vitamin D dose needed for decreasing the risk of gestational diabetes is not known yet.[14] Basic epidemiologic studies have shown a relationship between vitamin D deficiency and risk of gestational diabetes and diabetes type 2.[15] Although observational studies have proved the relationship between vitamin D deficiency and increased risk of gestational diabetes,[16] a reverse relationship has been identified between vitamin D deficiency and blood glucose in other studies.[17,18] In vivo studies on rats showed that vitamin D supplementation caused improved glucose tolerance and insulin secretion,[19,20] and in small trials vitamin D has caused blood glucose control in gestational diabetes.[20,21]
Considering the vitamin D deficiency in Iranian population, particularly in pregnant women, and its negative impact on pregnancy outcomes, the current study was designed to investigate the effect of vitamin D supplementation in the first and second trimesters on GDM in high-risk women.
MATERIALS AND METHODS
Study design and participants
This study was a randomized double-blind controlled clinical trial that was carried out on 90 pregnant high-risk women in the first trimester with in the Obstetrics and Gynecology section of Be‘sat hospital clinic offices in Sanandaj, Iran, in 2013. Sample size was calculated according to previous studies[2,3] considering the type one error rate α = 0.05, and statistical power 1-β = 0.9 for detecting a. At the beginning of the study, 110 participants were screened based on inclusion and exclusion criteria and 100 pregnant women were included and randomly divided into two groups using permuted block randomization of size two. The number of participants in each group was 50, but finally 46 and 44 participants per group remained in the analysis stage [Figure 1]. Pregnant women with the following criteria were included; at least one risk factor for gestational diabetes including body mass index (BMI; kg/m2) more than 25, history of macrosome neonate, positive family history for diabetes and gestational diabetes, history of gestational diabetes in previous pregnancies, and glycosuria. Exclusion criteria were as follows: Chronic diseases including renal, rheumatologic, hepatic, thyroid; pregnant women who had had diabetes before pregnancy; gestational diabetes at the time of admission; history of consuming vitamin D and drugs that interfere with glucose metabolism. Blinding was carried out in which drugs and placebos were prepared to be completely similar in appearance and taste and put in numbered pockets based on a randomization, list and neither the patients nor the physician knew the trend. The protocol was approved by the Institutional Review Board (IRB) of Kordestan University of Medical Sciences and carried out in agreement with the Declaration of Helsinki and its subsequent revisions. After complete explanation of the study details, written informed consent was obtained from eligible patients and their legally authorized representatives, informing the participants of their rights to withdraw from the trial at any time without any interruption in their health care benefits. This trial was registered at the Iranian Clinical Trials Registry (IRCT number 14140742; www.irct.ir).
Figure 1.
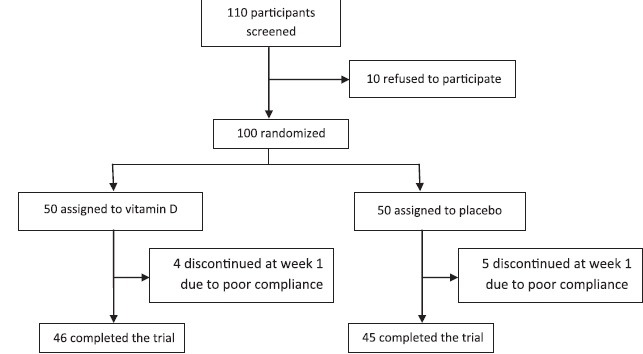
Flow diagram of the trial
Procedures and variables assessment
Then, randomization by simple randomization along with.
After entering the participants in the study, a glucose challenge test (GCT) was performed on all the patients in the first trimester. The women who were diagnosed with gestational diabetes were excluded from the study. Then, a blood sample was taken from women who had entered the study to measure the vitamin D level. Vitamin D level was determined in a laboratory by the Liebermann–Burchard method, in which the patient should fast for 12 h and not have a fatty dinner.
Women in the intervention group took vitamin D, 5000 units per week and women in the control group received placebo till the 26th week. Every month all the women were examined and their blood pressure were determined. GCT on the 26th week was carried out and gestational diabetes was determined.
Glucose tolerance test (GTT) was performed according to the following approach: Blood glucose was measured at baseline, then participants were given 75 g oral glucose, and blood glucose levels at 1 h and 2 h later were measured. The result for the glucose screening test was considered, as the fasting blood sugar was 100-120 mg/dL and 1 h after taking oral glucose equal or less than 140 mg/dL, while if blood glucose after 2 h was 140-200, it was considered to be impaired or positive GTT. Additionally, two impaired oral GTTs were considered as a positive test.[15]
All the patients were followed up till delivery. Type of delivery, Apgar score, and macrosome neonate were determined.
Statistical analysis
After collection the data were entered and analyzed using SPSS software. Quantitative data were expressed as mean (SD) and qualitative variables as frequency (percentage). Normality of quantitative data was evaluated using the Kolmogorov–Smirnov test and quantile-quantile (Q-Q) plot. Independent sample t-test was used for comparing normally distributed data between two groups, and chi-square or Fisher's exact test was used for comparing qualitative ones.
RESULTS
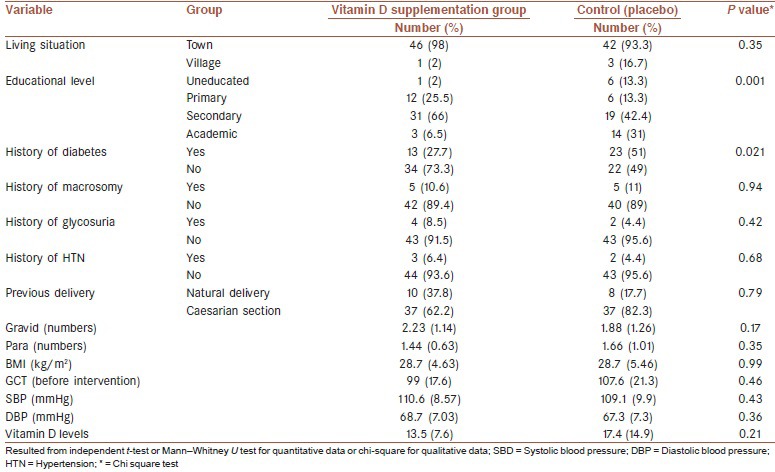
Table 1 presents the results of comparing the demographic and basic clinical variables between the two studied groups. Significant differences were found in two groups only in terms of educational levels (P < 0.01) and history of diabetes (P < 0.05), and the studied groups were similar based on other variables., residential place.
Table 1.
Demographic and basic baseline characteristics of study participants in two groups
The results of our study showed that at the end of the study intervention period there was no significant difference between the two studied groups in terms of blood pressure and birth weight outcome (P > 0.1) [Table 2]. In addition, no significant differences were observed before and after intervention in each groups in SBP and DBP (results not shown).
Table 2.
Results of comparing the SBP, DBP, and birth weight at end of study between the two study groups
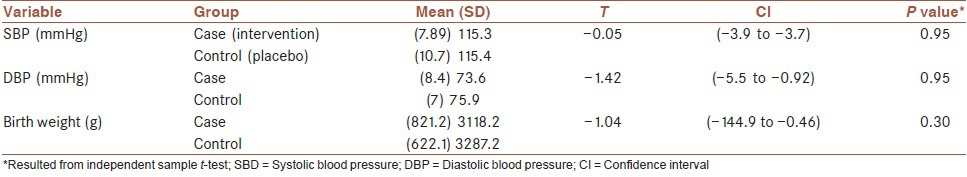
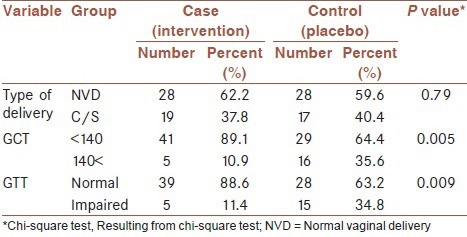
Table 3 presents the results of comparing the main study's outcomes, i.e., the abnormality in GTT and GCT levels between the two groups. As can be seen, significantly higher percentages of positive GTT (34.8% vs 11.4%) and GCT (35.6% vs10.9%) were observed in the control group compared with participants in the vitamin D supplementation group; both P < 0.01. Similar results were observed in stratified analysis based on the history of diabetes, in which the participants in the control group with and without history of diabetes experienced significant higher proportions of positive GTT and GCT than the intervention group (results not shown).
Table 3.
Main outcomes in intervention and control groups at the end of study
DISCUSSION
The current study was designed to investigate the effect of vitamin D supplementation in the first and second trimesters on GDM in high-risk women. Results showed that vitamin D supplementation in the first and second trimesters has an effect on GDM), in which significantly higher percentages of positive GTT (34.8% vs 11.4%) and GCT (35.6% vs 10.9%) were observed in the control group compared with participants in the vitamin D supplementation group; both P < 0.01. Pregnancy consequences including macrosome neonate and increased C/S were not different in intervention and placebo groups.
Evidences show that vitamin D metabolism is related to diabetes incidence and its exacerbations. In most studies on pancreas in vitro or in vivo,[22,23,24,25] results have shown that there are vitamin D receptors and vitamin D3 binding proteins in pancreatic beta cells and in this way vitamin D can be influential in insulin secretion.[25,26,27] Moreover, in studies on rats, vitamin D deficiency is associated with lower insulin secretion, and vitamin D supplementation therapy caused appropriate insulin secretion and improved glucose tolerance in these animals.[25,26,27]
In a study by Lacroix et al.(2013)[28] on low level of vitamin D in the first trimester and its relationship with increased gestational diabetes, results showed that women with lower vitamin D level were 1.48 times at higher risk for gestational diabetes than women who received vitamin D (P = 0.04), and our study results showed that pregnant women in the first trimester who received vitamin D had less GDM than women who received placebo, and this is statistically significant (P = 0.009).
In a cross-sectional study by Hoseinnejad et al.[29] that was done on 741 pregnant women, after adjustment for BMI, a significant relationship was observed between insulin resistance index and insulin sensitivity index with vitamin D serum concentrations. They concluded that gestational diabetes prevalence is higher in pregnant women with vitamin D deficiency (less than 10 ng/L) than in women with a normal level of vitamin. Particularly in severe deficiency, accordingly sufficient levels of vitamin D can be effective in blood glucose control in pregnant women.[28]
Asemi et al. investigated the effect of vitamin D and calcium on blood sugar control, inflammation, and oxidative stress in gestational diabetes in a clinical trial. Their results showed a significant reduction in plasma glucose tolerance and the insulin level in serum in comparison with the placebo group (P = 0.001).[30] Inappropriate metabolism of insulin during gestational diabetes can cause complications in pregnancy such as macrosome neonate, trauma in birth, and preeclampsia.[17,31,32] Asemi et al. also concluded that pregnancy outcomes such as neonate weight as well as C/S rate were not different between the intervention and placebo groups.[30] Other studies showed that women who had received less vitamin D were at higher risk of gestational diabetes, which caused macrosome neonate and thus more C/S[17] and more surgery risk as well as higher risk of type II diabetes.[14] Evidence declares that neonates of women with gestational diabetes are at risk of glucose intolerance, obesity, and metabolic syndrome, which cause a higher risk of cardiovascular diseases in their children.[15]
In a study by Farrant et al., the participants with vitamin deficiency received vitamin D and calcium supplementation at the 30th week; no association was found between vitamin D deficiency and gestational diabetes. However, it should be noted that this study was limited to women admitted to the hospital and its results could not be generalized to the whole population, and also, in line with our results, they did not find any relation with macrosome neonate.[18]
Clinical trials in different part of world have shown that the effect of vitamin D supplementation in pregnant women increases birth weight and height.[21,33,34,35] However, in a study on Asian women living in England, no relation was demonstrated between vitamin D and birth weight.[33] Different results in various trials can be attributed to the difference in supplementation period and levels of vitamin D in participants.
In a study by Rudnicki and Pedersen.[21] the effect of vitamin D caused reduction of glucose tolerance in gestational diabetes, which was statistically significant. In other studies, reduction of OGT was not seen after vitamin D injection, while some other studies showed possible effects of vitamin D on glucose metabolism because of the effect of insulin secretion on beta cells.[35,36] Zhou et al.[37] examined the effect of vitamin D on pregnancy outcomes, and their results showed that at the end of pregnancy, macrosome neonate, rate of C/S, birth weight, and congenital abnormalities were observed, consistent with our study's results; however, no decrease was observed in the Apgar score, while the prevalence of gestational diabetes and preterm delivery was higher in the group who had received higher levels of vitamin D than in women who had received low or moderate levels of vitamin D.[37,38]
The possible strengths of our study can be described as follows: This was the first study of high-risk pregnant women administered to vitamin D supplementation over the first two trimesters of pregnancy. Accordingly, it provides preliminary insight into the possible positive effects on gestational diabetes. On the other hand, the not-high patient compliance rate can be considered as a possible limitation. As there was the possibility for gynecology specialists to recommend patients to discontinue the drug, in order to prevent any misunderstanding, the purpose and procedure of the study were described to the specialists in a meeting.
CONCLUSION
Our study showed that vitamin D supplementation for high-risk pregnant women in the first and second trimesters has an effective impact on gestational diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
AUTHOR'S CONTRIBUTION
SS contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FF contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. BP contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
It is necessary to thank from Be‘sat hospital staffs and participants who have helped us in this research.
REFERENCES
- 1.Bouillon R. Non-classical actions of the vitamin D endocrine system. Bone. 2007;40(Suppl 1):S9. [Google Scholar]
- 2.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br Med J. 1980;280:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke OG, Brown IR, Cleeve HJ, Sood A. Observations on the vitamin D state of pregnant Asian women in London. Br J Obstet Gynaecol. 1981;88:18–26. doi: 10.1111/j.1471-0528.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32° N. Int J Endocrinol 2010. 2010 doi: 10.1155/2010/917428. 917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28:7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 6.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:366.e1–6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94:940–5. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–61. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. ; Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 10.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–12. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 11.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 12.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 2009;70:372–7. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 14.Vambergue A, Dognin C, Boulogne A, Rejou MC, Biausque S, Fontaine P. Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy: DIAGEST 2 study. Diabet Med. 2008;25:58–64. doi: 10.1111/j.1464-5491.2007.02306.x. [DOI] [PubMed] [Google Scholar]
- 15.Tai K, Need AG, Horowitz M, Chapman IM. Glucose tolerance and vitamin D: Effects of treating vitamin D deficiency. Nutrition. 2008;24:950–6. doi: 10.1016/j.nut.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–7. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- 17.Tsai PJ, Roberson E, Dye T. Gestational diabetes and macrosomia by race/ethnicity in Hawaii. BMC Res Notes. 2013;6:395. doi: 10.1186/1756-0500-6-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–99. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 19.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–52. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourlon PM, Billaudel B, Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J Endocrinol. 1999;160:87–95. doi: 10.1677/joe.0.1600087. [DOI] [PubMed] [Google Scholar]
- 21.Rudnicki PM, Mølsted-Pedersen L. Effect of 1, 25-dihydroxycholecalciferol on glucose metabolism in gestational diabetes mellitus. Diabetologia. 1997;40:40–4. doi: 10.1007/s001250050640. [DOI] [PubMed] [Google Scholar]
- 22.Jelsma JG, van Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: An European multicentre, randomised trial-study protocol. BMC Pregnancy Childbirth. 2013;13:142. doi: 10.1186/1471-2393-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gestational diabetes mellitus. Practice Bulletin NO. 137. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 24.Labriji-Mestaghanmi H, Billaudel B, Garnier PE, Malaisse WJ, Sutter BC. Vitamin D and Pancreatic islet function. I. Time course for changes in insulin secretion and content during vitamin deprivation and repletion. J Endocrinol Invest. 1988;11:577–84. doi: 10.1007/BF03350185. [DOI] [PubMed] [Google Scholar]
- 25.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29:142–5. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 26.Scragg R, Holdaway I, Singh V, Meltcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in Impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–8. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 27.Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: Effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology. 1983;113:1511–8. doi: 10.1210/endo-113-4-1511. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix M, Battista MC, Doyon M, Houde G, Ménard J, Ardilouze JL, et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014;51:609–16. doi: 10.1007/s00592-014-0564-4. [DOI] [PubMed] [Google Scholar]
- 29.Hossein-Nejad A, Maghbooli J, Arzaghi S, Shafaei A, Rahmani M, Larijani B. Relationship between vitamin D deficiency and gestational diabetes mellitus. IJDLD. 2006;5:226–35. [Google Scholar]
- 30.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium–vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia. 2014;57:1798–806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 31.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. ; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 32.Burris HH, Camargo CA., Jr Time for large randomised trials of vitamin D for women with gestational diabetes mellitus to improve perinatal health outcomes. Diabetologia. 2014;57:1746–8. doi: 10.1007/s00125-014-3314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol. 2013;29:396–9. doi: 10.3109/09513590.2012.752456. [DOI] [PubMed] [Google Scholar]
- 34.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1998;88:488–92. [PubMed] [Google Scholar]
- 35.Jovanovic L, Knopp RH, Brown Z, Conley MR, Park E, Mills JL, et al. ; National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study Group. Declining insulin requirement in the late first trimester of diabetic pregnancy. Diabetes Care. 2001;24:1130–6. doi: 10.2337/diacare.24.7.1130. [DOI] [PubMed] [Google Scholar]
- 36.Inomata S, Kadowaki S, Yamatani T, Fukase M, Fujita T. Effect of 1a (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986;1:187–92. [PubMed] [Google Scholar]
- 37.Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59:1083–7. doi: 10.1093/ajcn/59.5.1083. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Su L, Liu M, Liu Y, Cao X, Wang Z, et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. Eur J Clin Nutr. 2014;68:925–30. doi: 10.1038/ejcn.2014.99. [DOI] [PubMed] [Google Scholar]


