Abstract
Background:
Musculoskeletal manifestations (MSM) of inflammatory bowel diseases (IBDs) are usually the most frequent extraintestinal manifestations. However, they are not paid enough attention during regular office visits. This cross-sectional study aimed to draw a clinical picture of MSM and their relationships with other findings in patients with IBD.
Materials and Methods:
Patients of our IBD cohort between March 2012 and September 2013 were consecutively evaluated. Those with current or past history of any MSM were examined by a rheumatologist. The outcome of interest was different MSMs. Distribution of IBD manifestations between the two groups of patients with (n = 20) and without (n = 253) MSM was compared. Logistic regression analysis was employed to find the relationships of demographic, clinical, and laboratory findings with MSM.
Results:
Two hundred and seventy-three patients were enrolled. Forty-two patients (15.4%) had extraintestinal manifestations of which twenty patients (7.5%) had at least one MSM. 7/20 patients (35%) versus 22/253 (8.7%) had other extraintestinal manifestations (P = 0.0001). 12/20 patients (57%) had arthritis (polyarthritis, 33% and oligoarthritis, 67%). The most frequent involved joints were knee and ankle observed in 8 (40%) and 7 (35%) patients, respectively. The inflammatory back pain was recorded in 5/20 patients (25%) whereas two patients (10%) had ankylosing spondylitis. In regression analysis, oral aphthous (odds ratio [OR] =8.8 [95% confidence intervals (CI), 1.7–45], P = 0.009) and other extraintestinal manifestations (OR = 5.2 [95% CI, 1.3–20], P = 0.02) were significantly related with arthritis.
Conclusion:
The most frequent extraintestinal manifestations in patients with IBD were MSM. Knee and ankle were the most frequent involved joints. Extraintestinal manifestations were determinant variables of arthritis.
Keywords: Arthritis, Crohn's disease, musculoskeletal manifestations, ulcerative colitis
INTRODUCTION
The most common extraintestinal symptoms in inflammatory bowel disease (IBD) are musculoskeletal manifestations (MSM) presenting in up to 62% of patients.[1] They mainly include axial arthropathies and peripheral arthritis. Many episodes of musculoskeletal involvement may last up to 10 weeks[2] with frequent relapses coexistent mostly with gastrointestinal (GI) exacerbations.[3] However, the severity of GI and extraintestinal symptoms mostly impedes paying enough attention to MSM. Therefore, patients with IBD complain more frequently from GI and other prominent extraintestinal manifestations such as oral lesions, uveitis, and skin lesions.[4,5] Moreover, since administered medications to relieve GI involvements such as sulfasalazine are the usual treatments of MSM,[6] gastroenterologists do not routinely inquire about MSM in daily practice.[7] However, their effects on severe or long-lasting axial involvement are not significant.[8,9] Then, the patients experienced more devastating disease and decreased quality of life.[10,11] There are scant data on the prevalence of musculoskeletal presentations in patients with IBD in the Middle East.[12,13] The current study aimed to present a clinical picture of these manifestations and their relationships with GI, extraintestinal and laboratory findings in Crohn's disease (CD) and ulcerative colitis (UC).
MATERIALS AND METHODS
Design of study
This was a cross-sectional study carried out at Poursina Hakim outpatient clinic between March 2012 and September 2013. Poursina Hakim Research Institute is a gastroenterology research institute and clinic in Isfahan, the second largest city in the center of Iran with <2 million populations. It has an IBD registry with over 500 cases. The diagnosis of IBD was made by gastroenterologists based on clinical picture, endoscopic findings, and pathologic assessments. The study protocol was approved by Ethical Committee of Isfahan University of Medical Science. The Ethical Committee of Isfahan University of Medical Sciences approved the study design. The project number was 290089.
Data collection
Patients attending the clinic consecutively and diagnosed with IBD were enrolled in the study. The patients were interviewed by a trained medical student who filled out the proposed questionnaire. Presence or absence of extraintestinal involvements was questioned. The questionnaire also included specific questions about the current or history of joint pain, the joints involved, and the duration of involvement, the current or history of low back pain and widespread musculoskeletal pain. If the patient's answer to any of the latter questions were positive, he would be referred to the rheumatologist. The rheumatologist fully examined the patient's musculoskeletal system. Other characteristics of patients were collected from their recorded database of the Poursina cohort. They included demographic information, age when the first MSM appeared, history of other rheumatic diseases such as ankylosing spondylitis (AS), rheumatoid arthritis, Behcet's disease, reactive arthritis, psoriatic arthritis and gout, age at the time of IBD diagnosis, type of IBD, GI involvements including proctitis, left-sided colitis, extensive colitis, pancolitis, ileitis and ileocolitis, extraintestinal manifestations including mucocutaneous, oral, ocular, thromboembolic, and hepatopancreatobiliary manifestations, laboratory findings such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and therapeutic interventions such as colectomy and current medications including prednisolone, mycophenolate mofetil, infliximab, mesalamine, cyclosporine, azathioprine, sulfasalazine, and tacrolimus. In the case of inflammatory back pain, pelvic X-ray was done, because it was nonethical to expose all patients to pelvic radiation just for research purposes.
Definitions
The outcome of interest was MSM. Arthralgia, arthritis,[2] inflammatory back pain,[14] AS,[15] fibromyalgia,[16] CD and UC,[17,18,19] proctitis,[20] left-sided colitis,[20] extensive colitis,[20] and pancolitis[20] were defined according to practical definitions.
Independent variables included type of IBD, duration of IBD disease, IBD activity, GI involvements, thromboembolic events, oral aphthous, laboratory findings (ESR and CRP) and administered medications. Thromboembolic events and oral aphthous were observed in patients with and without MSM. Other extraintestinal manifestations were seen either in patients with or in those without MSM.
Statistical analysis
SPSS program (SPSS, Chicago, IL, USA) was employed for data analysis. Chi-square or Fisher exact tests were applied to compare categorical variables. Student's t-test was employed to compare continuous variables. Logistic regression analysis was applied to find the association of demographic, clinical, and laboratory variables with musculoskeletal picture (dependent variables). Statistical significant factors were used in multivariate regression analysis to estimate their adjusted effects on MSM. Odds ratios (OR) and the 95% of confidence intervals (CI) were calculated in regression analyses. P < 0.05 was considered significant.
RESULTS
Characteristics of patients according to the type of inflammatory bowel disease
A total of 273 patients were evaluated in the current study. Demographic characteristics between the patients with CD and those with UC were not significantly different [Table 1]. The mean (standard deviation) age at which the MSM reported first was 29.6 (7.5) and 39.2 (15) years, respectively (P = 0.1). Forty-five patients (16.5%) had active IBD during the study. Comparisons of extraintestinal manifestations, GI involvements, clinical and laboratory findings, and therapeutic interventions between the patients with CD and those with UC are presented in Table 2.
Table 1.
Demographic characteristics of patients
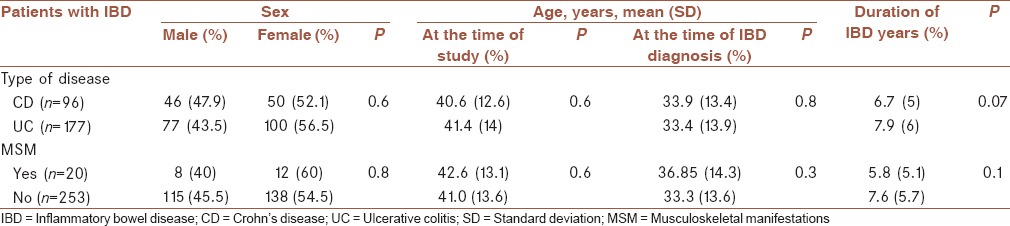
Table 2.
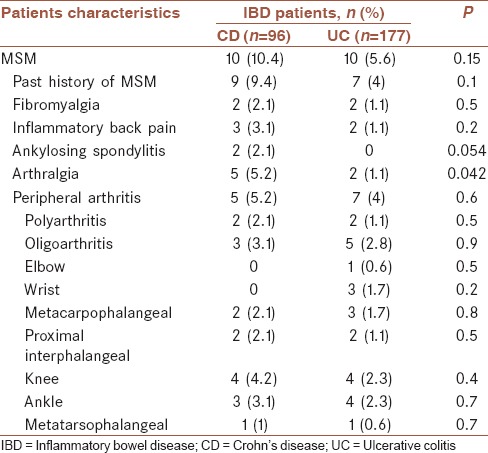
Distribution of musculoskeletal manifestations between the two groups of patients with ulcerative colitis and Crohn's disease
Characteristics of patients with extraintestinal manifestations other than musculoskeletal manifestations
Overall, 42 patients (15.4%) showed extraintestinal manifestations. Of these, 13 patients (4.8%) had no extraintestinal manifestations other than MSM whereas 29 patients (10.6%) had at least one extraintestinal manifestation other than MSM. 17/29 and 12/29 patients were diagnosed with CD and UC, respectively. It means 17.7% of all patients with CD and 6.8% of all patients with UC had extraintestinal manifestations other than MSM. Their distributions were significantly different (P = 0.005). 4/29 patients (13.8%) versus 8/244 patients (3.3%) had peripheral arthritis (P = 0.009). None of the 29 patients had AS. Furthermore, 5/29 patients (17.2%) versus 11/244 patients (4.5%) had history of MSM.
Characteristics of patients with musculoskeletal manifestations
Demographic characteristics of patients with MSM are presented in Table 1. Comparisons of extraintestinal manifestations, GI involvements, clinical and laboratory findings, and therapeutic interventions between the patients with and without MSM are presented in Table 3.
Table 3.
Distribution of inflammatory bowel disease manifestations between the two groups of patients with and without musculoskeletal manifestations
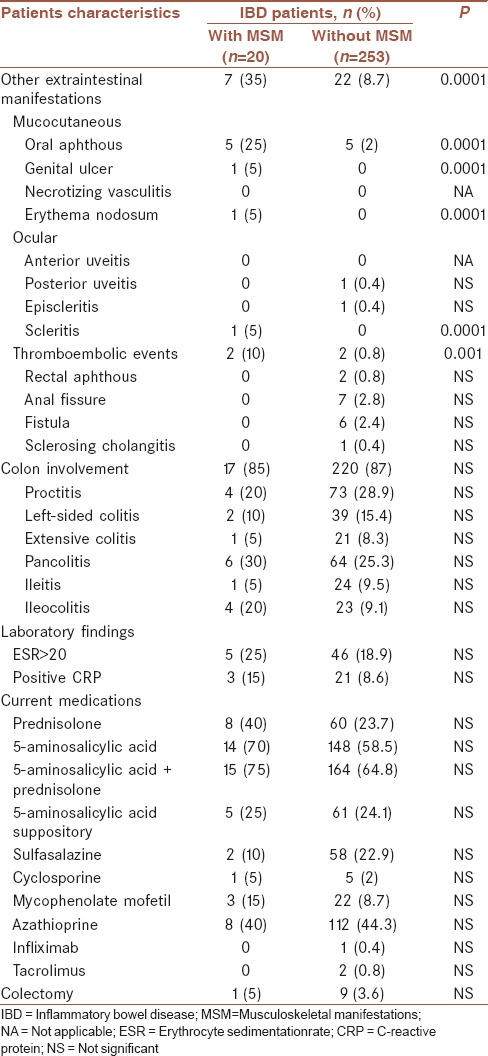
Overall, twenty patients (7.3%) had at least one MSM [Table 3]. 16/20 patients (80%) had history of MSM, and 4/20 patients (20%) were new cases with MSM. Therefore, the incidence rate of MSM was 4/(273–16) =1.55% per 18 months (roughly, 1%/year). 2/20 (10%) had AS. No patient had coincident other rheumatic diseases such as rheumatoid arthritis, Behcet's disease, reactive arthritis, psoriatic arthritis, and gout. 7 of 20 patients (35%) versus 22 of 253 (those with no MSM) (8.7%) had extraintestinal manifestations other than MSM (P = 0.0001). No significant differences were observed in the use of current medications between the patients with MSM and those with no MSM [Table 3].
12/20 patients (60%) had an active or history of peripheral arthritis. It means peripheral arthritis was recorded in 4.4% of total population. Half of them had current arthritis in physical examination of which only one was the new case of arthritis. Half of the patients with current arthritis had active IBD (3 patients) whereas none of those with history of arthritis but no current arthritis had active IBD). The polyarthritis and oligoarthritis patterns were observed in 4/12 (33%) and 8/12 (67%), respectively (P < 0.0001). The most frequent involved joints were knee and ankle [Table 3]. Just one patient had peripheral arthritis and AS, simultaneously. Extraintestinal manifestations other than MSM were recorded more significantly in patients with arthritis than in those with no arthritis; 4/12 patients (33.3%) versus 25/261 (9.6%), respectively (P = 0.009). These manifestations included oral ulcer, erythema nodosum, scleritis, and genital ulcer.
Univariate and multivariate analyses
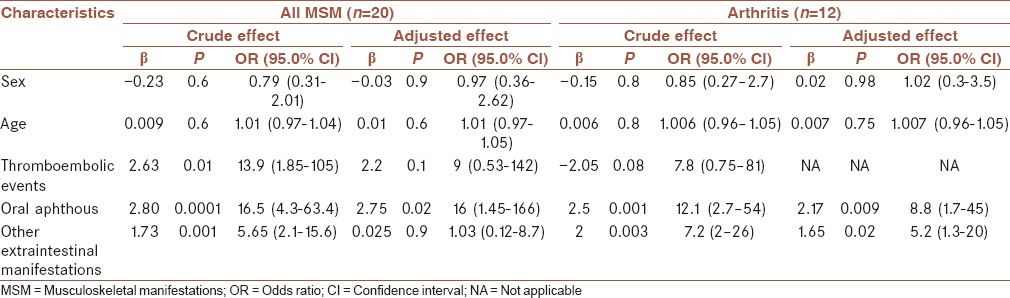
In univariate analyses, when arthritis was considered as the dependent variable, the associations of oral aphthous and other extraintestinal manifestations with arthritis were significant [Table 4]. When MSM was considered as the dependent variable, thromboembolic events showed significant association too. In multivariate analysis, oral aphthous and other extraintestinal manifestations kept their significance as determinant factors of arthritis. In the case of MSM, oral aphthous remained as the sole significant variable associated with the presence of MSM [Table 4].
Table 4.
Odds ratio of the association of demographic, clinical and laboratory variables with musculoskeletal manifestations
DISCUSSION
In the current study, the most frequent extraintestinal manifestations were MSM observed in 7.3% of patients. Peripheral arthritis was the most common MSM recorded in 4.4%. The prevalence of peripheral arthritis in patients with IBD was 5% in the USA,[21] 7.4% in the UK,[2] 12% in Norway,[22] 12.1% in Turkey,[23] 15.5% in Korea[24] and 28.1% in Germany.[25] Genetic backgrounds and different study designs may explain some of the variations seen in the prevalence of peripheral arthritis in different parts of the world.[26,27] Knee and ankle were the most frequent involved joints in our study and most other studies.[22,23,24,25] Oligoarticular arthritis was more frequent than polyarticular arthritis. This was in agreement with other investigations.[2,22] The rates of MSM were not different between the patients with UC and CD. Some studies agreed[2,17] with this finding and some did not.[22] Oral aphthous was >12 times more common in patients with MSM than in those with no MSM. Some extraintestinal manifestations such as erythema nodosum were seen specifically in patients with peripheral arthritis. That's why the extraintestinal manifestations and oral aphthous acted as determinant variables of arthritis in regression analysis. Likewise, other studies showed the significant association of oral aphthous and erythema nodosum with arthritis.[2,22,28]
The prevalence of AS in the current study was <1–10%, reported by other investigators.[4,6] Both patients with AS in our study had CD. Orchard et al. and Bourikas and Papadakisshowed also higher prevalence of AS in CD.[2,4] Arthralgia was recorded in 2.5% of patients and was observed more frequently in CD. The reported range varied from 0.15% in D’Incà et al. study[29] to 24% in Forrest and Russell.[28] The wide difference could be mainly explained by the design of study; retrospective studies carry the risk of recall bias. Fibromyalgia was observed in 1.5% of patients. The reported rate varied from 0.5%[29] to 49%.[30] In addition to the difference in designs of studies, the response to treatment of underlying disease (IBD) could probably explain the broad range of recorded prevalence of this psychosomatic syndrome.
This study had some limitations including the relatively low sample size and the short period of follow-up. Further investigations with larger sample size and longer follow-up are needed to better determine the behavior of MSM and their relationships with other characteristics of patients with IBD.
CONCLUSION
In the current study, the prevalence of MSM was at the bottom end of the worldwide reported range. Arthritis was the most common MSM. Its presence was significantly associated with oral aphthous and erythema nodosum. Multi-center cohort studies may provide clearer clinical picture of IBD-related MSM.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
AF contributed in study design, conduct of the study, data acquisition, drafting, revision of the draft, approval of the final version of the manuscript, and agreed regarding all aspects of the work
HHJ contributed in study design, conduct of the study, data acquisition, drafting, revision of the draft, approval of the final version of the manuscript, and agreed regarding all aspects of the work
MHE contributed in study design, conduct of the study, data acquisition, drafting, revision of the draft, approval of the final version of the manuscript, and agreed regarding all aspects of the work
AK conduct of the study, data acquisition, approval of the final version of the manuscript, and agreed regarding all aspects of the work
HT conduct of the study, data acquisition, approval of the final version of the manuscript, and agreed regarding all aspects of the work
AS contributed to study design, conduct of the study, analysis and interpretation of data, drafting, revision of the draft, approval of the final version of the manuscript, and agreed regarding all aspects of the work.
REFERENCES
- 1.Beslek A, Onen F, Birlik M, Akarsu M, Akar S, Sari I, et al. Prevalence of spondyloarthritis in Turkish patients with inflammatory bowel disease. Rheumatol Int. 2009;29:955–7. doi: 10.1007/s00296-008-0811-5. [DOI] [PubMed] [Google Scholar]
- 2.Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: Their articular distribution and natural history. Gut. 1998;42:387–91. doi: 10.1136/gut.42.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzeni F, Defendenti C, Ditto MC, Batticciotto A, Ventura D, Antivalle M, et al. Rheumatic manifestations in inflammatory bowel disease. Autoimmun Rev. 2014;13:20–3. doi: 10.1016/j.autrev.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1915–24. doi: 10.1002/ibd.20942. [DOI] [PubMed] [Google Scholar]
- 5.Salvarani C, Vlachonikolis IG, van der Heijde DM, Fornaciari G, Macchioni P, Beltrami M, et al. Musculoskeletal manifestations in a population-based cohort of inflammatory bowel disease patients. Scand J Gastroenterol. 2001;36:1307–13. doi: 10.1080/003655201317097173. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Reyna TS, Martínez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15:5517–24. doi: 10.3748/wjg.15.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolwijk C, Pierik M, Landewé R, Masclee A, van Tubergen A. Prevalence of self-reported spondyloarthritis features in a cohort of patients with inflammatory bowel disease. Can J Gastroenterol. 2013;27:199–205. doi: 10.1155/2013/139702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker H, Gaubitz M, Domschke W, Kucharzik T. Joint involvement in chronic inflammatory bowel disease – Current diagnostics and treatment options. Z Gastroenterol. 2006;44:497–502. doi: 10.1055/s-2006-926586. [DOI] [PubMed] [Google Scholar]
- 9.De Vos M. Joint involvement in inflammatory bowel disease: Managing inflammation outside the digestive system. Expert Rev Gastroenterol Hepatol. 2010;4:81–9. doi: 10.1586/egh.09.75. [DOI] [PubMed] [Google Scholar]
- 10.Bodur H, Ataman S, Bugdayci DS, Rezvani A, Nas K, Uzunca K, et al. Description of the registry of patients with ankylosing spondylitis in Turkey: TRASD-IP. Rheumatol Int. 2012;32:169–76. doi: 10.1007/s00296-010-1599-7. [DOI] [PubMed] [Google Scholar]
- 11.Arvikar SL, Fisher MC. Inflammatory bowel disease associated arthropathy. Curr Rev Musculoskelet Med. 2011;4:123–31. doi: 10.1007/s12178-011-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallahi GH, Moazzami K, Tabatabaeiyan M, Zamani MM, Asgar-Shirazi M, Najafi M, et al. Clinical characteristics of Iranian pediatric patients with inflammatory bowel disease. Acta Gastroenterol Belg. 2009;72:230–4. [PubMed] [Google Scholar]
- 13.Al-Jarallah K, Shehab D, Al-Azmi W, Al-Fadli A. Rheumatic complications of inflammatory bowel disease among Arabs: A hospital-based study in Kuwait. Int J Rheum Dis. 2013;16:134–8. doi: 10.1111/j.1756-185X.2012.01811.x. [DOI] [PubMed] [Google Scholar]
- 14.Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: A reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54:569–78. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 15.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 17.Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 19.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–46. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez VE, Costas PJ, Vazquez M, Alvarez G, Perez-Kraft G, Climent C, et al. Prevalence of spondyloarthropathy in Puerto Rican patients with inflammatory bowel disease. Ethn Dis. 2008;18(2 Suppl 2: S2):225–9. [PubMed] [Google Scholar]
- 22.Palm Ø, Moum B, Jahnsen J, Gran JT. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001;40:1256–61. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- 23.Yüksel I, Ataseven H, Basar O, Köklü S, Ertugrul I, Ulker A, et al. Peripheral arthritis in the course of inflammatory bowel diseases. Dig Dis Sci. 2011;56:183–7. doi: 10.1007/s10620-010-1260-z. [DOI] [PubMed] [Google Scholar]
- 24.Suh CH, Lee CH, Lee J, Song CH, Lee CW, Kim WH, et al. Arthritic manifestations of inflammatory bowel disease. J Korean Med Sci. 1998;13:39–43. doi: 10.3346/jkms.1998.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protzer U, Duchmann R, Höhler T, Hitzler W, Ewe K, Wanitschke R, et al. Enteropathic spondylarthritis in chronic inflammatory bowel diseases: Prevalence, manifestation pattern and HLA association. Med Klin (Munich) 1996;91:330–5. [PubMed] [Google Scholar]
- 26.Sheth T, Pitchumoni CS, Das KM. Musculoskeletal manifestations in inflammatory bowel disease: A revisit in search of immunopathophysiological mechanisms. J Clin Gastroenterol. 2014;48:308–17. doi: 10.1097/MCG.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 27.Orchard TR, Thiyagaraja S, Welsh KI, Wordsworth BP, Hill Gaston JS, Jewell DP. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology. 2000;118:274–8. doi: 10.1016/s0016-5085(00)70209-5. [DOI] [PubMed] [Google Scholar]
- 28.Forrest EH, Russell RI. Peripheral arthropathies in inflammatory bowel disease. Gut. 1999;44:439. doi: 10.1136/gut.44.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Incà R, Podswiadek M, Ferronato A, Punzi L, Salvagnini M, Sturniolo GC. Articular manifestations in inflammatory bowel disease patients: A prospective study. Dig Liver Dis. 2009;41:565–9. doi: 10.1016/j.dld.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Buskila D, Odes LR, Neumann L, Odes HS. Fibromyalgia in inflammatory bowel disease. J Rheumatol. 1999;26:1167–71. [PubMed] [Google Scholar]


