Abstract
Background:
The role of endothelial progenitor cells (EPCs) in the maintenance of vascularization following ischemic brain after experimental stroke has been established. Accordingly, in this study, we evaluated the role of circulating EPCs in transient ischemic attack (TIA) patients for future cerebrovascular (CV) events.
Materials and Methods:
The level of circulating EPCs (staining markers: CD34, CD309) were determined using flow cytometry at 24 h after TIA in thirty consecutive patients. The EPCs level was also evaluated once in thirty healthy volunteers. Over a period of 12 months, all patients were evaluated by an experienced neurologist for recurrent TIA, stroke or death induced by CV disorders.
Results:
Circulating EPCs increased in patients group following the first attack of TIA when compared with controls. By analysis of covariance, cardiovascular event history, hyperlipidemia, and statin therapy remained significant independent predictors of EPCs. The mean (standard deviation) duration of follow-up was 10.5 (3.1) months (range, 2–12 months). During follow-up, a total of three patients died due to CV accident and four patients experienced again recurrent TIA. By analyzing data with Cox regression, EPC did not predict the future CV events in TIA patients.
Conclusion:
Increased incidence of future CV events did not occur in those patients with elevated EPCs in the first attack of TIA. The significant predicting factors of EPCs were cardiovascular event history, hyperlipidemia, and statin therapy.
Keywords: Endothelial progenitor cell, prognosis, transient ischemic attack
INTRODUCTION
Endothelial damage and arteriosclerosis include the majority of the same basic etiologies of cerebrovascular (CV) and coronary artery disease.[1] Interestingly, it seems that activation of migratory endothelial progenitor cells (EPCs) in response to tissue ischemia results in formation of collaterals after myocardial ischemia.[2] In addition, the major role of EPCs in angiogenesis and regeneration of ischemic CV tissue have been established.[3,4] These cells are bone marrow-derived and subsequently characterize the expression of the specific marker for all cells, for example, CD34 + cells nonhematopoietic stem cells in humans and the endothelial marker like vascular endothelial growth factor receptor-2 or CD309+.[5] Furthermore, the role of EPCs in the maintenance of vascularization following ischemic brain after experimental stroke has been established. They act not only as the source of EPCs but also as the cause of growth and angiogenesis factors[6] and the likely increase the activity of matrix metalloproteinase.[7] On the other hand, with increased circulating EPCs levels, reduced infarct growth, and neurological improvement in patients after acute ischemic stroke (IS) is observed.[8,9]
Unlike a stroke, transient ischemic attack (TIA) is a period of focal ischemia, TIA symptoms typically precede in <1 h, while necrosis has not occurred in brain tissue. Stroke is often preceded by TIA attack as a warning sign, making it an important predictor of future ischemic incidents.[10] However, no study has addressed the role of circulating EPCs on outcomes prediction after TIA. Accordingly, in this study, we evaluated the role of circulating EPCs in TIA patients for prediction of future CV events (TIA).
MATERIALS AND METHODS
Study patients
This cohort study included consecutively admitted patients in Al-Zahra Hospital with acute TIA between November 2013 and October 2014. Thirty patient with a firstly attack of TIA according to TOAST-criteria[11] were included. Thirty controls were matched by age, gender with patients group. They were included from population that was referred to AL-Zahra Lab for performing the primary examination to employment in a public organization. Participants in the control group were healthy people and without any risk factors including hypertension, smoking, diabetes, cardiovascular event history, family history of cardiovascular, hyperlipidemia, and statin therapy at the baseline. Patients with a history of the following problems were not included in the study as well. Stroke or intracranial hemorrhage, surgery or trauma within the preceding 3 months, abnormal liver function, hematology disorders, renal insufficiency, malignancy, febrile disorders, acute or chronic inflammatory disease at study entry, atrial fibrillation, or congestive heart failure and consumption of angiotensin-converting inhibitor for hypertension treatment. Past medical history including diabetes (fasting blood sugar ≥126 mg/dL), hypertension (blood pressure ≥140/90 mmHg), hyperlipidemia (total cholesterol ≥240 mg/dL orandand triglyceride ≥200)[12] and smoking, family history of CV accident in the first degree was collected in both two groups. Informed consent form was obtained, and medical confidentiality issues were described from all study subjects. All of the information was explained to patients about taking of blood sampling and next visits in every 2 months by neurologist. If they did not refer in defined dates, our group followed up by telephone.
Neurological assessment
Over a period of 12 months, all patients were evaluated by an experienced neurologist for every recurrent TIA or stroke or death induced by CV disorders. Evaluation of neurological impairment of stroke patients was based on the National Institutes of Health Stroke Scale.[13]
Blood sampling and assessment of circulating endothelial progenitor cell level by flow cytometry
Blood samplings were obtained once at 24 h after TIA and assessment of circulating EPC level evaluated by flow cytometry.
Circulating EPCs were measured by phenotypic analysis in unselected peripheral blood cells. Blood samples were processed (2 h) after they were drawn. 50 μl of ethylenediaminetetraacetic acid-anticoagulated blood incubated (30 min, at 4°C) with 20 μl of fluorescein isothiocyanate (FITC)-conjugated anti-CD34 and 10 μl of phycoerythrin (PE)-conjugated anti-CD309. Appropriate isotype controls were used for each staining procedure. 1 ml of lysis solution was added (5 min, at room temperature). Then, samples were centrifuged, and pellets were resuspended (in 300 μl of PBS). Cells (2 × 105) were acquired by a FACS caliber flow cytometer and analyzed. Because the number of dead/apoptotic cells was negligible, the analysis was performed excluding cellular debris in a side scatter/forward scatter dot plot. CD34+ cells were electronically gated and the percentage of cells coexpressing CD309 was evaluated. For each sample, a minimum of 20,000 events was acquired. Detection of EPCs by flow cytometry was defined by the presence of at least 0.03% nucleated cells coexpressing, the three antigens over the background fluorescence. Results were expressed as a percentage of CD34 + cells that co-expressed CD309. On the basis of the peripheral blood nucleated cell count, we also calculated the absolute number of CD34+ CD309+ cells. The expression of cell surface antigens was determined by 2-color immunofluorescence staining. Briefly, 2 × 106 cells were incubated in buffer containing 2% bovine serum albumin with 20 µ Fc-blocking agent (10 min, at 25°C). Then, cells were incubated (30 min, at 4°C) with 20 µl of CD34-FITC and CD309-PE (total volume of 200 µl). The cells were washed twice before re-suspension in 400 µl stain buffer. FACS analysis was performed in triplicate for each sample.
Materials
The following were purchased from the sources indicated; antibodies, Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA); Lysis Solution (Dako, Glostrup, Denmark); Fetal Calf Serum (HyClone, Logan, UT, USA); Cell Quest Software, Stain buffer (BD Biosciences, San Diego, CA, USA); Fc-blocking agent (Miltenyi Biotech); CD34-FITC, CD309-PE (Miltenyi Biotech).
Statistical analysis
Data are expressed as mean standard deviation (SD). Continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test. Comparisons between groups were analyzed by t-test (two-sided) or ANOVA for normally distributed variables. Post hoc tests and pairwise multiple comparisons were done with the Tukey - t-test (two-sided). Comparison of categorical variables was performed with Chi-square and Fisher exact test. Analysis of covariance used for controlling variables in our model. A generalized linear model was performed for TIA risk factors on CD34+ CD309 to identify independent determinants of them. Cox proportional hazard ratio (HR) was used to estimate the HR and it is 95% CIs for TIA events and the association with identified variables. Statistical significance was considered at P < 0.05. All statistical analysis was performed with (SPSS version 15, SPSS, Inc., IL, USA).
RESULTS
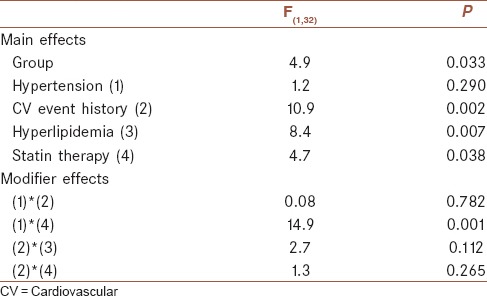
The baseline characteristics of the sixty subjects are summarized in Table 1. Circulating EPCs (CD34 + CD309) obviously increased in patients group following the first attack of TIA when compared with controls. Univariate analysis revealed that there were not statistically significant association between dependent factor (CD34 + CD309) and independent factors of age, smoking, diabetes, and family history (the results are not shown). By analysis of covariance variables such as cardiovascular event history, hyperlipidemia, and statin therapy remained significant independent predictors of CD34 + CD309. Despite the significant effect of hypertension on a dependent factor by univariate analysis, there was not significant association, but there was statistically modifier effect of hypertension and statin therapy on CD34 + CD309 [Table 2].
Table 1.
Patients’ baseline characteristics
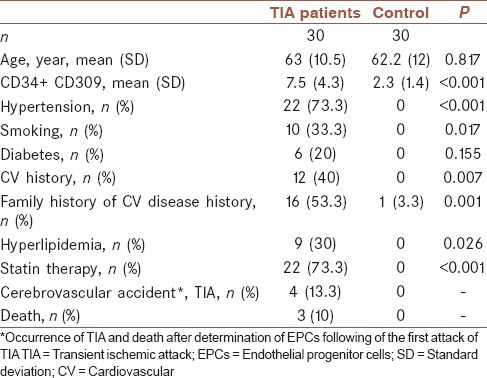
Table 2.
Analysis of covariance between transient ischemic attack risk and protective factors and CD34+ CD309+ (n=60)
The mean (SD) duration of follow-up was 10.5 (3.1) months (range, 2–12 months). During the follow-up, a total of three patients died due to CV accident and four patients experienced again recurrent TIA. Table 3 illustrates that the HR measured by the Cox proportional hazard regression model for risk factors of TIA in thirty patients during follow-up including hyperlipidemia and cardiovascular event were 1.8, 1.1, respectively and statin therapy act as a protective factor against risk of TIA occurrence (0.71) in which noun of them were not statistically significant. Figure 1 illustrated the event-free survival based on TIA patients’ duration of 6 months follow-up. Three patients did not refer to defined dates and after following it became clear to us that these subjects had died in this period. Four TIA recurrences have been dedicated between patients and did not find any correlation between this accident and level of EPCs. During follow-up revealed that increased incidence of CV events did not occur in those patients with elevated EPCs in the first attack of TIA and determination of EPCs did not act as a predictive factor in our population [Figure 1].
Table 3.
Risk factor-adjusted hazard ratios of transient ischemic attack event in patients group (n=30)
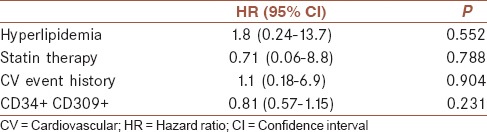
Figure 1.
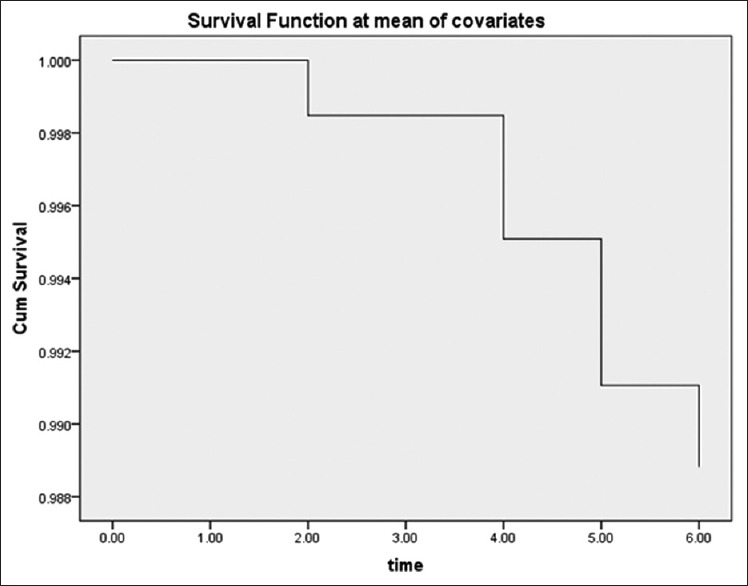
Event-free survival based on transient ischemic attack patients (n = 30) duration of 6 months
DISCUSSION
This study first demonstrated an increased mobilization of EPC in patients with the first attack of TIA compared with healthy individuals. Second, for determination the role of confounding factors on EPCs level in TIA patients, some variables were considered and was shown that cardiovascular event history, hyperlipidemia, and statin therapy remained a significant independent predictors of CD34 + CD309 level. Third, to our knowledge, this is the first study to detect cEPC level, defined as CD34 + CD309 + cells, in peripheral blood of TIA patients as a predictive value for future attacks. During the follow-up, it was revealed that increased incidence of CV incidents did not occur in those patients with elevated EPCs in the first attack of TIA patients and determination of EPCs did not act as a predictive factor in our population.
One of the most important stimuli for the progression of atherosclerotic lesions is endothelial cell injury.[14] In fact, impaired endothelial function could act as a prediction marker for risk of subsequent cardiovascular[15,16] and CV incidents.[3,8]
The CD34 + and CD309 + markers are easily detected using flow cytometry. In the study of Taguchi et al., the role of circulating CD34 + cells in maintenance of the cerebral circulation in ischemic stress condition has been confirmed. In addition, converse correlation between the number of circulating CD34 + cells and cerebral infarction has been shown.[17]
In previous studies, rapidly rising level of circulating EPCs in acute coronary syndrome and traumatic vascular injury compared with healthy controls has been presented.[18,19,20] The experimental results of this study also determined a rapid increase in the acute phase of patients with first attack of TIA. Similar results were observed in Yip et al.[9] following acute phase of IS. Indeed, mobilization of EPCs from bone marrow to circulation is a rapid reaction to tissue ischemia.[4] In contrast to our report, Ghani et al.[21] reported declining number of circulating EPCs without any difference between acute and chronic phase in IS patients. We could not be sure why our and recent findings[19,20] are inconsistent with Ghani et al.'s results.[21] This discrepancy could be attributed to serial changes of circulating level of EPCs, found in patients after acute IS. Furthermore, this diversity could be explained by different time intervals for blood sampling in the present study and that of Ghani et al.'s. In another study, the authors reported that the level of CD34 + cells increased at 7th day poststroke and this increase continued significantly to the 14th day compared to the prestroke baseline, while it returned to the baseline levels by day 30.[17] This could clarify the fluctuation level of EPCs in similar diseases in different studies. In addition, we could not completely rule out the effect of related confounding variables, for example, dissimilar method had been used in different experimental research works. In the research conducted by Ghani et al.,[21] according to utilized techniques, cells with a mature endothelial phenotype collected that may have an origin other than EPCs.
Although in our study, elevated EPCs in first attack of TIA patients did not act as a predictive factor in our population, Cuadrado-Godi et al. showed that basal EPCs associated with 6 months new accident events in patients with myocardial infarction and stroke.[22] Elevation of EPCs in our study has been observed in stroke patients in another study when compared with controls, but 30 days after stroke, no significant change in EPCs was indicated.[23] In another study, the number and migratory activity of circulating EPCs were shown to have an inverse correlation with risk factors for coronary artery diseases[2] and Framingham risk factor score.[21] In Sobrino et al.'s study, the magnitude of EPC increments in response to acute IS patients was directly correlated to a better functional outcome and a negative correlation was shown to exist between EPCs level and infarct volume growth.[8]
On the other hand, in patients with recurrent IS, a significantly lower level of circulating EPCs was reported compared to patients with the first IS.[9] It seems that EPCs work as angiogenesis, vasculogenesis factor, and improve repairing of endothelial injury following ischemic events.[24]
However, we did not find a prognostic effect of EPCs at baseline after TIA. Furthermore, the role of risk factors in modulation of homing, and differentiation of circulating EPCs has been elucidated. Atherosclerosis and age synergistically cause the most prominent effect of risk factors and induce decreased levels of circulating EPCs.[25] Hypercholesterolemia,[26] smoking habit,[2] and diabetes are other risk factors for alteration in EPCs level. In addition, statin[27] therapy and physical exercise[28] lead to beneficial effects in primary and secondary prevention of atherosclerosis and subsequently enhance the number and function of EPCs.
In addition, in our study, there was statistically modifier effect of hypertension and statin therapy on CD34 + CD309. However, in Diamant et al. study such interaction had been observed, indeed after statin therapy, the number of endothelial microparticles in diabetic and hypertensive subjects was declined.[29] Accordingly, this would be a further support to our findings, so some variables including cardiovascular event history, hyperlipidemia, and statin therapy remain significant independent predictors of CD34 + CD309 level in TIA patients. However, cardiovascular event history and hyperlipidemia act as nonsignificant risk factors, but statin therapy proceeds as a protective factor for recurrence of TIA.
Although in our study, monitoring the levels of circulating EPCs could not be considered as a surrogate biological marker for future accidents in TIA patients, further prospective studies are needed to evaluate and identify novel therapeutic approaches targeted at enhancing circulating EPCs post-TIA attack.
Financial support and sponsorship
This project has been supported by grants 291172 from the Isfahan Neurosciences Research Center, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
RM: Conceive and design of study and coordinated the study, participated in most of the experiments, wrote the paper.
HN: provide assistance in the design of the study, participated in manuscript preparation.
LD: Performed all steps of experiments.
MT: cooperation in preparation of samples for flowcytometry.
MD: writing proposal and blood sample collection.
MGh: blood sample collection.
BA: diagnosis and confirmation of TIA in patients.
MS: diagnosis and confirmation of TIA in patients.
Acknowledgments
This work was founded by grant no 291172. From the Neurosciences Research Center, University of Medical Sciences, Isfahan, Iran. We are grateful to all of the patients who helped us in our project.
REFERENCES
- 1.Baines CP, Pass JM, Ping P. Protein kinases and kinase-modulated effectors in the late phase of ischemic preconditioning. Basic Res Cardiol. 2001;96:207–18. doi: 10.1007/s003950170051. [DOI] [PubMed] [Google Scholar]
- 2.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–8. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108:3122–7. doi: 10.1161/01.CIR.0000105722.96112.67. [DOI] [PubMed] [Google Scholar]
- 5.Rafat N, Beck GC, Peña-Tapia PG, Schmiedek P, Vajkoczy P. Increased levels of circulating endothelial progenitor cells in patients with moyamoya disease. Stroke. 2009;40:432–8. doi: 10.1161/STROKEAHA.108.529420. [DOI] [PubMed] [Google Scholar]
- 6.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–85. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 7.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;25(93):e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 8.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 9.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 10.van Rooy MJ, Pretorius E. Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb Res. 2015;135:434–42. doi: 10.1016/j.thromres.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Amort M, Fluri F, Weisskopf F, Gensicke H, Bonati LH, Lyrer PA, et al. Etiological classifications of transient ischemic attacks: Subtype classification by TOAST, CCS and ASCO – A pilot study. Cerebrovasc Dis. 2012;33:508–16. doi: 10.1159/000337236. [DOI] [PubMed] [Google Scholar]
- 12.Ghobadzadeh M, Demerath EW, Tura Y. Prevalence of blood pressure, blood glucose and serum lipids abnormalities among Ethiopian immigrants: A community-based cross-sectional study. J Immigr Minor Health. 2015;17:1070–7. doi: 10.1007/s10903-014-0051-6. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–2. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Tepper OM, Galiano RD, Capla JM, Baharestani S, Kleinman ME, et al. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg. 2004;113:284–93. doi: 10.1097/01.PRS.0000091169.51035.A5. [DOI] [PubMed] [Google Scholar]
- 15.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 16.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–5. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 18.Bonello L, Basire A, Sabatier F, Paganelli F, Dignat-George F. Endothelial injury induced by coronary angioplasty triggers mobilization of endothelial progenitor cells in patients with stable coronary artery disease. J Thromb Haemost. 2006;4:979–81. doi: 10.1111/j.1538-7836.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, et al. Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur Heart J. 2004;25:1003–8. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 21.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–3. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 22.Cuadrado-Godia E, Regueiro A, Núñez J, Díaz-Ricard M, Novella S, Oliveras A, et al. Endothelial progenitor cells predict cardiovascular events after atherothrombotic stroke and acute myocardial infarction. A PROCELL Substudy. PLoS One. 2015;10:e0132415. doi: 10.1371/journal.pone.0132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regueiro A, Cuadrado-Godia E, Bueno-Betí C, Diaz-Ricart M, Oliveras A, Novella S, et al. Mobilization of endothelial progenitor cells in acute cardiovascular events in the PROCELL study: Time-course after acute myocardial infarction and stroke. J Mol Cell Cardiol. 2015;80:146–55. doi: 10.1016/j.yjmcc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: From biology to treatment. Trends Cardiovasc Med. 2002;12:88–96. doi: 10.1016/s1050-1738(01)00152-9. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 26.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–80. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 27.Sobrino T, Blanco M, Pérez-Mato M, Rodríguez-Yáñez M, Castillo J. Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. Eur J Neurol. 2012;19:1539–46. doi: 10.1111/j.1468-1331.2012.03770.x. [DOI] [PubMed] [Google Scholar]
- 28.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 29.Diamant M, Tushuizen ME, Abid-Hussein MN, Hau CM, Böing AN, Sturk A, et al. Simvastatin-induced endothelial cell detachment and microparticle release are prenylation dependent. Thromb Haemost. 2008;100:489–97. [PubMed] [Google Scholar]


