Abstract
Background:
Serum procalcitonin (PCT) levels differ in patients with bacterial or fungal infections and are significantly elevated in patients with Gram-negative bacteremia. We evaluated the diagnostic accuracy of different inflammatory markers to discriminate sepsis caused by different pathogens.
Materials and Methods:
We included 328 episodes of bacteremia from 292 patients with sepsis and 31 patients with suspected sepsis in this study. Medical records of patients who had bacteremia caused by Gram-negative bacteria (Gram-negative), Gram-positive bacteria (Gram-positive) or fungi were reviewed, and information about PCT and other inflammatory markers was recorded. The diagnostic performance of inflammatory markers was calculated via receiver operating characteristic (ROC) curves.
Results:
Serum PCT levels in Gram-negative, Gram-positive, and fungal sepsis were 7.47 (interquartile range [IQR]: 1.09–41.26) ng/mL, 0.48 (IQR: 0.15–2.16) ng/mL, and 0.60 (IQR: 0.14–2.06) ng/mL, respectively (P < 0.001). ROC analysis revealed an optimal cut-off value of 2.44 ng/mL for PCT in discriminating Gram-negative sepsis from Gram-positive sepsis, which yielded a sensitivity of 68.4% and a specificity of 77.1%. An optimal cut-off value of 3.11 ng/mL for PCT in discriminating Gram-negative sepsis from fungal sepsis, led to a sensitivity of 63.9% and specificity of 93.3%. Neither PCT nor other inflammatory markers could be used to distinguish between Gram-positive and fungal sepsis.
Conclusion:
Serum PCT levels were significantly higher in patients with Gram-negative sepsis than in those with Gram-positive or fungal sepsis. PCT is a potential sensitive biomarker for distinguishing Gram-negative sepsis from Gram-positive and fungal sepsis.
Keywords: Fungi, Gram-negative bacteria, procalcitonin, sepsis
INTRODUCTION
Sepsis is defined as a systemic inflammatory response to confirmed or suspected infection.[1] Sepsis is the major cause of morbidity and mortality in critically ill patients. Epidemiological data indicate that mortality is higher than 25–30% in patients with sepsis and 40–50% in patients with septic shock.[2] Delayed diagnosis and inadequate antimicrobial therapy are associated with excess mortality.[3,4] Choosing optimized antimicrobial therapy according to the causative pathogen of sepsis can drive significant improvements in the prognosis of sepsis.[5] Therefore, identifying the causative pathogen is critical for the successful antimicrobial treatment of sepsis. Microbiological examinations represent the current gold standard to identify causative pathogens. However, bacteremia is confirmed via microbiological examinations in only about 30% of patients with sepsis.[3] Systemic inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), have poor sensitivity and specificity in diagnosing bacterial infections. Hence, a biomarker to rapidly and accurately identify causative agents is warranted for use in the clinical setting.
Procalcitonin (PCT), the protein precursor of calcitonin, is synthesized and released by C-cells in the thyroid gland. Serum PCT levels have been found to increase within 4 h, reach peak levels within 6 h and maintain a plateau through 24 h after healthy volunteers are injected with Gram-negative bacterial endotoxin.[6] PCT appears to have higher sensitivity and specificity for predicting bacterial infection than other markers.[7,8] Whereas, PCT levels are normal in patients without infection.[9] Furthermore, serum PCT levels correlate with the severity of the systemic inflammatory response.[10,11] Hence, PCT is a useful biomarker to predict systemic inflammatory response syndrome (SIRS), bacteremia, and even sepsis.[3]
Serum PCT levels differ in patients with bacterial or fungal infections and are significantly elevated in patients with Gram-negative bacteremia.[12,13] PCT levels have been shown to be able to distinguish Gram-negative bacterial (Gram-negative) sepsis from Gram-positive bacterial (Gram-positive) and fungal sepsis.[10,14,15,16] Here, we evaluated the diagnostic accuracy of different inflammatory markers to discriminate sepsis caused by different pathogens within a large patient population.
MATERIALS AND METHODS
Study design and participants
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University, China. Written informed consent was provided by each participant. Personal information of all subjects was destroyed after data collection was completed.
The researchers retrospectively analyzed medical records from January 2013 to September 2014 in Zhongnan Hospital of Wuhan University, China, and all patients with sepsis were included. Patients were diagnosed with sepsis if they met the following criteria.[17] (1) Confirmation of infection based on microbiological evidence (positive blood culture results) and (2) a diagnosis of SIRS, which required the presence of at least two of the following clinical symptoms: Fever (≥38°C) or hypothermia (<36°C), tachycardia (heart rate ≥ 90bpm), tachypnea (frequency ≥ 20/min) or hyperventilation (pCO2 ≤4.3 KPa [33 mmHg]), and leukocytosis (≥12 × 109/L) or leukopenia (≤4 × 109/L). Patients were divided into three distinct groups according to the results of blood culture and Gram-staining tests: Gram-negative group, Gram-positive group, and fungi group. Patients with suspected sepsis whose blood cultures were negative for pathogen were considered the control group. Essential information, such as age, sex, and vital signs, was recorded. Mortality within the first 28 days in patients was also recorded.
Laboratory examinations
Blood culture samples (aerobic and anaerobic) were collected by sterile venipuncture and processed using the BACTEC 9240 automated blood culture system (Becton Dickinson, USA). Bacteria were identified using the VITEK 2 Compact system (bioMérieux, France). One episode of bacteremia was defined as the recovery of any bacterial or fungal species, other than coagulase-negative staphylococci (CoNS), in one or more blood cultures. CoNS were considered contaminants when isolated from only one sample of blood culture. If two blood samples from the same patient with at least 7 days of appropriate antibiotic treatment tested positive, two episodes were recorded.
Serum PCT levels were measured by a PCT kit (bioMérieux) using a VIDAS B.R.A.H.M.S automatic analyzer (bioMérieux) following manufacturer's instructions. The lower limit of detection was 0.05 ng/mL. Serum CRP concentrations were measured by immunoturbidimetric assays using an Olympus AU5400 chemistry analyzer (Olympus, Japan). Serum ESRs were measured by an infrared barrier method using a Monitor-20 analyzer (Vital Diagnostics, Italy). Inflammatory marker dosages had been obtained with a 24-h window when patients were diagnosed with sepsis.
Statistical analysis
Statistical analysis was performed using the software SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR) unless otherwise stated. Kruskal–Wallis nonparametric analysis of variance was used for multi-group comparisons, and Bonferroni Mann–Whitney U-tests were used to detect differences between two groups. Categorical variables were compared using the Chi-square test. All tests were performed as two-tailed tests. P < 0.05 was considered statistically significant, except when Bonferroni Mann–Whitney U-tests were used (P < 0.01).
The diagnostic accuracy of each inflammatory marker was described by the following parameters: Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The area under the curve (AUC) was used to assess diagnostic accuracy. Youden's index (Youden's index = sensitivity + specificity − 1) was calculated to find the optimal cut-off value.
RESULTS
Characteristics of the study population
Over 21 months, 439 cases of bacteremia and fungemia in 363 patients were recorded. Thirty cases of polymicrobial infection and 81 cases without sepsis were excluded. In all, 328 cases of bacteremia from 292 septic patients, and 31 patients with suspected sepsis whose blood culture was negative for pathogen were included in this study. Overall, 218 (60.7%) men and 141 (39.3%) women were included. The mean age of the patients was 63.7 ± 19.5 years. Within the rirst 28 days, 46 (29.1%), 26 (18.6%), 7 (23.3%), and 2 (6.5%) patients with Gram-negative sepsis, Gram-positive sepsis, fungal sepsis and suspected sepsis, respectively, died. The mortality of patients with Gram-negative sepsis was higher than that of other patients (P = 0.021). [Table 1].
Table 1.
Characteristics of patients with Gram-negative bacterial sepsis, Gram-positive bacterial sepsis, fungal sepsis, and suspected sepsis
Distribution of causative pathogens
Among 328 cases of sepsis, 158 (48.2%) were caused by Gram-negative bacteria, 140 (42.7%) by Gram-positive bacteria, and 30 (9.1%) by fungi. The most frequent causative bacterial species were CoNS (n = 70), Escherichia coli (n = 52), and Staphylococcus aureus (n = 39). Candida parapsilosis (n = 8) and Candida albicans (n = 8) were the most frequent causative fungal species [Table 2].
Table 2.
Distribution and serum procalcitonin levels corresponding to different causative pathogens
Measurements of inflammatory markers
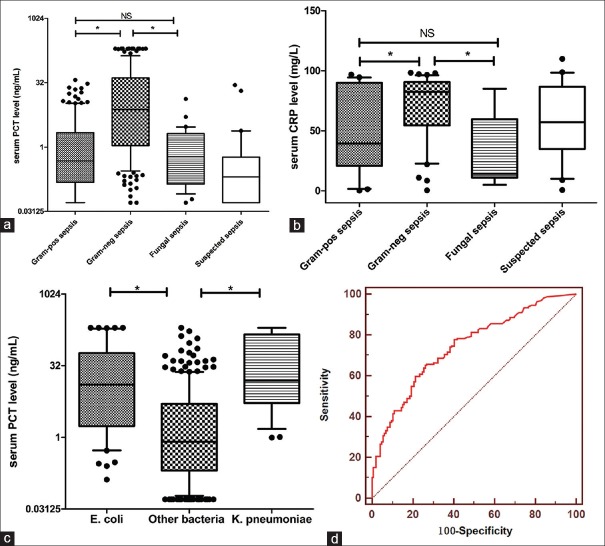
Serum PCT concentrations in patients with Gram-negative sepsis were significantly greater than in patients with Gram-positive, fungal or suspected sepsis (7.47 ng/mL [IQR: 1.09–41.26], 0.48 ng/mL [IQR: 0.15–2.16], 0.60 ng/mL [IQR: 0.14–2.06] and 0.20 ng/mL [IQR: 0.05–0.58], respectively, P < 0.001). Concentrations of other inflammatory markers are shown in Table 1. Furthermore, serum PCT levels in patients with E. coli sepsis and Klebsiella pneumoniae sepsis were 12.74 ng/mL (IQR: 1.73–58.88 ng/mL) and 15.46 ng/mL (IQR: 5.21–144.48), respectively [Figure 1a–c].
Figure 1.
(a and b) Serum procalcitonin and C-reactive protein levels in patients with Gram-positive bacterial sepsis, Gram-negative bacterial sepsis, fungal sepsis and suspected sepsis. (c) Serum procalcitonin levels in patients with sepsis caused by Escherichia coli, Klebsiella pneumoniae, or other bacteria. Data are presented as a box plot with median lines 25th and 75th percentile boxes, and 10th and 90th percentile error bars. The Y-axis is a log two scale in (a and c). NS: Not statistically significant, *P < 0.05. (d) Receiver operating characteristic curve for procalcitonin levels to distinguish Gram-negative sepsis from Gram-positive sepsis
Diagnostic accuracy of inflammatory markers
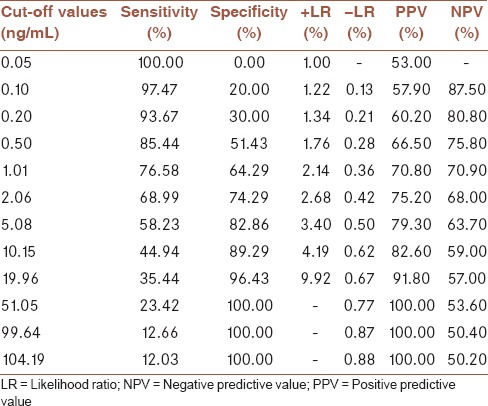
Receiver operating characteristic (ROC) analysis was performed to reveal the diagnostic accuracy of using PCT or CRP concentrations to distinguish Gram-negative sepsis from Gram-positive sepsis. An optimal PCT cut-off value of 2.44 ng/mL resulted in an AUC value of 0.793 (95% confidence interval [CI], 0.743–0.843, P < 0.001), sensitivity of 77.1%, specificity of 68.4%, PPV of 74.0%, and NPV of 65.1%. An optimal CRP cut-off value of 59.25 mg/L resulted in a sensitivity of 74.4%, specificity of 65.4%, PPV of 71.3%, and NPV of 68.2% with an AUC value of 0.678 (0.541–0.814, P < 0.05) [Figure 1d and Table 3].
Table 3.
Different cut-off values of procalcitonin in distinguishing Gram-negative sepsis from Gram-positive sepsis, (area under the curve=0.793 (95% confidence interval 0.743-0.843, P<0.001)). Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio are listed
In addition, an optimal PCT cut-off value of 2.32 ng/mL could be used to distinguish E. coli sepsis from other bacterial (including Gram-negative/Gram-positive bacteria) sepsis with a sensitivity of 76.2%, specificity of 65.4%, PPV of 36.6%, and NPV of 91.5%. An optimal PCT cut-off value of 2.81 ng/mL could be used to distinguish K. pneumoniae sepsis from other bacterial sepsis with a sensitivity of 89.3%, specificity of 69.4%, PPV of 25.5%, NPV of 98.3%, and AUC of 0.859 (95% CI, 0.800–0.917, P < 0.001) [Table 4].
Table 4.
Results of receiver operating characteristic curve for procalcitonin levels in discriminating sepsis caused by different pathogens
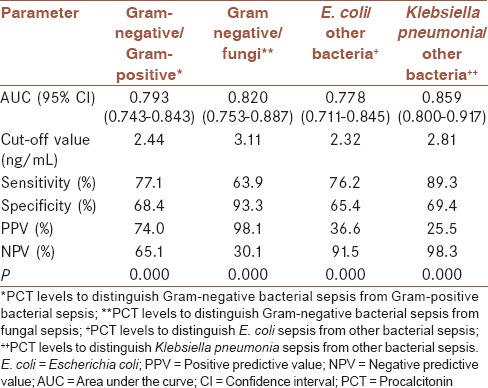
The diagnostic accuracy of using PCT and CRP levels to distinguish Gram-negative sepsis from fungal sepsis was assessed by ROC analysis. An optimal PCT cut-off value of 3.11 ng/mL resulted in sensitivity, specificity, PPV, and NPV of 63.9%, 93.3%, 98.1%, and 30.1%, respectively. An optimal CRP cut-off value of 52.25 mg/L. Resulted in sensitivity, specificity, PPV, and NPV of 79.1%, 83.3%, 96.4%, and 42.4%, respectively, with an AUC of 0.841 (95% CI, 0.687–0.995, P < 0.01).
None of these inflammatory markers could be used to distinguish Gram-positive sepsis from fungal sepsis.
DISCUSSION
Despite improvements in antimicrobial therapies and supportive treatments,[18] mortality in critically ill patients remains high. Here, we observed 24.1% mortality in sepsis patients, which, increased with the severity of sepsis, with the mortality in patients with septic shock being 55.2% (data not shown). Empirical antimicrobial therapy is crucial for the prognosis of patients with sepsis, and inappropriate or inadequate antimicrobial therapy reduces the probability of survival.[5,19] Therefore, the identification of causative pathogens of sepsis is critical for physicians to choose the appropriate antimicrobial therapy.
PCT levels might be used as a surrogate marker to distinguish Gram-negative sepsis from Gram-positive or fungal sepsis. Serum PCT levels were significantly higher in patients with Gram-negative sepsis than in patients with Gram-positive or fungal sepsis. A PCT cut-off value of 2.41 ng/mL yielded a sensitivity and specificity of 68.4% and 93.5%, respectively, for distinguishing Gram-negative sepsis from suspected sepsis with an AUC of 0.870 (95% CI 0.804–0.936, P < 0.001, data not shown). Moreover, a cut-off value of >2.44 ng/mL for PCT could distinguish Gram-negative sepsis from Gram-positive sepsis with a sensitivity, specificity, PPV, and NPV of 77.1%, 68.4%, 74.0%, and 65.1%, respectively. Hence, significantly higher PCT levels can serve as a marker to diagnose Gram-negative sepsis. Similar findings have been demonstrated in other studies as well. Charles et al.[16] reported that an optimal PCT cut-off value of 16 ng/mL resulted in 75.0% sensitivity and 82.2% specificity in distinguishing Gram-negative from Gram-positive sepsis.
The mechanism underlying different PCT levels in response to Gram-negative and Gram-positive bacteria remains confusing. Differences in the membrane composition of Gram-negative and Gram-positive bacteria might partly explain this. The major membrane component of Gram-negative bacteria is lipopolysaccharide (LPS) (the major component of endotoxin) and that of Gram-positive bacteria is peptidoglycan (PGN). LPS and PGN, as pathogen-associated molecular patterns (PAMPs), are recognized by pattern recognition receptors (PRRs) in the innate immune response. PRRs include several families such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs). The majority of TLRs play a vital role in recognizing bacteria. The ligation of PAMPs to TLRs initiates signaling pathways and induces the release of pro-inflammatory cytokines.[20] LPS is sensed as a ligand by TLR4, and PGN is sensed by TLR2.[21,22] LPS binding to TLR4 activates the classical MyD88-dependent signaling pathway and induces the production of cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).[22] Oberhoffer et al.[23] demonstrated that both LPS and sepsis-related cytokines increased PCT expression in human peripheral blood mononuclear cells (PBMCs). In PBMCs cultured with LPS, TNF-α, or IL-6, PCT mRNA was detected at levels 4- to 230-fold, 2- to 90-fold, or 2- to 35-fold, respectively, higher than in control cultures. In addition, Gram-positive pathogens induce relatively poor cytokine levels (TNF-α and IL-6) than Gram-positive ones do, owing to TLR2-dependent simulation.[24,25] These factors might mediate the expression of serum PCT concentrations in response to Gram-negative bacterial infections.
E. coli and K. pneumoniae are the most common causative pathogens of Gram-negative bacterial infections. Approximately 54% of E. coli and 27% of K. pneumoniae were confirmed to possess extended-spectrum beta-lactamase (ESBL) in Zhongna Hospital (unpublished data). ESBL-producing E. coli and K. pneumoniae are resistant to beta-lactam antimicrobials. Moreover, these pathogens might markedly prolong hospital stays and exacerbate the financial burden. In this study, PCT levels in patients with E. coli (12.74 ng/mL [IQR: 1.73–58.88]) or K. pneumoniae (15.46 ng/mL [IQR: 5.21–144.48]) sepsis were significantly higher than in patients with other Gram-negative, Gram-positive or fungal sepsis. Brodská et al.[14] reported similar ranges of PCT levels in E. coli and K. pneumoniae sepsis, namely 15.30 ng/mL (IQR: 2.70–38.0 ng/mL) and 15.23 ng/mL (IQR: 5.45–43.61 ng/mL), respectively. Elson et al.[21] demonstrated that for E. coli and K. pneumonia, TLR4/myeloid differentiation factor-2 (MD-2) ligands were dominant. These bacteria strongly simulated cells expressing TLR4/MD-2 and induced increased PCT levels. Furthermore, serum PCT levels could be used to distinguish E. coli or K. pneumoniae sepsis from other bacterial sepsis. For E. coli, 76.2% sensitivity and 91.5% NPV were achieved with an optimal cutoff value of 2.32 ng/mL. For K. pneumoniae, 89.3% sensitivity and 98.3% NPV were achieved with an optimal cut-off value of 2.81 ng/mL. Hence, higher-than-average PCT concentrations might indicate E. coli or K. pneumoniae infection. Further studies are warranted to elucidate the mechanisms underlying the significant increase in PCT concentrations in patients with E. coli or K. pneumoniae sepsis.
According to several studies, low concentrations of PCT in critically ill patients might suggest fungal infection.[10,15,26] In this study, PCT levels could be used to sensitively and specifically distinguish Gram-negative sepsis from fungal sepsis. The diagnostic accuracy of PCT for this differentiation was determined by ROC analysis, revealing an optimal cut-off value of 3.11 ng/mL, resulting in 63.9% sensitivity and 93.3% specificity. In a similar study, Leli et al.[10] found that an optimal PCT cut-off value of 1.6 ng/mL could be used to distinguish Gram-negative infections from fungal infections with 77% sensitivity and 96% specificity. Martini et al.[26] also demonstrated that an optimal PCT cut-off value of 2 ng/mL could be used to distinguish Candida sepsis from bacterial sepsis with 92% sensitivity and 93% specificity.
PCT levels in patients with Gram-positive (0.48 ng/mL [IQR: 0.15-2.16 ng/mL]) and fungal (0.60 ng/mL [IQR: 0.14–2.06 ng/mL]) sepsis differed, albeit not significantly (P = 0.602). Although serum PCT concentrations were moderately elevated in fungal and Gram-positive sepsis, mechanisms of PCT expression were different. TLRs were sensed by both Gram-positive bacteria and fungi, but the major receptors recognizing fungi are CLRs.[27] The secretion of cytokines, such as IL-1β, IL-10, and IL-6, is induced by the activation of the CLR/Syk-CARD9 signaling pathway, resulting in moderately elevated PCT levels after fungal infections.[26,28,29] In addition, serum interferon-gamma (IFN-γ) levels are markedly elevated in patients with fungal infections,[30,31] IFN-γ is a critical factor for defense against fungal infections.[31,32,33] In vitro, PCT secretion has been found to be inhibited by IFN-γ.[34] Thus, PCT concentrations in fungal sepsis might be regulated by IFN-γ levels.[35] Furthermore, patients treated with broad spectrum antibiotics and invasive surgeries have a greater risk of developing fungal infections,[26] and such patients with elevated PCT levels should, therefore, be screen for fungal infections.
CRP can be used as a sensitive indicator of systemic inflammation during early stages of infection. Here, CRP concentrations were significantly elevated in all groups and could be used to distinguish between different causative pathogens. An optimal cut-off value of 59.25 mg/L for CRP levels resulted in 74.4% sensitivity and 65.4% specificity, in discriminating Gram-negative sepsis from Gram-positive sepsis. An optimal cut-off value of 52.25 mg/L for CRP levels resulted in 79.1% sensitivity and 83.3% specificity in discriminating Gram-negative sepsis from fungal sepsis. Although CRP levels can be used to accurately distinguish causative pathogens, confounding factors limit the broader application of this marker. Serum CRP concentrations were Influenced by myocardial diseases[36] and autoinflammatory diseases.[37] These confounding factors were not excluded in this research. Hence, CRP levels were considered inferior to PCT levels in distinguishing causative pathogens of sepsis. ESR is also influenced by inflammation, serum concentrations of fibrinogen and immunoglobulin and erythrocytes characteristics.[38] In this study, we did not observe any significant correlation between ESR and the causative pathogen of sepsis.
We acknowledge some limitations of our study. First, the majority of the patients were treated at different departments, and only some of the patients received a sequential organ failure assessment score. The diagnosis of severe sepsis and septic shock was defined by the guidelines of the German Sepsis Society.[17] Patients in each group were divided into independent subgroups according to the severity of sepsis (sepsis subgroup, severe sepsis subgroup, and septic shock subgroup). No differences in PCT levels were observed in the severe sepsis subgroup, and PCT levels could not be used to distinguish between patients with severe sepsis caused by different pathogens (P = 0.308, data not shown). We cannot explain whether the severity of sepsis would influence the diagnostic accuracy of PCT in discriminating between different causative pathogens. Further research is warranted. Second, CoNS are common contaminants in blood cultures.[39] Because of technical limitations, distinguishing between CoNS contaminants and CoNS infections was difficult. False-positive CoNS infections may affect the quantification of serum PCT concentrations. Seventy cases of CoNS bacteremia were identified in our study, and some of these cases might actually represent CoNS contamination. More rigid inclusion criteria should be adopted in future research to obviate the influence of CoNS contaminants.
CONCLUSION
Serum PCT levels were significantly higher in patients with Gram-negative sepsis than in patients with Gram-positive or fungal sepsis with the exception of patients with severe sepsis. We provide evidence that PCT is a sensitive biomarker that can be used to distinguish Gram-negative sepsis from Gram-positive and fungal sepsis. It should be noted that the importance of microbiological examinations remains unchanged. Blood culture and antimicrobial susceptibility testing are required in clinical applications to positively identify causative microorganisms and assess their sensitivity to different antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SL contributed to the conception of the work, conducting the study, recruiting patients, processing data, and writing the manuscript
HR contributed to the conception of the work, recruiting patients, processing data, and preparing the tables and figures
QG contributed to the conception of the work and collecting the results of blood-culture and serum inflammatory biomarkers
YC contributed to the conception of the work, processing data, drawing ROC curves, and preparing the figures
GZ contributed to the conception of the work and recruitment of patients
JY contributed to the conception of the work and revising the draft
All authors agree to all aspects of the study and approve the final version of the manuscript for publication.
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: A roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 3.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426–35. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 4.Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, Aldabó-Pallás T, Cayuela-Dominguez A, Marquez-Vacaro JA, et al. Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: A matched cohort study. J Antimicrob Chemother. 2008;61:436–41. doi: 10.1093/jac/dkm460. [DOI] [PubMed] [Google Scholar]
- 5.Vazquez-Grande G, Kumar A. Optimizing antimicrobial therapy of sepsis and septic shock: Focus on antibiotic combination therapy. Semin Respir Crit Care Med. 2015;36:154–66. doi: 10.1055/s-0034-1398742. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–8. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 7.Hattori T, Nishiyama H, Kato H, Ikegami S, Nagayama M, Asami S, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol. 2014;141:43–51. doi: 10.1309/AJCP4GV7ZFDTANGC. [DOI] [PubMed] [Google Scholar]
- 8.Chirouze C, Schuhmacher H, Rabaud C, Gil H, Khayat N, Estavoyer JM, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis. 2002;35:156–61. doi: 10.1086/341023. [DOI] [PubMed] [Google Scholar]
- 9.Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60:409–16. doi: 10.1016/j.jinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Leli C, Ferranti M, Moretti A, Al Dhahab ZS, Cenci E, Mencacci A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis Markers 2015. 2015:701480. doi: 10.1155/2015/701480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29. doi: 10.1016/j.cccn.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima A, Yazawa J, Sugiki D, Mizuguchi M, Sagara H, Fujisiro M, et al. Clinical utility of procalcitonin as a marker of sepsis: A potential predictor of causative pathogens. Intern Med. 2014;53:1497–503. doi: 10.2169/internalmedicine.53.1785. [DOI] [PubMed] [Google Scholar]
- 13.Marková M, Brodská H, Malícková K, Válková V, Cetkovský P, Kolár M, et al. Substantially elevated C-reactive protein (CRP), together with low levels of procalcitonin (PCT), contributes to diagnosis of fungal infection in immunocompromised patients. Support Care Cancer. 2013;21:2733–42. doi: 10.1007/s00520-013-1844-1. [DOI] [PubMed] [Google Scholar]
- 14.Brodská H, Malícková K, Adámková V, Benáková H, Štastná MM, Zima T. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med. 2013;13:165–70. doi: 10.1007/s10238-012-0191-8. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Chen J, Cai B, Zhang J, Li L, Liu C, et al. The use of PCT, CRP, IL-6 and SAA in critically ill patients for an early distinction between candidemia and Gram positive/negative bacteremia. J Infect. 2012;64:438–40. doi: 10.1016/j.jinf.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008;8:38. doi: 10.1186/1471-2334-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, et al. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI)) Ger Med Sci. 2010;8 doi: 10.3205/000103. Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng G, Lyu J, Huang J, Xiang D, Xie M, Zeng Q. Experimental treatments for mitochondrial dysfunction in sepsis: A narrative review. J Res Med Sci. 2015;20:185–95. [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–46. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 21.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–83. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 23.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134:49–55. doi: 10.1016/s0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 24.Beran O, Potmešil R, Holub M. Differences in Toll-like receptor expression and cytokine production after stimulation with heat-killed gram-positive and gram-negative bacteria. Folia Microbiol (Praha) 2011;56:138–42. doi: 10.1007/s12223-011-0001-9. [DOI] [PubMed] [Google Scholar]
- 25.Tavares E, Maldonado R, Ojeda ML, Miñano FJ. Circulating inflammatory mediators during start of fever in differential diagnosis of gram-negative and gram-positive infections in leukopenic rats. Clin Diagn Lab Immunol. 2005;12:1085–93. doi: 10.1128/CDLI.12.9.1085-1093.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martini A, Gottin L, Menestrina N, Schweiger V, Simion D, Vincent JL. Procalcitonin levels in surgical patients at risk of candidemia. J Infect. 2010;60:425–30. doi: 10.1016/j.jinf.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106. doi: 10.1007/s00281-014-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akin H, Akalin H, Budak F, Ener B, Ocakoglu G, Gürcüoglu E, et al. Alterations of serum cytokine levels and their relation with inflammatory markers in candidemia. Med Mycol. 2015;53:258–68. doi: 10.1093/mmy/myu084. [DOI] [PubMed] [Google Scholar]
- 29.Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58:89–99. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Sood BG, Shankaran S, Schelonka RL, Saha S, Benjamin DK, Jr, Sánchez PJ, et al. Cytokine profiles of preterm neonates with fungal and bacterial sepsis. Pediatr Res. 2012;72:212–20. doi: 10.1038/pr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, et al. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-gamma release. J Immunol. 2011;187:1369–76. doi: 10.4049/jimmunol.1003593. [DOI] [PubMed] [Google Scholar]
- 32.Gozalbo D, Maneu V, Gil ML. Role of IFN-gamma in immune responses to Candida albicans infections. Front Biosci (Landmark Ed) 2014;19:1279–90. doi: 10.2741/4281. [DOI] [PubMed] [Google Scholar]
- 33.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis. J Immunol. 2009s;182:4306–12. doi: 10.4049/jimmunol.0803462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: A novel product of human adipose tissue. Endocrinology. 2003;144:5578–84. doi: 10.1210/en.2003-0854. [DOI] [PubMed] [Google Scholar]
- 35.Dou YH, Du JK, Liu HL, Shong XD. The role of procalcitonin in the identification of invasive fungal infection-a systemic review and meta-analysis. Diagn Microbiol Infect Dis. 2013;76:464–9. doi: 10.1016/j.diagmicrobio.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Saito I, Maruyama K, Eguchi E. C-reactive protein and cardiovascular disease in East Asians: A systematic review. Clin Med Insights Cardiol. 2015;8(Suppl 3):35–42. doi: 10.4137/CMC.S17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56:131–42. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 38.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 39.Elzi L, Babouee B, Vögeli N, Laffer R, Dangel M, Frei R, et al. How to discriminate contamination from bloodstream infection due to coagulase-negative staphylococci: A prospective study with 654 patients. Clin Microbiol Infect. 2012;18:E355–61. doi: 10.1111/j.1469-0691.2012.03964.x. [DOI] [PubMed] [Google Scholar]