Abstract
Background:
Additional high-quality prospective studies are needed to better define the objective criteria used in relation to return-to-sport decisions after synthetic (ligament advanced reinforcement system [LARS]) and autograft (hamstring tendon [2ST/2GR]) anterior cruciate ligament (ACL) reconstruction in active populations.
Purpose:
To prospectively investigate and describe the recovery of objective clinical outcomes after autograft (2ST/2GR) and synthetic (LARS) ACL reconstructions, as well as to investigate the relationship between these clinimetric test outcomes and return-to-sport activity (Tegner activity scale [TAS] score) at 12 and 24 months postoperatively.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 64 patients who underwent ACL reconstruction (32 LARS, 32 2ST/2GR autograft) and 32 healthy reference participants were assessed for joint laxity (KT-1000 arthrometer), clinical outcome (2000 International Knee Documentation Committee [IKDC] knee examination), and activity (TAS score) preoperatively and at 12, 16, 20, and 24 weeks and 12 and 24 months postoperatively.
Results:
There was no significant correlation observed between clinical results using the 2000 IKDC knee examination and TAS score at 24 months (r s = 0.188, P = .137), nor were results for side-to-side difference (r s = 0.030, P = .814) or absolute KT-1000 arthrometer laxity of the surgical leg at 24 months postoperatively (r s = 0.076, P = .553) correlated with return-to-sport activity. Nonetheless, return-to-sport rates within the surgical cohort were 81% at 12 months and 83% at 24 months, respectively. No statistically significant differences were observed between physiological laxity of the uninjured knee within the surgical group compared with healthy knees within the reference group (P = .522).
Conclusion:
The results indicate that although relatively high levels of return-to-sport outcomes were achieved at 24 months compared with those previously reported in the literature, correlations between objective clinical tests and return-to-sport outcomes may not occur. Clinical outcome measures may provide suitable baseline information; however, the results of this study suggest that clinicians may need to place greater emphasis on other outcome measures when seeking to objectively promote safe return to sport.
Keywords: LARS, ACL, knee, autograft, hamstring
Anterior cruciate ligament (ACL) injury during sport is an occurrence that can be devastating for the athlete. The ultimate aim of a surgical ACL reconstruction (ACLR) after injury is to replicate the function of an intact ACL, whereas the ultimate aim of rehabilitation is to return an individual to their chosen level of activity in a timely and safe manner.21 Return to sport after ACLR depends on several different patient-, knee-, and ligament-specific variables, with validated, reliable, and responsive subjective and clinical outcome scores described and advocated in the literature.15 Despite this, a recent meta-analysis15 of randomized controlled trials (N = 4178 patients) observed that up to 90% of research failed to use any objective criteria for return to sport, with time elapsed after surgery being the most commonly used measure for return to activity. The potential weakness of such a method is that it does not account for a large percentage of identified risk factors for a safe resumption of activity.34 The ability to return to sport is broadly and variably defined, based on the preinjury competitive level played, the goals of the patient after injury, and the level of sport achieved after ACLR.2,15 Although scores may be high on clinical measures, the ability to return to sport and performance on return may not meet patient expectations, thus making the surgery unsuccessful in the patient’s opinion. Although the concept of accelerated ACL rehabilitation has existed since the 1990s,11,35 there is not yet complete agreement on the ideal rehabilitative guidelines and return-to-sport outcomes for ACLR.20 Ardern et al5 reported that of participants who underwent autograft ACLR (N = 5770), 63% had returned to their preinjury level of participation at a mean follow-up of 41.5 months. Some of the concern regarding return-to-sport outcomes has been attributed to an infrequent use of clinimetric or functional performance outcome measures in clinical practice after ACLR.15 Postsurgical activity levels after ACLR are also infrequently reported in the literature27 (24% of 119 studies). Despite the wealth of peer-reviewed research on the topic of ACL, no conclusive guidelines exist on the outcome measures most closely attributed to return-to-sport outcomes.
The purpose of this prospective study was therefore to describe and correlate the prospective activity-based and clinical outcomes after 2 different ACL procedures: autologous (hamstring tendon [2ST/2GR]) and synthetic (ligament advanced reinforcement system [LARS]) ACLR from time of surgery to 24 months postoperatively. Outcome measures included the 2000 International Knee Documentation Committee (IKDC) examination criteria, Tegner activity scale (TAS) results, and KT-1000 arthrometer joint laxity. The secondary purpose was to examine the strength of relationships between objective clinical tests (2000 IKDC and KT-1000 arthrometer joint laxity) and return-to-sport outcomes (TAS scores) at 12 and 24 months postoperatively to assess the utility of these tests in clinical practice. Given the implications of different rehabilitation protocols between surgical procedures, our intention in this study was not to directly compare surgical procedures but rather to look at participants who had selected 2 different ACL procedures in parallel to one another with reference to the outcome measures associated with recovery of function.
Methods
The study received ethical approval from the Human Research Ethics Committee at the University of Canberra. Patients who satisfied the inclusion criteria were considered eligible to be enrolled into the study from February 2012 to June 2014 (Table 1). Participants who met the inclusion criteria for the 2 different surgical groups, having self-selected to undergo either a LARS or a 2ST/2GR autograft ACLR procedure, were recruited. An additional group consisting of healthy matched participants from the general population was recruited to act as a performance reference for the surgical group.
TABLE 1.
Inclusion and Exclusion Criteriaa
| Inclusion criteria: |
|
|
|
|
|
| Exclusion criterion: |
|
aACL, anterior cruciate ligament.
A total of 64 surgical participants were recruited into the study. There were also 32 participants in the healthy reference group. The age range of participants varied from 18 to 33 years. Independent-samples t tests, with concomitant Levene tests for equality of variance, were performed for age, height, and weight and were found to be equivalent between groups (Table 2). Given that previous literature28 has not found an effect of limb dominance on the objective functional tests used in this study, limb dominance was not considered a covariate.
TABLE 2.
Participant Demographicsa
| Group | Age, y | Sex, Male:Female | BMI, kg/m2 | Injured Limb |
|---|---|---|---|---|
| Healthy controls (n = 32) | 26.31 | 16:16 | 25.73 | N/A |
| LARS group | 26.9 | 19:13 | 25.2 | 23 dominant (72%), 9 nondominant (28%) |
| 2ST/2GR group | 28.9 | 25:7 | 24.6 | 22 dominant (69%), 10 nondominant (31%) |
a2ST/2GR, hamstring tendon; BMI, body mass index; LARS, ligament advanced reinforcement system; N/A, not applicable.
Surgical Technique
Surgery was performed by 3 senior orthopaedic consultants, each with more than 10 years of experience. An endoscopic technique with anteromedial portal femoral tunnel drilling was used. Two types of grafts were used: an autologous doubled semitendinosus/gracilis (2ST/2GR) or a double-bundle ACL LARS graft using an AC DB40 or AC DB50 graft, depending on patient size. All 3 surgeons performed 2ST/2GR procedures, whereas only 1 surgeon performed the LARS procedure.
In the surgical group, patients were allowed to fully bear weight immediately, and no brace was used. A standardized accelerated early rehabilitation protocol was then commenced, though rehabilitation time frames differed between the 2 graft types to allow for the neoligamentization phenomenon of the 2ST/2GR graft. Table 3 illustrates the rehabilitation time frames for each respective graft. It is important to note again that progressions for rehabilitation were goal oriented and not specifically time oriented.
TABLE 3.
Rehabilitation Protocols: 2ST/2GR and LARS Graftsa
| Postoperative Goals | |||
|---|---|---|---|
| Stage of Recovery | Benchmark Goal | 2ST/2GR Group | LARS Group |
| 1. Early postoperative | Full extension, reduce swelling, early quadriceps activation | 0-14 d | 0-14 d |
| 2. Functional strength | ∼80% leg strength symmetry during rehabilitation exercises, full extension, able to do bodyweight lunge pain free | 2-10 wk | 2-6 wk |
| 3. Rehabilitation running—volume | ∼90% leg strength symmetry during rehabilitation exercises, able to tolerate >2 km total running volume, no increased swelling | 11-16 wk | 7-9 wk |
| 4. Running intensity and plyometrics | >95% leg strength symmetry during rehabilitation exercises, able to tolerate >3 km total running volume, normal plyometrics, >95% hop test, no increased swelling | 17-22 wk | 10-12 wk |
| 5. Integrate into sport | Must complete minimum 2 wk full-team training before return to sport | Week 23+; return to sport permitted after 6 mo | Week 13+; return to sport permitted after 14 wk |
a2ST/2GR, hamstring tendon; LARS, ligament advanced reinforcement system.
Immediate Postoperative Considerations
Each participant received an inpatient physical therapy visit before discharge, as well as standardized postoperative initial rehabilitation aimed at reduction of swelling and early restoration of active range of motion and local muscle activation around the knee.
Concomitant Therapy
Preoperative
The surgical group had an initial physical therapy consultation during which the postoperative rehabilitation process was explained. Patients were given a standardized preoperative rehabilitation program aimed at maintenance of full range of motion and muscle function and consisting of wall co-contractions, calf raises, exercise bike, straight-leg raise, heel slides, and hamstring bridge exercises.
Postoperative
The surgical group had their first outpatient consultation within 1 week postoperatively. Participants had 1 to 2 physical therapy sessions per week until full active knee extension was achieved, then emphasis shifted toward a standardized land-based strength and reconditioning protocol according to the time frames described in Table 3.
Clinical Assessment
Participants in the study were assessed pre- and postoperatively by clinical examination using the 2000 IKDC examination form, KT-1000 arthrometer (MEDmetric Co) measurement of joint laxity during the manual-maximum Lachman test, and self-reported activity scores using the TAS13 preoperatively and at 12, 16, 20, and 24 weeks and 12 and 24 months postoperatively.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 23 (SPSS Inc). A power analysis was derived from Cohen,9 and sample size was determined so that the study had 80% power to detect an effect size as large as 0.7 standard deviations on the dependent variables of interest in the study, with an alpha value of 0.05 (2-tailed). This analysis determined that 32 participants were required in each group for sufficient statistical power to be achieved. In Cohen’s terms, the effect size that the study was set up to detect is medium-large. For each test, means and SDs were obtained for each group, and a repeated-measures analysis of variance was used to assess change over time. An interquartile range (IQR) was used as the measure of variation for the ordinal data of the TAS. The nonparametric Spearman correlation coefficient was calculated to assess association of each clinical test with the TAS. As recommended by Cohen, a small correlation effect was deemed as r = 0.1 to 0.3, a medium correlation effect as r = 0.3 to 0.5, and a large correlation effect as r > 0.5.
A separate reliability study was performed (n = 9), with interrater reliability (ICC) for the manual Lachman tests found to be 0.83 and intrarater reliability observed at 1.0 for both examiners, which, according to the criteria established by Shrout and Fleiss,36 demonstrated excellent reliability (>0.75). The validity and reliability of the 2000 IKDC knee examination and TAS outcomes after ACL injury have previously been established.25
Results
2000 IKDC Knee Examination
When examining the surgical group over time, there was a significant improvement in IKDC results (P < .001) compared with preoperative, and the function had a significant quadratic (concave) component (P = .007), indicating a flattening of the observed clinical improvement after the 12-month follow-up. IKDC rating at 12 months had a strong correlation with observed results in the same test at 24 months postoperative (r s = 0.867, P < .001), suggesting that clinical examination at 1 year was generally maintained at the 2-year follow-up. The ordinal IKDC scores throughout the follow-up period are highlighted in Table 4. Preoperative IKDC results did not correlate significantly with return-to-sport outcomes at 12 months (r s = 0.146, P = .250) or 24 months (r s = 0.252, P = .70), indicating that preoperative score is not an adequate indicator of postoperative return-to-sport outcome. Furthermore, IKDC results at the 12-month follow-up were significantly correlated neither with return-to-sport levels (TAS) at the 12-month follow-up (r s = 0.235, P = .062) nor with return-to-sport outcomes at 24 months (r s = 0.188, P = .137).
TABLE 4.
2000 IKDC Knee Examination Resultsa
| Postoperative | |||||||
|---|---|---|---|---|---|---|---|
| IKDC Score | Preoperative | 12 wk | 16 wk | 20 wk | 24 wk | 12 mo | 24 mo |
| A | 0 | 10 | 10 | 17 | 25 | 29 | 28 |
| B | 56 | 52 | 52 | 46 | 38 | 33 | 34 |
| C | 8 | 2 | 2 | 1 | 1 | 2 | 2 |
| D | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
aData are reported as number of patients. IKDC, International Knee Documentation Committee.
Tegner Activity Scale
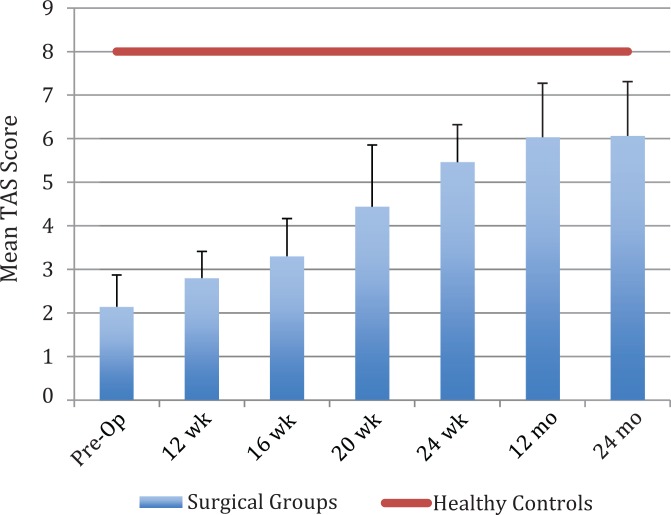
Participants from the surgical group preoperatively varied from national elite (level 10) to competitive and recreational sports (level 5). Figure 1 illustrates the mean pre- and postoperative TAS scores across the surgical group and the healthy reference group at each time point. Across the surgical group, 81% of participants had returned to their preoperative level of activity by 12 months, and 83% had returned to preoperative levels of sport by 24 months. Preoperative Tegner scores had a moderate correlation with postoperative activity levels at 12 (r s = 0.347, P = .005) and 24 months (r s = 0.326, P = .009). Tegner scores at 12 months were strongly correlated with results in the same test at 24 months (r s = 0.896, P < .001), indicating that activity scores were generally maintained after the 12-month follow-up.
Figure 1.
Mean Tegner activity scale (TAS) scores for hamstring tendon (2ST/2GR) and ligament advanced reinforcement system (LARS) anterior cruciate ligament reconstruction. Error bars represent interquartile range. Pre-Op, preoperative.
KT-1000 Arthrometer Joint Laxity During Manual Lachman Test
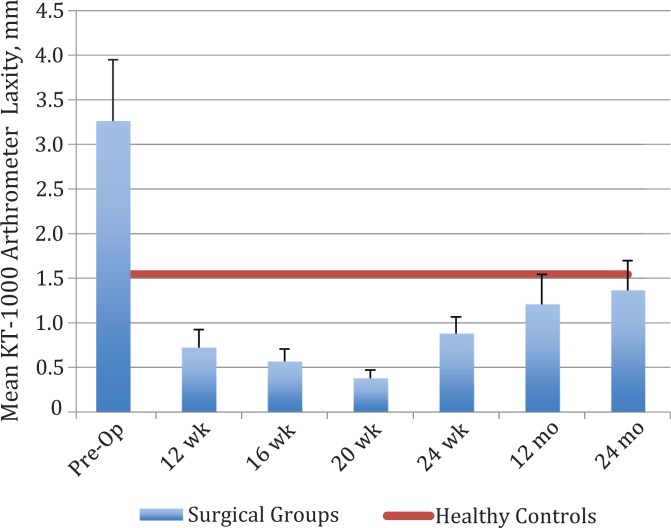
Figure 2 shows the KT-1000 arthrometer side-to-side differences during the follow-up period. In absolute terms, instrumented laxity during the Lachman test remained significantly higher for the operated leg in comparison with the uninjured leg within the surgical group (P < .001); however, throughout postoperative follow-up, the magnitude of change did not vary by more than 1 mm, which is a clinically small change. The mean side-to-side differences of the surgical group remained <3 mm.
Figure 2.
Mean difference in manual Lachman KT-1000 arthrometer side-to-side laxity for the surgical groups compared with healthy controls. Error bars represent SD. Pre-Op, preoperative.
When examining relationships between KT-1000 arthrometer testing and TAS scores across the surgical group, KT-1000 arthrometer differences at 24 months did not have a statistically significant correlation with activity levels at 24 months (r s = 0.030, P = .814), and absolute KT-1000 laxity of the surgical leg similarly did not correlate with return-to-sport activity at 24 months (r s = 0.076, P = .553). Pooled KT-1000 arthrometer absolute laxity values within the healthy reference group were a mean (± SD) of 7.64 ± 1.56 mm, whereas for the uninjured leg of the surgical group it was 7.38 ± 2.192 mm. An independent-samples t test was applied to the surgical group with equal variance not assumed (Levene test for equality of variance: F = 15.1, P < .001) and was not statistically significant (t = 0.643, df = 93, P = .522), indicating that laxity of the uninjured legs in the surgical group was not statistically different from healthy uninjured legs.
Discussion
This study identified 2 important clinically relevant findings. First, elevated return-to-sport outcomes can be achieved 12 months after ACLR and maintained at 2 years postoperatively. Second, despite a trend toward higher level sport participation in this cohort, no significant correlation was observed between results of the 2000 IKDC knee examination and the manual Lachman KT-1000 arthrometer scores for return-to-sport outcomes up to 24 months postoperatively. These findings may help guide appropriate use of clinimetric tests in postoperative ACLR.
Return to preinjury activity is one of the most important outcomes after ACLR, and arguably an important reason behind a patient’s decision to elect for surgery after an ACL injury. The pooled return-to-sports rates within this study at 12 and 24 months remained above 80% in the surgical group at each period, a value that is greater than the 63% previously indicated in the literature.5 Importantly, preinjury sports participation in the surgical group continued to improve between 1 and 2 years postoperatively, which is also in contrast to previous findings where sports activity was not maintained in the medium term after ACLR.2 Although influences are often multifactorial—most notably, adequate rehabilitation,23 compliance level during rehabilitation,14 and psychological variables1,3,4 (from a rehabilitation perspective)—the results of this study appear to endorse the concept of “goal-oriented” rehabilitation protocols used in this study rather than the “time-oriented” protocols prevalent in current clinical practice.27 Although the present study did not measure compliance specifically, it might be hypothesized that an emphasis on supervised postsurgical rehabilitation in the study design and the frequent scheduling of objective testing in the first 6 months postoperatively helped maximize rehabilitation participation. Time safely spent in higher risk activities has been shown to reduce fears of reinjury postoperatively,12 and although optimal return-to-sport outcomes ultimately require a high level of patient motivation to achieve, goal-oriented rehabilitation may help increase recovery of physical function and promote graduated exposure to fear-enhancing tasks.
Clinical and functional performance measures are increasingly utilized in clinical practice and ACL research to objectively assess recovery of function. This study focused specifically on clinimetric tests, including the 2000 IKDC knee examination criteria, and instrumented laxity using a KT-1000 arthrometer in comparison with return-to-sports outcomes. Regarding the 2000 IKDC knee examination results, the surgical group demonstrated improvement during follow-up comparable to that previously described in the literature. In our surgical cohort, 96% of participants were rated as an IKDC A or B at 24 months postoperatively. Ardern et al5 previously reported that 85% of pooled autograft participants had knee scoring of IKDC A or B at follow-up, and Chen et al7 reported that 96% of LARS participants were rated as an IKDC A or B in their study. Although clinical knee impairment may be a useful indicator of self-reported function in activities of daily living,24,29 the data within this study suggest that these clinical criteria do not correlate with higher functional tasks, including return-to-sport level (as measured by the TAS), a finding that is in agreement with previous studies.18 Although 2000 IKDC knee examination outcomes arguably form an important initial clinical reference point, the results of this study suggest that emphasis needs to be placed on additional measures that have stronger associations with successful return-to-sport outcomes.
To comprehensively examine graft integrity in situ in the surgical group, we utilized a manual maximum Lachman test using the KT-1000 arthrometer. Similar to clinical IKDC outcomes, we did not observe clinically relevant correlations between knee joint laxity (KT-1000 arthrometer) and return-to-sport level (TAS score), which is also in agreement with previous research.18 There is still a paucity of evidence relating to remodeling of an autograft procedure, with most studies conducted as in vivo animal studies or in vitro cadaveric studies,8 and currently, minimal guiding evidence exists regarding knee laxity parameters and successful activity outcomes, particularly within active populations.37,38 It is important to note that mean knee joint laxity of the surgical group remained below a 3-mm difference side-to-side throughout the postoperative period, which is the long recommended surgical benchmark for structural stability of the knee.19 The results of this study reaffirm this value as a suitable reference point for interpretation of laxity values in the postoperative period. It is, however, possible that mean values that approximate or exceed a 3-mm difference may be associated with suboptimal postoperative activity levels. When looking at absolute side-to-side differences, the surgical group had a mean difference of 1.36 mm. This is less than the pooled results of 1.7 mm reported in a meta-analyses of autograft ACLRs by Ardern et al5 and comparable to the 1.2- to 2.38-mm side-to-side differences reported in previous LARS cohorts.7,26,31,33 Increased translation with a KT-1000 arthrometer has been associated with anterior-to-posterior (AP) joint laxity more so than rotatory instability, and both are considered important for coupled motion of the knee.10 Janssen et al18 observed a significant relationship between pivot-shift laxity (grades 2 and 3) and reduced activity levels, so it is plausible that rotatory laxity might be a more crucial factor in determining higher level sports participation than purely AP joint laxity. Future research might examine the relative absolute benchmarks and strength of relationships between AP and rotatory knee joint laxity in reference to suboptimal activity levels postoperatively. Although not a major focus of this study, further work is also needed to quantify changes of synthetic grafts in situ over much longer periods of time (>5 years) to assess graft utility in clinical practice.
Excessive physiological anterior joint laxity is also assumed to be a risk factor for initial ACL injury.34 We did not observe a significant difference between KT-1000 AP laxity in healthy knees in the reference group and contralateral uninjured knees in the surgical group. However, we did not control for injury mechanism in our analysis (noncontact versus contact injuries). Contralateral noninjured knees of patients with current noncontact ACL injuries have been observed to display greater mean static anterior and internal rotational knee laxity scores than healthy control knees,30 and the combination of anterior and rotational laxity has been reported as a 3.18-times greater risk factor for suffering an ACL injury.30 Further work is needed to better define this as a risk factor, including the absolute thresholds associated with injury risk for both anterior and rotational physiological laxity in both contact and noncontact injuries.
A notable strength of this study lies in the objective assessment of clinical function at regular time points within the first 24 months postoperatively. However, this study was limited to a single-blinded prospective case-controlled series. When considering clinimetric outcomes after ACLR, a number of determinants have been identified in the literature, including patient age,6,16,22,32 body mass index,22 preinjury function,17 and sex,6 which were all controlled for in this study. Fear of reinjury is a well-described restrictor of return-to-sport activity,1,3,5 and although we did not account for this in our study design, our return-to-sport rates were greater than those reported previously in the literature. Future research could investigate the rehabilitation factors that most strongly influence this psychological barrier.
Conclusion
The results of this study examined commonly used clinimetric tests in relation to return to sport and did not observe a significant correlation between the results of these tests (2000 IKDC knee examination and KT-1000 arthrometer laxity) and activity outcomes as measured by the TAS up to 24 months postoperatively. The pooled return-to-sports rates at 12 and 24 months were greater than 80% in the surgical group, a value that is above indicative rates at similar time periods previously reported in the literature.
Acknowledgment
The authors thank Mr Kristian Waller and Mrs Anne Freeman (née Southgate) for their assistance with data collection during this project.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this project was received in the form a PhD Scholarship to T.M.M. at the University of Canberra.
References
- 1. Ardern CL, Taylor NF, Feller JA, Webster KE. Fear of re-injury in people who have returned to sport following anterior cruciate ligament reconstruction surgery. J Sci Med Sport. 2012;15:488–495. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40:41–48. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Taylor NF, Feller JA, Webster KE. A systematic review of the psychological factors associated with returning to sport following injury. Br J Sports Med. 2013;47:1120–1126. [DOI] [PubMed] [Google Scholar]
- 4. Ardern CL, Taylor NF, Feller JA, Whitehead TS, Webster KE. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2013;41:1549–1558. [DOI] [PubMed] [Google Scholar]
- 5. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. [DOI] [PubMed] [Google Scholar]
- 6. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40:2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Gu A, Jiang H, Zhang W, Yu X. A comparison of acute and chronic anterior cruciate ligament reconstruction using LARS artificial ligaments: a randomized prospective study with a 5-year follow-up. Arch Orthop Trauma Surg. 2015;135:95–102. [DOI] [PubMed] [Google Scholar]
- 8. Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39:2476–2483. [DOI] [PubMed] [Google Scholar]
- 9. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 10. Collette M, Courville J, Forton M, Gagnière B. Objective evaluation of anterior knee laxity; comparison of the KT-1000 and GNRBÒ arthrometers. Knee Surg Sports Traumatol Arthrosc. 2012;20:2233–2238. [DOI] [PubMed] [Google Scholar]
- 11. De Carlo MS, McDivitt R. Rehabilitation of patients following autogenic bone-patellar tendon-bone ACL reconstruction: a 20-year perspective. N Am J Sports Phys Ther. 2006;1:108–123. [PMC free article] [PubMed] [Google Scholar]
- 12. Gignac MA, Cao X, Ramanathan X, et al. Perceived personal importance of exercise and fears of re-injury: a longitudinal study of psychological factors related to activity after anterior cruciate ligament reconstruction. BMC Sports Sci Med Rehabil. 2015;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamido F, Misfer AK, Al Harran H, et al. The use of the LARS artificial ligament to augment a short or undersized ACL hamstrings tendon graft. Knee. 2011;18:373–378. [DOI] [PubMed] [Google Scholar]
- 14. Han F, Banerjee A, Shen L, Krishna L. Increased compliance with supervised rehabilitation improves functional outcome and return to sport after anterior cruciate ligament reconstruction in recreational athletes. Orthop J Sports Med. 2015;3(12):2325967115620770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris JD, Abrams GD, Bach BR, et al. Return to sport after ACL reconstruction. Orthopedics. 2014;37:e103–e108. [DOI] [PubMed] [Google Scholar]
- 16. Hartigan EH, Zeni J, Jr, Di Stasi S, Axe MJ, Snyder-Mackler L. Preoperative predictors for noncopers to pass return to sports criteria after ACL reconstruction. J Appl Biomech. 2012;28:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heijne A, Ang BO, Werner S. Predictive factors for 12-month outcome after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2009;19:842–849. [DOI] [PubMed] [Google Scholar]
- 18. Janssen RP, du Mée AW, van Valkenburg J, Sala HA Tseng CM. Anterior cruciate ligament reconstruction with 4-strand hamstring autograft and accelerated rehabilitation: a 10-year prospective study on clinical results, knee osteoarthritis and its predictors. Knee Surg Sports Traumatol Arthrosc. 2013;21:1977–1988. [DOI] [PubMed] [Google Scholar]
- 19. Jardin C, Chantelot C, Migaud H, Gougeon F, Debroucker MJ, Duquennoy A. Reliability of the KT-1000 arthrometer in measuring anterior laxity of the knee: comparative analysis with Telos of 48 reconstructions of the anterior cruciate ligament and intra- and interobserver reproducibility [in French]. Rev Chir Orthop Reeparatrice Appar Mot. 1999;85:698–707. [PubMed] [Google Scholar]
- 20. Joreitz R, Lynch A, Rabuck S, Lynch B, Davin S, Irrgang J. Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. Int J Sports Phys Ther. 2016;11:264–278. [PMC free article] [PubMed] [Google Scholar]
- 21. Kline PW, Johnson DL, Ireland ML, Noehren B. Clinical predictors of knee mechanics at return to sport after ACL reconstruction. Med Sci Sports Exerc. 2016;48:790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krych AJ, Woodcock JA, Morgan JA, Levy BA, Stuart MJ, Dahm DL. Factors associated with excellent 6-month functional and isokinetic test results following ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23:1053–1059. [DOI] [PubMed] [Google Scholar]
- 23. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50:946–951. [DOI] [PubMed] [Google Scholar]
- 24. Lentz TA, Tillman SM, Indelicato PA, Moser MW, George SZ, Chmielewski TL. Factors associated with function after anterior cruciate ligament reconstruction. Sports Health. 2009;1:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Letchford R, Sparkes V, van Deursen RWM. Assessing participation in the ACL injured population: Selecting a patient reported outcome measure on the basis of measurement properties. Knee. 2015;22:262–269. [DOI] [PubMed] [Google Scholar]
- 26. Liu Z-T, Zhang X-I, Jiang Y, Zeng BF. Four-strand hamstring tendon autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Int Orthop. 2010;34:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makhni EC, Padaki AS, Petridis PD, et al. High variability in outcome reporting patterns in high-impact ACL literature. J Bone Joint Surg Am. 2015;97:1529–1542. [DOI] [PubMed] [Google Scholar]
- 28. McGrath TM, Waddington G, Scarvell JM, et al. The effect of limb dominance on lower limb functional performance—a systematic review. J Sports Sci. 2016;34:289–302. [DOI] [PubMed] [Google Scholar]
- 29. Möller E, Weidenhielm L, Werner S. Outcome and knee-related quality of life after anterior cruciate ligament reconstruction: a long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2009;17:786–794. [DOI] [PubMed] [Google Scholar]
- 30. Mouton C, Theisen D, Meyer T, et al. Noninjured knees of patients with noncontact ACL injuries display higher average anterior and internal rotational knee laxity compared with healthy knees of a noninjured population. Am J Sports Med. 2015;43:1918–1923. [DOI] [PubMed] [Google Scholar]
- 31. Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. Two-year follow-up of a randomised trial. J Bone Joint Surg Br. 2002;84:356–360. [DOI] [PubMed] [Google Scholar]
- 32. Øiestad BE, Holm I, Gunderson R, Myklebust G, Risberg MA. Quadriceps muscle weakness after anterior cruciate ligament reconstruction: a risk factor for knee osteoarthritis? Arthritis Care Res. 2010;62:1706–1714. [DOI] [PubMed] [Google Scholar]
- 33. Pan X, Wen H, Wang L, Ge T. Bone-patellar tendon-bone autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Eur J Orthop Surg Traumatol. 2013;23:819–823. [DOI] [PubMed] [Google Scholar]
- 34. Serpell BG, Scarvell JM, Ball NB, Smith PN. Mechanisms and risk factors for noncontact ACL injury in age mature athletes who engage in field or court sports: a summary of the literature since 1980. J Strength Cond Res. 2012;26:3160–3176. [DOI] [PubMed] [Google Scholar]
- 35. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18:292–299. [DOI] [PubMed] [Google Scholar]
- 36. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 37. Smith TO, Postle K, Penny F, McNamara I, Mann CJ. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee. 2014;21:462–470. [DOI] [PubMed] [Google Scholar]
- 38. Weiler R, Monte-Colombo M, Mitchell A, Haddad F. Non-operative management of a complete anterior cruciate ligament injury in an English Premier League football player with return to play in less than 8 weeks: applying common sense in the absence of evidence. BMJ Case Rep. 2015;2015:bcr2014208012 doi:10.1136/bcr-2014-208012. [DOI] [PMC free article] [PubMed] [Google Scholar]