Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout the world. Exacerbation of COPD has negative effect on quality of life. Therapeutic effect of nebulized antibiotics in pulmonary infections has been reported previously. Hence, we evaluated the effect of nebulized gentamicin in acute exacerbation of COPD (AECOPD).
Materials and Methods:
In this clinical trial study, 86 hospitalized patients with AECOPD were divided into two groups for using nebulized gentamicin twice daily (case group) and placebo (control group) for 5 days in addition to standard treatment. On admission and on the 6th day, respiratory rate (RR), white blood cell (WBC), spirometry, and SPO2 (arterial O2 saturation by pulse oxymetry) were measured in groups. The severity of dyspnea was evaluated by the Medical Research Council scale.
Results:
In both groups, changes of SpO2, RR, forced an expiratory volume of first second (FEV1), and forced vital capacity (FVC) were significant during the times of intervention (P < 0.05). However, changes of FEV1 and FVC were significantly different between two groups (P < 0.05). So that increments of FEV1 and FVC were higher in the case group than control group. WBC decreased significantly in the case group (P < 0.05) compared to control group. There was no significant difference between groups in severity of dyspnea after intervention (P > 0.05).
Conclusion:
Treatment with Nebulized Gentamicin in AECOPD exacerbation resulted in further improvement of FVC and FEV1 on the 6th day.
Keywords: Chronic obstructive lung disease, gentamicin, spirometry
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases. Exacerbations and comorbidities contribute to the overall severity in individual patients.[1] COPD, the fourth leading cause of death in the world,[2] represents an important public health challenge that is both preventable and treatable. Globally, the COPD burden is projected to increase in coming decades because of continued exposure to COPD risk factors and aging of the population.[3]
Acute exacerbation of COPD (AECOPD) is triggered by infection with bacteria or viruses (which may coexist), environmental pollutants, or unknown factors. During respiratory exacerbations, there is increased hyperinflation and gas trapping, with reduced expiratory flow, thus accounting for the increased dyspnea.[4] Although the infectious agents in COPD exacerbations can be viral or bacterial,[5,6] the use of antibiotics in exacerbations remains controversial. In several studies, colonization and infection with Pseudomonas aeruginosa (PA) have been shown.[7,8,9,10,11,12,13] Different studies have been done on the effect of nebulized antibiotics on different chronic lung diseases,[14,15,16] but there are controversy results and the data are not conclusive to prove the therapeutic effect of this treatment. Therefore, we evaluated the effect of nebulized gentamicin in AECOPD.
MATERIALS AND METHODS
This study was a double-blinded placebo-controlled clinical trial conducted on a sample of hospitalized patients with AECOPD. All patients older than 40 years and known case of COPD were included in the study. Patients with a history of heart failure (ejection fraction <40%), renal failure, ongoing ischemia (based on electrocardiography), hearing problems, lung cancer, lung abscess, bronchiectasis, lung resection surgery, and hypersensitivity to aminoglycosides were excluded. The sampling method was based on convenience sampling. The sample size was calculated based on the result of a pilot study to obtain a 95% of confidence and a power of 80% to see a difference between two groups equal to 60% of standard deviation in the mean increasing of forced expiratory volume (FEV) parameter during the intervention. The sample size obtained as 43 patients in each group. The study was approved by the Ethical Committee of the Shahrekord University of Medical Sciences and was registered at ClinicalTrials.gov, number IRCT2015081723659N1. Informed consent was obtained from all participants.
Participants were randomly assigned to two groups of placebo (control) and gentamicin (case). Both patients and investigators were blinded to study groups. In all patients after hospitalization, on admission, SpO2 (oxygen saturation), respiratory rate (RR) and white blood cell (WBC) were measured and spirometry was performed. Patients in case group received gentamicin (Osveh factory, Iran) 80 mg (2 cc) plus 1 cc sodium chloride 0.9% nebulization[17] and patients in control group received 2 cc distilled water plus 1 cc sodium chloride 0.9% nebulization twice daily for 5 days were used. Both groups were under treatment with supplemental O2, tablet amoxicillin-clavulanic acid 625 mg every 8 h, capsule doxycycline 100 mg twice a day, salbutamol nebulization, ipratropium bromide nebulization, and tablet prednisolone 30 mg daily. On the 6th day, RR, SpO2, WBC, and spirometry were repeated in two groups. Spirometry in all patients was done by spirometer custo vit m R 2010.
To evaluate the effect of treatment on dyspnea in both groups, the Medical Research Council (MRC) scale [Table 1] was used.[10] The best value of forced vital capacity (FVC) and FEV of first second (FEV1) and FEV1/FVC ratio were recorded.
Table 1.
Medical research council scale

Data analysis
For continuous variables, data are given as means ± standard deviation (SD) and for categorical ones, as number with percent. Comparisons between two groups were done using the Chi-square or Fisher exact test for categorical variables and independent t-test for continuous ones. Paired - t-test was used for comparing the changes between two periods for continuous variables. Wilcoxon signed rank test was used to compare the severity of dyspnea before and after study. Statistical analysis was done by SPSS 16 (Version 16.0, 2007, SPSS Inc., Chicago, IL, USA). and P < 0.05 was statistically significant.
RESULTS
In this study, of 86 patients, 43 patients were included in the case and 43 patients in the control group. The age of patients in the case group was from 49 to 82 years with mean (SD) of 65.5 ± 9.7 years and in the control group from 43 to 89 years with mean of 66 ± 12 years (P = 0.82). 27 (62.8%) patients in the case group and 24 (55.8%) in the control group were female (P = 0.51). The height of patients in the case group was from 157 to 180 cm with mean of 167.2 ± 6.4 cm and in the control group from 156 to 180 cm with mean of 166.7 ± 7.4 cm (P = 0.75).
Mean of interested variables including SpO2, RR, FEV1, FVC, and WBC on the first and 6th day of intervention are presented in Table 2.
Table 2.
Mean and standard deviation of variables during the study in both groups
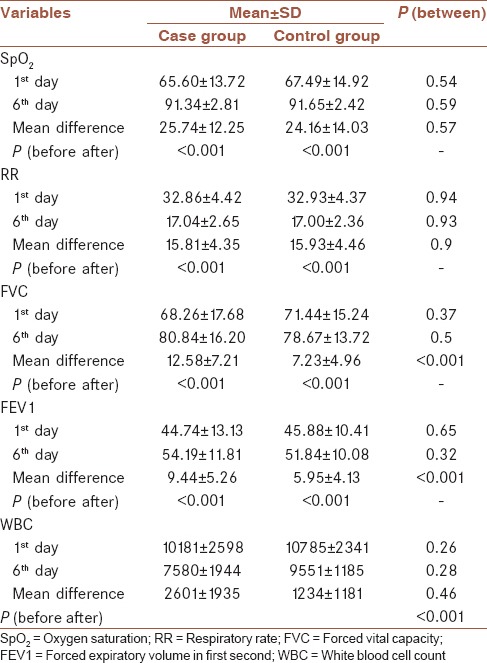
Changes of all variables except WBC after intervention were significant in the both groups. However, changes of SpO2 and RR were not significantly different between two groups, but changes of FEV1 and FVC were significantly different between two groups, so that, increments of FEV1 and FVC were higher in the case group than control group. WBC decreased significantly in the case group, but it did not change in the control group.
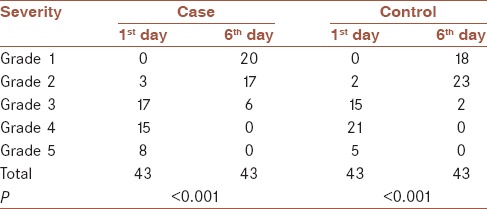
Severity frequency of dyspnea in the case and control groups based on MRC scale was shown in Table 3. Based on MRC scale, on the 1st day, there were three patients in Grade 2, 17 patients in Grade 3, 15 patients in Grade 4, and eight patients in Grade 5 of dyspnea in the case group. In the control group, there were two patients in Grade 2, 15 patients in Grade 3, 21 patients in Grade 4 and 5 patients in Grade 5. There was no patient in Grade 1 in the both group on the 1st day of intervention. On the 6th day, there were twenty patients in Grade 1, 17 patients in Grade 2, six patients in Grade 3 of dyspnea in the case group. In the control group, there were 18 patients in Grade 1, 23 patients in Grade 2, two patients in Grade 3. There was no patient in Grade 4 and 5 in both groups after intervention. There was no significant difference between groups in severity of dyspnea on the 1st day (P = 0.60) and 6th day (P = 0.27). Wilcoxon signed rank test showed a significant reduction on the grade of dyspnea during the study [Table 3].
Table 3.
Severity frequency of dyspnea based on medical research council scale
DISCUSSION
Different studies have been done on the effect of nebulized antibiotics on different chronic lung diseases, but there are controversy results and the data are not conclusive to prove the therapeutic effect of this treatment. Our results showed strong association between treatment with nebulized gentamicin and better spirometric parameters (FVC and FEV1).
Bacterial infection is important etiology of AECOPD. In a clinical trial was done by Schienberg and Shore, treatment with tobramycin solution inhalation was associated with significant improvement in mean pulmonary total symptom severity score, a composite score that assesses the severity of cough, dyspnea, sputum production, fatigue, and wheezing in patients with severe bronchiectasis.[18] However, in our study, improvement of dyspnea in two groups was not different significantly. This may be due to different patients evaluated (COPD versus bronchiectasis).
Similar to our study Murray et al., showed that nebulized gentamicin in non-CF bronchiectasis for 12 months had significant benefit in noncystic fibrosis bronchiectasis.[19] In another study, researchers demonstrated that long-term therapy with inhaled gentamicin could eradicate the infection or reduce the bacterial load, decrease the risk of subsequent infections and improve the quality of life in patients with non-CF bronchiectasis with a minimal risk of side effects.[14]
In previous studies, the role of PA infection in AECOPD have been clarified.[8,9,10,11,12,13] Although we did not check the sputum culture for detecting PA, beneficial effect of nebulized gentamicin may be due to therapeutic effect on PA.
CONCLUSION
Treatment with Nebulized Gentamicin in AECOPD exacerbation resulted in improvement of FVC and FEV1 on the 6th day.
Limitations
The small sample size and short evaluation time could be a limitation of this study for more conclusions about the efficacy of gentamicin. In addition, the lack of microbiologic study of sputum in patients was another limitation to be able to discuss the PA infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
FS contributed to the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work as a first author and supervisor. SA contributed to the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work as a supervisor. SKH contributed to the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work as a supervisor. RH contributed to the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work as a consultant. AA contributed to the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease; 2016 [Google Scholar]
- 2.World Health Report. Geneva: World Health Organization; 2000. Available from: http://www.who.int/whr/2000/en/statistics.htm . [Google Scholar]
- 3.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 4.Parker CM, Voduc N, Aaron SD, Webb KA, O’Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26:420–8. doi: 10.1183/09031936.05.00136304. [DOI] [PubMed] [Google Scholar]
- 5.Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–80. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 6.Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: A systematic review and metaanalysis. Chest. 2008;133:756–66. doi: 10.1378/chest.07-1207. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–71. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–60. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Vidal C, Almagro P, Romaní V, Rodríguez-Carballeira M, Cuchi E, Canales L, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: A prospective study. Eur Respir J. 2009;34:1072–8. doi: 10.1183/09031936.00003309. [DOI] [PubMed] [Google Scholar]
- 10.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–64. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler K, Mühlemann K, Garzoni C, Pfahler H, Geiser T, von Garnier C. Colonisation with Pseudomonas aeruginosa and antibiotic resistance patterns in COPD patients. Swiss Med Wkly. 2012;142:w13509. doi: 10.4414/smw.2012.13509. [DOI] [PubMed] [Google Scholar]
- 12.Groenewegen KH, Wouters EF. Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1-year prospective study. Respir Med. 2003;97:770–7. doi: 10.1016/s0954-6111(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47:1526–33. doi: 10.1086/593186. [DOI] [PubMed] [Google Scholar]
- 14.Antoniu SA, Trofor AC. Inhaled gentamicin in non-cystic fibrosis bronchiectasis: Effects of long-term therapy. Expert Opin Pharmacother. 2011;12:1191–4. doi: 10.1517/14656566.2011.563735. [DOI] [PubMed] [Google Scholar]
- 15.Steinfort DP, Steinfort C. Effect of long-term nebulized colistin on lung function and quality of life in patients with chronic bronchial sepsis. Intern Med J. 2007;37:495–8. doi: 10.1111/j.1445-5994.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 16.Dal Negro R, Micheletto C, Tognella S, Visconti M, Turati C. Tobramycin nebulizer solution in severe COPD patients colonized with Pseudomonas aeruginosa: Effects on bronchial inflammation. Adv Ther. 2008;25:1019–30. doi: 10.1007/s12325-008-0105-2. [DOI] [PubMed] [Google Scholar]
- 17.McCorquodale C, Grady D. Gentamicin for Nebulisation areas for responsibility for the sharing of care, EJ 34shared care guidline. 2013. [Last accessed on 2016 Jul 13]. p. 5. Available from: http://www.cambsphn.nhs.uk/Libraries/Shared_Care_Guidance/Gentamicin_for_Nebulisation_SCG.sflb.ashx .
- 18.Scheinberg P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest. 2005;127:1420–6. doi: 10.1378/chest.127.4.1420. [DOI] [PubMed] [Google Scholar]
- 19.Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2011;183:491–9. doi: 10.1164/rccm.201005-0756OC. [DOI] [PubMed] [Google Scholar]


