Abstract
Background:
It is supposed that human colorectal cancer consists of a phenotypically distinct population of tumorigenic cancer cells known as cancer stem cells (CSCs) which play a pivotal role in cancer progression, maintenance, metastasis, and the relapse. The aim of this effort was to investigate and compare biological characterizations of CD133+ with CD133− cell subsets isolated from both primary and metastatic human colorectal tumors.
Materials and Methods:
Using our optimized protocols, unfixed colorectal tumors were enzymatically and mechanically dissociated into single cells followed by evaluation of postdigestion viability. The obtained single cell suspensions were then subjected to cell sorting using magnetic beads according to CD133 marker. The resultant CD133+ and CD133− cell subsets were cultured in specific cell culture medium followed by aldehyde dehydrogenases (ALDH) activity assessment and flow cytometric analyses.
Results:
The results demonstrate that CD133+ cells have smaller size and lower complexity of intracellular structure, sphere formation ability, and ALDH enzyme activity while CD133− cells isolated from primary colon cancer samples were not able to form a sphere and did not show ALDH enzyme activity. Intriguingly, CD133− cells isolated from metastatic colorectal cancer specimen were able to form a sphere and shown ALDH enzyme activity. The present study indicates that our results are in agreement with SC theory and possibility of the existence of cellular plasticity among cancer subpopulations should be portrayed.
Conclusion:
We also conclude that this cellular plasticity is greatly affected by tumor microenvironment cues and the role of CSCs niche in cancer therapeutic strategies should be precisely considered.
Keywords: Cancer stem cells, cancer therapy, cellular plasticity, colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is one of the most commonly diagnosed and lethal cancers worldwide.[1,2,3] In many years, CRC accomplishment has been explained as a multistep process that requires the accumulation of genetic/epigenetic aberrations in signal transduction pathways.[4,5,6] A growing body of evidence is increasingly supporting the idea that human cancers can be considered as a stem cell (SC) disorder.[7,8] The existence of cancer SCs (CSCs) was established in hematologic malignancies and recently in several human solid tumors including CRC.[9,10,11,12] According to the CSC concept, CRC originates from a small fraction of cancer cells that show self-renewal and pluripotency and is capable of initiating and sustaining tumor growth.[10,13]
Recent studies demonstrate that CD133, a cell surface antigen, has been widely used as a marker for identification and isolation of colorectal CSCs from malignant colorectal tissues.[10,14,15] These studies suggest that CRC clone is organized as a hierarchy that originates from rare SCs with CD133+ phenotype. These cells described to be responsible for tumor initiation, progression, maintenance, metastasis, and the relapse of the CRC.[14,15,16,17] Furthermore, since the most of the current cellular data of colorectal CSCs such as cell size, complexity of intracellular structure, sphere formation ability, and aldehyde dehydrogenases (ALDH) enzyme activity have been restricted to cell line studies as well as little is known about these features in CSCs separated from colorectal tumor tissues, therefore, a better realizing of cellular properties of CSCs isolated from patient specimen is aiding in understanding of the behavior of colorectal CSCs, as the root of cancer, and devising innovative and novel therapeutic strategies.[6,18] The present study aimed to investigate and compare cellular characteristics of both CD133+ and CD133− cell subsets isolated from both human primary and liver metastatic colorectal cancers.
MATERIALS AND METHODS
Collection of colon specimens
Patients were selected from referrals to Alzahra Hospital of Isfahan University of Medical Sciences. Human tumor tissues were obtained from twenty consenting patients (age range: 45–79 years) undergoing colorectal resection [Table 1] according to the Internal Review and the Ethics Boards of the Isfahan University of Medical Sciences, Isfahan, Iran. The patients’ cancer status in both primary tumor site and liver was carefully evaluated for the location and extent of the cancer by a surgeon during surgery. Once the disease was localized to a surgically resectable area of the liver, patients were treated by wedge excision, segmentectomy, lobectomy, or extended lobectomy (trisegmentectomy) depending on the extent of disease. Twenty colorectal cancer specimens from the histologic diagnostic assessment and sampled by pathologists were collected in serum free-Dulbecco's modified Eagle's medium (DMEM) containing 25 units/ml of penicillin and 25 μg/ml of streptomycin (at 4°C) and transferred to the laboratory within 30 min of surgery and immediately mechanically and enzymatically disaggregated.
Table 1.
Patient description and tumor characteristics
Tissue disaggregation
In the laboratory, cancer tissues were extensively washed in phosphate-buffered saline (PBS) containing antibiotics penicillin (500 IU/ml), streptomycin (500 μg/ml), and amphotericin B (1.25 μg/ml). To prepare single-cell suspension, tumor tissues were minced into tiny fragments (~2 mm3), followed by enzymatic digestion with 1.5 mg/mL collagenase, 20 μg/ml hyaluronidase for 1 h at under continuous shaking at 37°C. Cells were then filtered sequentially through 70 μm and 40 μm cell strainers, followed by a wash with excess MACS buffer. Red blood cells were lysed during a brief exposure to ammonium chloride (5 min at 4°C) and washed again with excess MACS buffer. All materials were obtained from Sigma, USA, exception MACS buffer from Miltenyi Biotec, Germany. Then, cells were subjected to magnetic bead separation.[19]
Magnetic cell sorting
All analyses and cell isolations were performed using freshly dispersed cell suspensions. Cell suspensions were incubated with a monoclonal CD133 antibody labeled with MicroBeads (Miltenyi Biotech) for 30 min at 4°C, and CD133+ cells were enriched using a MiniMACS magnet and MS columns (Miltenyi Biotech). Briefly, magnetic labeling with 1 μl CD133/1 microbeads/1 million cells was performed using the Miltenyi Biotec CD133 cell isolation kit. Ten microliters of CD133-2-PE (fluorochrome-conjugated mouse monoclonal IgG1, clone 293C3; Miltenyi Biotec) was added for an additional 30 min to evaluate the efficiency of magnetic separation by flow cytometry. After incubation, cells were washed in MACS buffer (Miltenyi Biotec GmbH, Germany) and centrifuged at 300 ×g for 10 min. Magnetic cell separation was carried out on the MiniMACS column (Miltenyi Biotec) and the column was washed with MACS buffer to remove unbound cells. Positive and negative fractions were eluted with a double-sensitive mode. After magnetic sorting, both cell subsets were counted on a neubauer counting chamber and cell viability was assessed using trypan blue exclusion. Aliquots of CD133+ and CD133− sorted cells were evaluated for purity by flow cytometry with an FACSCalibur machine (BD Biosciences, San Diego, USA). CD133+ and CD133− sorted cell populations were resuspended in DMEM/F12 with growth hormones and supplements. All MACS procedures were performed according to the manufacturer's instructions. The purity of isolated CD133+ regularly exceeded 95%.[19]
Sphere formation assay
Both postsorted CD133+ and CD133− cell compartments, as described above, were plated in 96-well plates (Orange Inc., United Kingdom.) at a density of 100,000 cells per well in specific SC medium DMEM/F12 supplemented with glucose 6 mg/ml, NaHCO3 1 mg/ml, HEPES 5 mM, L-glutamine 2 mM, heparin 4 μg/ml, BSA 4 mg/ml, bFGF 10 ng/ml, EGF 20 ng/ml, apotransferrin 100 μg/ml, insulin 25 μg/ml, putrescin 9.6 μg/ml, sodium selenite anhydrous 30 nM, and progesterone 20 nM (Sigma, USA) without serum. Plates were gently spun at 500 rpm for 5 min at room temperature following plating. Plates were cultured in vitro (37°C and 5% CO2) for more than 28 days and media was replaced every 2 days. After reaching a confluency of 70%, CSCs colonies were splitted by the mechanical method through several ups and downs. Then, CSCs were centrifuged at 800 rpm for 5 min. Following centrifugation, cell pellet was resuspended in 3 mM EDTA plus 0.05 mM dithiothreitol in PBS or in 0.05% trypsin plus 0.02% EDTA until the cells were not well dissociated and incubated at 37°C. After an incubation time of 5 min, cell suspension was centrifuged at 800 rpm for 5 min again. Specific medium for supernatant was discarded and the pellet containing CSCs was resuspended in the specific medium for next subcultures and passages. Images of each well were captured using an inverted microscope (Olympus Inc., USA). Moreover, to evaluate cell colony formation capacity in a specific medium, sphere formation assay was carried out. Colony formation in such specific medium is representative of self-renewal activity of given cells.[20]
Analysis of aldehyde dehydrogenases enzyme activity
ALDH activity is a marker commonly used to isolate SCs, particularly CSCs and it was originally designed to isolate viable hematopoietic SCs. To describe ALDH activity of both CD133+ and CD133− cancer cell subsets, ALDEFLUOR® assay was performed. The assay is thought to specifically detect ALDH isoform ALDH1A1 activity. To assess ALDH activity of the two cell subsets, the ALDEFLUOR® assay kit (StemCell Technologies, Canada) was utilized. The basis for this assay is that uncharged ALDH substrate (BODIPY-aminoacetaldehyde [BAAA]) is taken up by living cells via passive diffusion. Once inside the cell, BAAA is converted into negatively charged BODIPY-aminoacetate (BAA) by intracellular ALDH. BAA − is then retained inside the cell, causing the cell to become highly fluorescent. Only cells with an intact cell membrane can retain BAA−, so only viable cells can be identified. The ALDEFLUOR® assay was conducted as described previously. Briefly, CD133− and CD133+ cells were harvested, placed in ALDEFLUOR® assay buffer (2 × 106/ml) and incubated with the ALDEFLUOR® substrate for 45 min at 37°C to allow substrate conversion. As a negative control for all experiments, an aliquot of ALDEFLUOR® -stained cells was immediately quenched with 1.5-mM diethylaminobenzaldehyde, a specific ALDH inhibitor. Cells were analyzed using the green fluorescence channel on a BD FACSCalibour flow cytometer (BD Inc., USA). K562 cell line as an ALDH-positive SC fraction was labeled and assayed for ALDH activity in order to optimize and validate the flow cytometry protocol.[21,22]
RESULTS
The present effort enrolled 20 patients, aged 45 years or older (mean age, 63.25 years; range: 45–79) [Table 1].
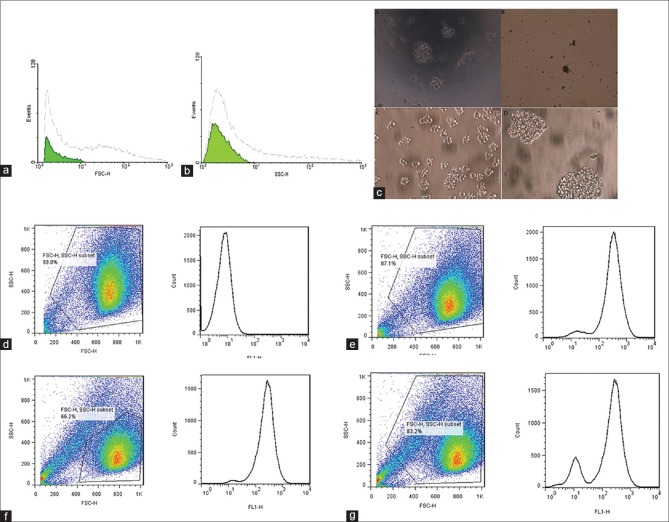
To assess cell size and complexity of intracellular structure of CD133+ and CD133−, sorted CD133+ and CD133− cell subsets were analyzed by flow cytometry. Forward-angle light scatter (FSC) and side-angle light scatter (SSC) were used for cell size and complexity of intracellular structure, respectively. Our results show that CD133− cells are bigger and more complex than CD133+ cells. Figure 1a and b represent cell size and complexity of intracellular structure analyses, respectively. Culturing of both cell compartments revealed that CD133+ cells isolated from primary colon cancers were able to grow in specific medium while CD133− cells were unable to form colonies in specific serum free medium. Interestingly, both CD133+ and CD133− cells isolated from liver metastatic colon cancers were grown in specific serum-free medium [Figure 1c].
Figure 1.
(a) Tumor cell size. (b) Complexity of intracellular structure of a tumor. (c) Sphere formation assay. (d) Aldehyde dehydrogenases enzyme activity of CD133− cell subset isolated from primary colorectal cancer sample, (e) aldehyde dehydrogenases enzyme activity of CD133− cell subset isolated from liver metastatic colorectal cancer sample, (f) aldehyde dehydrogenases enzyme activity of CD133+ cell subset isolated from primary colorectal cancer sample, and (g) aldehyde dehydrogenases enzyme activity of CD133+ cell subset isolated liver metastatic colorectal cancer sample
The assay demonstrated that CD133+ cells show ALDH enzyme activity while CD133− cells isolated from primary colon cancer samples do not show ALDH enzyme activity. Intriguingly, CD133− cells isolated from metastatic colorectal cancer specimen exhibit ALDH enzyme activity. Figure 1d–g are representatives of flow cytometric analysis of ALDH enzyme activity in both CD133− and CD133+ cell subsets.
DISCUSSION
In this study, we showed that phenotypic isolation of colon CSCs is not reliable and it should be further validated by their growth capacity in a specific cell culture. Moreover, herein, we also described the possibility of an interconversion between differentiated colon cancer cells and colon CSCs, a phenomenon that is more likely due to tumor microenvironment cues. This highlight the importance of cancer microenvironment cues in this phenomenon and potential of tumor microenvironment-modulating agents in cancer therapy.
SCs are undifferentiated cells that can differentiate into specialized cells. Some important distinct features of SCs are slowly cycling or fast cycling during homeostasis in vivo, low intracellular complexity (low SSC values), small size (low FSC values) with poor differentiation, and potential of high proliferation ability after injury or placement.[23,24]
A great deal of study revealed that hematological cancers and most of the solid tumors such as colorectal cancer contain a subpopulation which possess characteristics associated with normal SCs specifically the ability to give rise to all cell types found in a particular cancer sample.[9,10,11,15] This subpopulation is also known of CSCs. In fact, this subset of cancer cells in some cancers exhibited higher tumorigenicity and proposed to cause relapse and metastasis by giving rise to new tumors. These cells were isolated and identified by different approaches from cell surface markers and cell culture methods to ability of dye elimination.[12,25,26,27] Currently, identification and isolation of CSCs are largely dependent on the presence of specific cell surface markers, although the expression of such markers depends on various factors (e.g., the cell differentiation states and microenvironment factors).[28,29,30,31] Many of the markers utilized to identify CSCs are derived from the surface markers known to be present on normal SCs. One of these markers, CD133, is a well-described CSCs marker in various types of cancers including colorectal cancer.[15,25]
In this study, we speculated that if we could evaluate some cellular characteristics in two different cell subsets, an appropriate estimation would be available for cancer therapies and progression. Moreover, the study of these cellular characteristics in cancer cell subsets might be a suitable indicator for the evaluation of treatment outcome and even prediction of cancer relapse. In our study, we used the CD133 antigen as a CSC marker in order to identify, isolate, and further investigate some cellular characteristics within human colon cancer tissues. In this study, we reported cell size and complexity of intracellular structure of two (CD133+ and CD133−) cell subsets isolated from human colorectal cancers. The results reported here clearly show that CD133− cells are bigger and more complex than CD133+ cells. These observations are in agreement with previous SC theory demonstrating SCs are small size with poor differentiation, and low complexity of intracellular structure.[23,24] Furthermore, intriguingly obtained results from sphere formation and ALDH enzyme activity assays revealed that contrary to our first assumption, CD133− cell subsets isolated from liver metastatic colorectal cancer samples, similar to CD133+ cell subsets isolated from both primary and liver metastatic colorectal cancer samples, were able to form sphere in vitro and exhibited ALDH enzyme activity. Our latter result would be indicated the ability for CD133+ cell subsets to produce CD133− cell subsets and surprisingly also CD133− cells to produce CD133+ cells, especially in metastatic state. These findings would be consistent with an earlier investigation where it exhibited that during the metastatic transition, CD133+ tumor cells might give rise to the more aggressive CD133- subset, which is also capable of tumor initiation in NOD/SCID mice.[14] Such a dynamic evokes one possibility, there is a dynamic relation between CD133+ and CD133− subpopulations and allows for interconversion between the two states. This equilibrium may be switched bidirectionally by tumor niche cues that influence the probability of interconversion between the CD133+ and CD133− cell compartments.[32,33] Another more recent study showed that there is a dynamic relationship among subpopulations of human breast cancer for a given phenotypic state over time. This study was explained by observations using Markov model in which cancer cells can be transit between phenotypic states (herein, for example, CD133+ and CD133− subpopulations) stochastically. According to this model, it has predicted that given certain conditions, any subpopulation of cancer cells come back to equilibrium based on phenotypic compartments over time and CSCs originate from non-SCs (herein, CD133− cancer subpopulation). Collectively, these findings demonstrate that cancers are heterogeneous and also a phenotypic interconversion may be existing in populations of cancer cells which are greatly affected by tumor microenvironment cues.[34] The role of CSCs niche in cancer therapeutic strategies should be precisely considered and targeted. It seems that targeting of CSCs niche is as pivotal as or more pivotal than targeting of CSCs themselves. Nowadays, current cancer therapies are emphasizing on eradication or differentiation of CSCs as a therapeutic strategy in tumor-derived CSCs; however, it would be predicted that recurrence of cancers will be inevitable because although they may target CSCs, the cancer niche is in the original state, in turn, normal tissue-resident SCs or progenitors will be exposed to already existing transforming cues and maybe lead to new CSCs formation and finally cancer relapse.
CONCLUSION
It seems that new therapies should be directed against both CSCs niche and neoplastic SCs or their niche at least. The concept of the role of CSCs niche and its targeting appear to be a pivotal approach for treatment and/or cure of cancers. Ultimately, an understanding of the CSCs niche in addition to the CSCs themselves lead to a better understanding of the therapeutic approaches for future cancer therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
All authors contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
The authors deeply appreciate all patients who were involved in this study. This study was approved by the Mashhad University of Medical University (Grant number: 188120).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Veeranki SP, Zheng S. Trends and determinants of up-to-date status with colorectal cancer screening in tennessee, 2002-2008. Int J Prev Med. 2014;5:865–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Mohajeri G, Mohajeri MR, Afshar-Moghaddam N, Aslanpour A. The significance of clinicopathological aspects of tumor for the detection of liver micrometastasis in patients with colorectal cancer. J Res Med Sci. 2014;19:410–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 5.Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–60. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Ieta K, Tanaka F, Haraguchi N, Kita Y, Sakashita H, Mimori K, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15:638–48. doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 7.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 8.Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363–71. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 12.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097–104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 14.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 16.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, et al. A subpopulation of CD26+cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 18.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharagozloo M, Mirzaei HR, Bagherpour B, Rezaei A, Kalantari H, Sanei MH, et al. Cell cycle analysis of the CD133+and CD133-cells isolated from human colorectal cancer. J Cancer Res Ther. 2012;8:399–403. doi: 10.4103/0973-1482.103520. [DOI] [PubMed] [Google Scholar]
- 20.Du L, Wang H, He L, Zhang J, Ni B, Wang X, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–60. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 21.Hess DA, Craft TP, Wirthlin L, Hohm S, Zhou P, Eades WC, et al. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–20. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–55. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 23.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: Entity or function? Cell. 2001;105:829–41. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 24.De Paiva CS, Pflugfelder SC, Li DQ. Cell size correlates with phenotype and proliferative capacity in human corneal epithelial cells. Stem Cells. 2006;24:368–75. doi: 10.1634/stemcells.2005-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 28.Mannelli G, Magnelli L, Deganello A, Busoni M, Meccariello G, Parrinello G, et al. Detection of putative stem cell markers, CD44/CD133, in primary and lymph node metastases in head and neck squamous cell carcinomas. A preliminary immunohistochemical and in vitro study. Clin Otolaryngol. 2015;40:312–20. doi: 10.1111/coa.12368. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, et al. CD133+tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20:2388–99. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klevebring D, Rosin G, Ma R, Lindberg J, Czene K, Kere J, et al. Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in vivo . Breast Cancer Res. 2014;16:R72. doi: 10.1186/bcr3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalla Pozza E, Dando I, Biondani G, Brandi J, Costanzo C, Zoratti E, et al. Pancreatic ductal adenocarcinoma cell lines display a plastic ability to bi-directionally convert into cancer stem cells. Int J Oncol. 2015;46:1099–108. doi: 10.3892/ijo.2014.2796. [DOI] [PubMed] [Google Scholar]
- 32.Till JE, McCulloch EA, Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci U S A. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]