Abstract
Background:
Limited data available about the mechanisms of dysphagia and areas involving swallow after brain damage; accordingly it is hard to predict which cases are more likely to develop swallowing dysfunction based on the neuroimaging. The aim of this study was to investigate the relationship between brain lesions and dysphagia in a sample of acute conscious stroke patients.
Materials and Methods:
In a cross-sectional study, 113 acute conscious stroke patients (69 male mean [standard deviation (SD)] age 64.37 [15.1]), participated in this study. Two neurologists and one radiologist localized brain lesions according to neuroimaging of the patients. Swallowing functions were assessed clinically by an expert speech pathologist with the Mann Assessment of Swallowing Ability (MASA). The association of brain region and swallowing problem was statistically evaluated using Chi-square test.
Results:
Mean (SD) MASA score for the dysphagic patients was 139.61 (29.77). Swallowing problem was significantly more prevalent in the right primary sensory (P = 0.03), right insula (P = 0.005), and right internal capsule (P = 0.05).
Conclusion:
It may be concluded from these findings that the right hemisphere lesions associated with occurring dysphagia. Further studies using more advanced diagnostic tools on big samples particularly in a perspective structure are needed.
Keywords: Brain lesion, dysphagia, neuroimaging, stroke
INTRODUCTION
Anatomical-clinical correspondence of swallowing disorder (dysphagia) has been poorly studied in acute phases of stroke.[1] In addition, incomplete understanding of spatial and temporal features of cortical processing during swallowing have led to limited insight into the mechanisms explaining dysphagia after brain damage[2] and hence it is hard to predict which cases are likely to develop swallowing dysfunction based on their neuroimaging.[3] On the other hand, utilizing new rehabilitation techniques such as transcranial magnetic stimulation for dysphagia depends on knowledge about specific areas of the motor cortex supportive of swallowing functions.[4,5] Recent studies which have revealed parallel cortical networks functioning during volitional swallow need to clarify individual areas involved in swallowing.[6] However, the information regarding these individual areas is not as solid.[7]
Various brain regions have been shown to control swallowing. The most cited brain areas which are unfolded mainly through lesion studies include the primary sensorimotor cortex, sensorimotor integration areas, the insula and frontal operculum, the anterior cingulate cortex, parietooccipital region, basal ganglia, thalamus, cerebellum, and supplementary motor areas (SMAs).[6,7,8,9,10,11,12,13,14] In particular, the insula is shown to play a role in swallowing, due to the evidence that a lesion in the insular cortex produces profound dysphagia.[8] Some studies have revealed the bilateral involvement of the insula,[6,9,10,11] while other researchers have reported activation of the right insula during swallow.[12,13,14,15,16] There is also some evidence about the presence of anterior insula engagement in dysphagic patients.[16,17] Lowell and other researchers showed that the interactions of the left insula with other brain areas were more prominent than the right one during volitional swallowing.[7,18] In addition, there is conflicting evidence about the involvement of other areas such as cerebellum in dysphagia problem, making a study of the issue necessary.[3,5,15,16]
The objective of current study was to determine the possible clinical-anatomical correspondence of dysphagia in acute conscious stroke patients based on the involvement of cortical brain lesion locations including primary sensory cortex, the primary motor cortex, SMA, the insula, prefrontal cortex, inferior frontal cortex, operculum, and supramarginal gyrus; and the subcortical brain lesion locations comprising cingulate cortex, basal ganglia, thalamus, and cerebellum. Moreover, this research aimed at exploring other dysphagia-related brain lesions in neuroimaging of the patients.
MATERIALS AND METHODS
Study design and participants
In a cross-sectional study, 113 conscious first stroke adult patients who satisfied the study inclusion criteria from April 2014 to September 2014 participated in our study. Patients were selected using convenience sampling from two teaching hospitals clinics in Isfahan, Iran. All patients with a cerebrovascular accident who had consecutively admitted into the internal neurology units of teaching hospitals were assessed by a neurologist to confirm the diagnosis of stroke and assessed for evaluating the inclusion criteria. The inclusion criteria were age 18 years and over, stroke for the first time in the acute phase; no previous history of swallowing disorder. The exclusion criteria were subject who could not cooperate in swallow examination because of low consciousness, low comprehension (such as Wernicke aphasia or mental retardation), using ventilator or orogastric tube, subject who suffered from transient ischemic attack or small vessel disease and did not have revealed a focal lesion in neuroimaging were excluded too. During the study course, the patients who revealed the clinical symptoms of vertebrobasilar ischemia, without neuroimaging evidence were excluded.
After explaining the purposes of study for patients, a written informed signed consent was obtained participate in the study. The study was approved by the Ethic Committee of University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (the ethical code = USWR. REC.1392.91).
Outcome evaluation: Neuroimaging acquisition and interpretation
Checklists were prepared for recording both main study outcomes, i.e., dysphasia and lesion as being acute in both hemorrhagic and ischemic lesions and involved brain region as well as demographic and medical conditions of the patients including stroke symptoms (hemiplegia, dysarthria, low consciousness, vertigo, vomiting, headache, confusion, vision problems, and falling), stroke risk factors, and comorbidities (hypertension, diabetes mellitus, hyperlipidemia, smoking, and kidney disease) were obtained from their medical report.
The patients who underwent magnetic resonance imaging (MRI) (preferable if accessible) and computed tomography (CT) scan or both within 24–72 h after stroke were included in the study (CT: Somatom Definition AS+; Siemens Healthcare; 120 kV, 340 mA s, 5.0 mm slice reconstruction, 1.0 mm increment, 0.6 mm collimation, 0.8 pitch, and H30s soft kernel. MRI: 1.5 T Intera Gyroscan, Philips Achieva, The Netherlands; diffusion-weighted images (DWI) with time to repetition (TR) 4.25 ms, echo time (TE) 95 ms, matrix 256 9 256, field of view 230 9 230 mm, transversal 5 mm thick slices, and b values 0 and 1.0 mm2/s; fluid-attenuated inversion recovery (FLAIR) with TR 8.00 ms, TE 120 ms, transversal 5 mm thick slices). At first, CT scan images were performed to confirm hemorrhagic or nonhemorrhagic stroke patients; all CT scans were studied before discharge. FLAIR images were studied to localize ischemic lesions. Then, DWI images were used to confirm lesion as being acute in both hemorrhagic and ischemic lesions. Eventually, FLAIR and DWI images were correlated to demonstrate the exact anatomical location of the acute infarct areas based on the FLAIR images.
Twelve brain regions which were cited from different authors as relevant areas of dysphagia were entered into a checklist.[18] These regions were observed from both hemispheres which summed up to a total of 24 selected brain regions. The specified brain regions consisted of left and right primary sensory cortex, the primary motor cortex, SMA, cingulate cortex, insula, prefrontal cortex, inferior frontal cortex, basal ganglia, thalamus, cerebellum, operculum, and supramarginal gyrus. The checklist from diagnostic of lesion as being acute in both hemorrhagic and ischemic lesions, as well as location of involved brain region aspects, was completed by three observers (consisting of two neurologists and one radiologist) independently; inter-rater agreement was evaluated using kappa statistics leading to an acceptable to high levels of consistency; (range kappa: 0.2–1). They scored the checklists based on a binary scoring system with (1) for the presence and (0) for the absence of the lesion in each region. An area was declared as impaired if at least two of three observers agreed on its impairment. Lesions in more than one position were multicounted. The observers added additional areas to the above list according to the individual profiles of the patients as a heuristic aspect of the research.
In the current study, the Mann Assessment of Swallowing Ability (MASA) as a validated tool for diagnostics of dysphasia was used; its sensitivity and specificity have been reported as 91% (95% confidence interval [CI]: 82–95%) and 74% (95% CI: 64–80%), respectively, and positive predictive value of 95% and inter-rater reliability evaluated with Cohen kappa k = 0.76.[19,20] MASA was performed by an expert speech pathologist with good experience in dysphagia in the first 20 days from onset of stroke; two dysphagia experts SLPs have scored the videos of 29 of patients. A high degree of agreement was found between two raters. The average measure intraclass correlation coefficient was obtained as 0.992 (95% CI: 0.983–0.996). This tool helps to diagnose all grades of dysphagia. It evaluates 24 subskills of patients’ swallowing behaviors. The test quantifies each item according to a scoring system with 5 Likert ordinal scale. For example, lip seal was scored from 1 to 5 as follows: (1) For no closure or unable to assess, (2) for incomplete seal, (3) for unilaterally weak or poor maintenance, (4) for mild impairment and occasional leakage, and (5) for no abnormality detected on screening. The total score is 200 with a cutoff point of ≤ 177 representing dysphagia.[21]
Statistical analysis
Quantitative data were expressed as a mean ± standard deviation [SD] and qualitative variables as frequency (percentage). The Chi-square or Fisher exact (as appropriate) test was used to assess the possible association between the brain region and dysphagia. Independent sample's t-test was used for comparing the quantitative data between groups. All statistical analysis was performed using Statistical Package for Social Sciences version (SPSS, Inc., Chicago IL, USA; version 15).
RESULTS
One-hundred and thirty-six conscious stroke patients participated in this study. Twenty-three patients (%16.9) who suffered from small vessel ischemic stroke were excluded from the study. Hence, the data of 113 first acute stroke patients (44 female and 69 male) were finally analyzed. The mean age of the subjects was 64.37 years (SD: 15.08). The type of stroke in 92 (81.4) patients was ischemic, and in other 21 (18.6) was hemorrhagic. Of the 113 subjects, forty (35.39) were left hemisphere damaged, 59 (52.21) had right hemisphere stroke, and 15 (13.27) had brainstem lesion. Seven patients suffered from bilateral lesions. Eight patients suffered from both brainstem and hemispheric stroke. Fifty-four patients (47.8%) were diagnosed as dysphagic. Mean MASA score for the dysphagia group was 139.61 (SD: 29.77). The test was performed within a mean of 3.8 (SD: 2.9) days postonset. Table 1 presents the difference of main demographic and clinical characteristics of study participants in two dysphagic and nondysphagic patients. As can be seen, there are significant differences between two groups in terms of age and prevalence of aphasia. Thirteen (9.3%) participants were diagnosed as mild dysphagic (MASA score 168–177), moderate dysphagia (MASA score 139–167) was observed in 16 (11.4%) subjects, and 23 (16.4%) patients were diagnosed as severe dysphagic (the MASA score <138). Aphasia more prevalent among patient in severe category (20.2%) than patients in categories of moderate (0%) and mild (5.5%) (P = 0.024). No significant differences were found among the patients in mentioned categories in terms of stroke type (P = 0.59). No statistically significant relationship was observed between the presence of dysphagia and the type of stroke 8 (8.7%) for ischemic versus 1 (4.3%) (P = 0.47).
Table 1.
Basic and clinical characteristics of study participants in dysphagic and nondysphagic patients
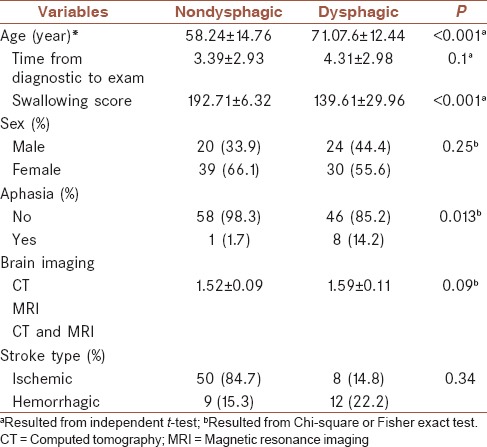
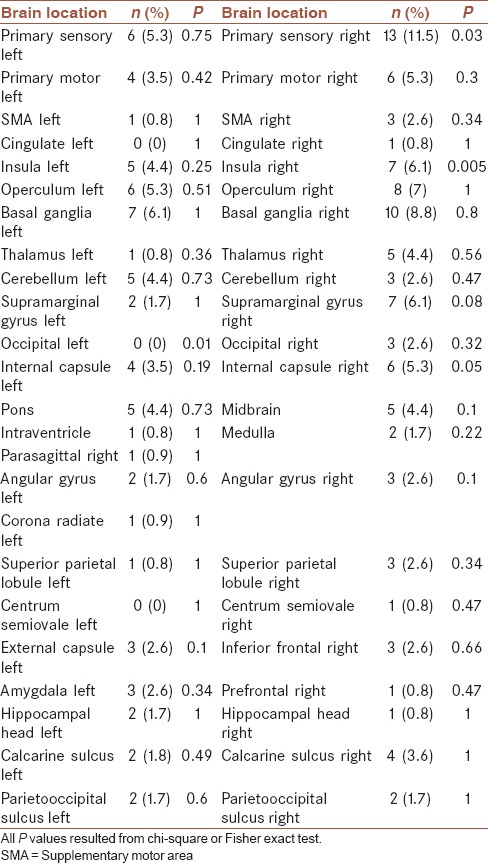
Table 2 present the frequency and corresponding percentages of dysphagia in different studied brain sites. As can be seen the presence of dysphasia more significantly higher among patients with involved right primary sensory area (P = 0.03), right insula (P < 0.005), and right internal capsule (P < 0.05). In the other hand, when midbrain, pons, and medulla were categorized as brainstem lesion, a significant relation between brainstem lesion and dysphagia was found (P = 0.01).
Table 2.
Association of dysphagia and involved brain regions in stroke patients
DISCUSSION
The relationship between different variables such as site and type of lesion and the presence of dysphagia in the acute phase of stroke is controversial. Yet, the lesion localization as a predictor of dysphagia has recently obtained more interest. While some studies have detected an association between lesion site and dysphagia,[22] others have not found a significant relationship between the location of lesion and presence of dysphagia.[23,24]
Hemispheric dominancy in controlling swallow is not clear. The most important finding of this research was the higher frequency of dysphagia in the right hemisphere in all investigated areas than the left hemisphere. As shown by descriptive findings, in most of the proposed regions related to dysphagia, the right hemisphere lesions can predict swallowing disorder, which is in line with some other previous studies.[12,15,25,26,27,28]
As previously mentioned in the results section, there is a significant relationship between the presence of dysphagia and the right primary sensory cortex lesion. Other researchers such as Mosier and Bereznaya, Martin et al., Hamdy et al., Toogood et al., Malandraki et al., and Gonzalez-Fernandez et al.[3,6,15,25,29,30,31,32] also confirmed the role of this area in swallowing. The higher occurrence of primary sensory cortex lesion in the dysphagic patients may be attributed to the role of sensory inputs in controlling complex swallowing movements. Moreover, based on the association of sensory regions with motor cortex in the process of voluntary movement, it is necessary to study the function of primary motor cortex in future studies. Some research studies have shown the role of primary motor cortex in controlling normal swallow in functional magnetic resonance imaging studies and presence of dysphagia in lesion studies.[3,6,12,15,16,30,31,33,34] Although some dysphagic patients with primary motor area impairment were observed (left primary motor cortex: n = 4, 3.5%; right primary motor cortex: n = 6, 5.3%), the number was not statistically significant, which may emphasize a more critical role of sensory versus motor inputs for controlling swallowing.
The other important result is involving right insula in swallowing. Insula integrates sensorimotor inputs and also is involved in speech and auditory process[35] (cited by Ertekin, 2003). In primates, swallowing was observed after stimulating insula[14,36] (cited by Ertekin, 2003). Moreover, tasting a food stimulates primate insular neurons[37] (cited by Ertekin, 2003). Hamdy and Aziz believe that interaction of insula as a modulator of motor function with primary sensory cortex lead to control of swallowing[15,38] (cited by Ertekin, 2003). The result of this study also showed patients who suffer from lesions in primary sensory cortex or insula revealed dysphagia. Further studies are necessary to clear the exact role of these two areas and their relation in controlling swallow.
Our findings revealed the relation of right insula lesion and presence of dysphagia. Zald et al. and have found the dominancy of the right insula in swallow function. Besides, Martin et al., Hamdy et al., and Mosier pointed to the role of the right insula in swallow.[12,13,14,15,16] Furthermore, Lowell et al. pointed to activation of posterior part of the left insula.[18] Other researchers found activation of the left insula.[33] These results show that activation of anterior and posterior areas of insula in right and left hemispheres are different during swallow as observed in a previous case study.[39] In this study, insula in each hemisphere was explored, and anterior and posterior insula were not studied separately. Further studies need to focus on the role of posterior and anterior insula in swallow.
In line with the findings of previous studies about the dysphagia diagnosed 3–4 weeks after onset of CVA following basal ganglia and internal capsule stroke by videofluoroscopy (VF), results of our study showed a significant correlation between the right internal capsule and swallowing disorder.[40] It should be noted our findings was based on diagnostics of within a mean of 3 days after stroke by a bedside examination. This outcome strongly suggests that basal ganglia and left internal capsule stroke patients need to be assessed about 1 month after stroke instrumentally. It differs from other researchers’ opinion which suggests that regardless of lesion site, all stroke patients need to be screened about dysphagia at the onset of stroke.[41] On the other hand, significant relation of right internal capsule and dysphagia shows the necessity of further studies about the different role of right and left internal capsule in swallowing.
Some areas have been listed as not significant in the results section. From one point of view, the low number of cases in each area may have led to no significant relationship. For instance, when we considered areas of the brainstem (midbrain, pons, and medulla oblongata) individually, the relationship was not significant, but when brainstem was considered as a category, the results became significant.
Regarding cerebellum and diencephalon which our study did not show a significant relationship with dysphagia, due to low number of patients because of high mortality of them in the acute phase. But it is noteworthy that some researchers have shown bilateral activation of cerebellum during swallow.[15,16,31,33,42] Hence, our results may not interpreted that bilateral diencephalon and cerebellum stroke patients would not suffer from dysphagia. It seems that lesion study is not a fruitful method for studying regions of posterior cerebral artery bloodshed. These areas activities are better examined by functional neuroimaging technologies.
Swallowing disorder after stroke shows a spontaneous recovery. In this study, the MRI was done in the first 3 days after stroke and three observers (two neurologists and one radiologist) were reported the images. To our knowledge, the rare lesion studies have explored brain controlling of swallowing in the acute phase of stroke in such a large sample.[25] Dysphagia in 105 (%93/8) was evaluated within 8 days after the stroke. Eight other subjects had low consciousness and have been assessed 9–19 days after stroke (when they could cooperate in MASA). These setting made it possible to research these subjects in a prospective study with minimal changes due to spontaneous recovery or treatment. Furthermore, excluding small vessel ischemic stroke increased the accuracy of brain lesions localization.
Swallowing assessment is a problematic issue in the acute phase of stroke. Performing VF, as the gold standard of dysphagia, was not possible for most of this population. Some researchers were used other instrumental assessments such as fiberoptic endoscopic evaluation of swallowing (FEES) in acute phase, but FEES only assesses pharyngeal phase of swallowing which leads to biased results.[25] In the present work, dysphagia in all cases was evaluated by MASA which assesses both oral and pharyngeal phases to control biased previous results.
CONCLUSION
Dealing with feeding and respiration problems of acute stroke patients requires prediction of likelihood of developing swallowing dysfunction based on clinical and paraclinical data. This study showed the relation between the right insula, right internal capsule, right primary sensory cortex lesions, and the presence of dysphagia. It also found that in all statistically significant and not significant areas, right hemisphere was involved more than left hemisphere in dysphagic patients.
Financial support and sponsorship
This project was funded by the University of Social Welfare and Rehabilitation Sciences.
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
SED contributed in the conception of the work, designing the study, definition of intellectual content, literature review, data gathering, data analysis, manuscript preparation, and agreed for all aspects of the work
FY contributed in the conception of the work, designing the study, definition of intellectual content, manuscript editing, manuscript review, and agreed for all aspects of the work
AA contributed in the design of the work, definition of intellectual content, statistical model development, data analysis, and agreed for all aspects of the work
AC and MK contributed in the data acquisition and agreed for all aspects of the work.
Acknowledgments
The authors thank the technical help of the lecturers and students of internal neurology of Isfahan University of Medical Sciences and nursing personnel of internal neurology units of Alzahra Hospital and Kashani Hospital, who supported this paper in data gathering phase. Moreover, this project was funded by the University of Social Welfare and Rehabilitation Sciences.
REFERENCES
- 1.Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40:1903–6. doi: 10.1161/STROKEAHA.108.535468. [DOI] [PubMed] [Google Scholar]
- 2.Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, et al. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–55. doi: 10.1016/j.neuroimage.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Fernandez M, Kleinman JT, Ky PK, Palmer JB, Hillis AE. Neuroanatomical basis of swallowing disorders after stroke: A pilot study. Stroke. 2008;39:3022–8. doi: 10.1161/STROKEAHA.108.518969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingram JC. NY, USA: Cambridge University Press; 2007. Neurolinguistics. [Google Scholar]
- 5.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–71. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 6.Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–9. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- 7.Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein EB, et al. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20:135–44. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- 8.Bass N. The neurology of swallowing. In: Groher M, editor. Dysphagia Diagnosis and Management. Boston: Butterworth-Heinemann; 1997. pp. 23–33. [Google Scholar]
- 9.Teismann IK, Suntrup S, Warnecke T, Steinsträter O, Fischer M, Flöel A, et al. Cortical swallowing processing in early subacute stroke. BMC Neurol. 2011;11:34. doi: 10.1186/1471-2377-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, et al. Neurophysiology of swallowing: Effects of age and bolus type. Neuroimage. 2009;44:982–91. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teismann IK, Steinstraeter O, Schwindt W, Ringelstein EB, Pantev C, Dziewas R. Age-related changes in cortical swallowing processing. Neurobiol Aging. 2010;31:1044–50. doi: 10.1016/j.neurobiolaging.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–50. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 13.Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–43. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- 14.Martin RE, Gati JS, Fox A, Menon RS. Cortical activation associated with human swallowing: A fMRI study. Soc Neurosci Abstr. 1997;23:1275. [Google Scholar]
- 15.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, et al. Cortical activation during human volitional swallowing: An event-related fMRI study. Am J Physiol. 1999;277(1 Pt 1):G219–25. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 16.Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15) O PET activation. J Neurophysiol. 1999;81:1917–26. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- 17.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–56. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- 18.Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp Brain Res. 2012;219:85–96. doi: 10.1007/s00221-012-3069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schepp SK, Tirschwell DL, Miller RM, Longstreth WT., Jr Swallowing screens after acute stroke: A systematic review. Stroke. 2012;43:869–71. doi: 10.1161/STROKEAHA.111.638254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmiaston J, Connor LT, Loehr L, Nassief A. Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19:357–64. doi: 10.4037/ajcc2009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann G. Cambridge, UK: Delmar Cengage Learning; 2002. MASA: The Mann Assessment of Swallowing Ability. [Google Scholar]
- 22.Moon HI, Pyun SB, Kwon HK. Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Ann Rehabil Med. 2012;36:347–55. doi: 10.5535/arm.2012.36.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMicken BL, Muzzy CL. Prognostic indicators of functional outcomes in first time documented acute stroke patients following standard dysphagia treatment. Disabil Rehabil. 2009;31:2196–203. doi: 10.3109/09638280902956894. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder MF, Daniels SK, McClain M, Corey DM, Foundas AL. Clinical and cognitive predictors of swallowing recovery in stroke. J Rehabil Res Dev. 2006;43:301–10. doi: 10.1682/jrrd.2004.12.0154. [DOI] [PubMed] [Google Scholar]
- 25.Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, et al. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: Dysphagia incidence, severity and aspiration. Eur J Neurol. 2015;22:832–8. doi: 10.1111/ene.12670. [DOI] [PubMed] [Google Scholar]
- 26.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G354–60. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 27.Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, et al. Oropharyngeal dysphagia after stroke: Incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18:329–35. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis. 1996;6:30–4. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- 29.Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–41. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- 30.Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: A functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161:81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]
- 31.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–26. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teismann IK, Steinsträter O, Warnecke T, Suntrup S, Ringelstein EB, Pantev C, et al. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009;10:71. doi: 10.1186/1471-2202-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: Functional implications. Laryngoscope. 1999;109:1417–23. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–39. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 36.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- 37.Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 2002;87:1068–75. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- 38.Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, et al. Cortical processing of human somatic and visceral sensation. J Neurosci. 2000;20:2657–63. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riecker A, Gastl R, Kühnlein P, Kassubek J, Prosiegel M. Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. 2009;24:114–8. doi: 10.1007/s00455-008-9164-1. [DOI] [PubMed] [Google Scholar]
- 40.Logemann JA, Shanahan T, Rademaker AW, Kahrilas PJ, Lazar R, Halper A. Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia. 1993;8:230–4. doi: 10.1007/BF01354543. [DOI] [PubMed] [Google Scholar]
- 41.Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–6. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–7. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]


