Abstract
Background:
Given the importance of the role of depression in predicting the outcome of cardiovascular disorders, current medications for treating depression, particularly selective serotonin reuptake inhibitors (SSRIs), are taken into consideration. This study aimed to systematically review the published findings in the use of SSRIs and the risk for cardiac events.
Materials and Methods:
An independent review of the Web of Science, PubMed, Scopus, Cochrane, CINAHL, index Copernicus, and Google Scholar, up to 2014, was performed. We identified studies evaluating the effect of SSRIs, on cardiovascular events. Articles in English with full text availability, review articles, and experimental studies were included in the study. Among 150 studies reviewed based on the included keywords, 17 met the study criteria and were finally reviewed.
Results:
The use of some types of SSRIs may prevent platelet adhesion and aggregation; control the cardiovascular risk profile including hypertension, insulin resistance, and body weight; and also inhibit inflammatory processes. The appearance of adverse cardiac events, including cardiac arrhythmias (torsade de pointes and QT prolongation), syncope, increased systolic and diastolic right ventricular volume, and the production of pro-inflammatory cytokines leading atherosclerosis development, has also been expected with the chronic use of some types of SSRIs.
Conclusion:
According to our systematic review, both beneficial and adverse cardiovascular events can be established following the chronic use of various types of SSRIs. Therefore, when taking SSRIs, the cardiovascular effect of each SSRI has to be carefully considered, based on patients’ cardiovascular risk profiles.
Keywords: Cardiovascular, drug, selective serotonin reuptake inhibitor
INTRODUCTION
Cardiovascular disorders and major depression are two global problems, leading to a higher cost burden, loss of productivity, and an elevation in health services utilization.[1] Both problems also result in a considerably lower quality of life, frequently with physical, psychological, and social components; lost productivity, and functional disability.[2,3] More importantly, not only do a notable number of patients who suffer from cardiovascular disorders experience major depression because of disease-related disability, a fear of being unable to work, and even an inability to communicate effectively with others (particularly with partners), the appearance of depression can lead to serious adverse events in those affected patients.[4,5,6] Cardiovascular diseases should be carefully considered as a major adverse event.
Overall, the prevalence of depression in patients with cardiovascular diseases has been reported to be widely varied. About two-thirds of these patients are diagnosed with a mild form of depression, especially those who experience acute myocardial infarction while major debilitating depression can be found in about 15% of these patients.[7,8] The prevalence of major depression has been reported to be even higher in chronic heart failure, so depending on the level of physical dysfunction, it can range between 10% in heart failure patients with mild physical dysfunction to 40% in those with severe physical impairment.[9] The importance of this issue is that depression has been well identified as an independent determinant in lowering patients’ survival.
Given the importance of the role of depression in predicting the outcome of cardiovascular disorders, current medications for treating depression in these patients are taken into consideration. In this regard, employing newer antidepressants may lead to adverse cardiovascular consequences.[10] Thus, the choice of these drugs should be considered based on their purported mechanism of action and their related side effects.[10] It seems that the interactive role of both cardiovascular disorders and antidepressants on triggering or inhibiting hormonal pathways, including serotonin, norepinephrine, and dopamine, may account for these effects.[11,12]
One of the main drug groups commonly used to treat depression that affects cardiovascular function is selective serotonin reuptake inhibitors (SSRIs), which were primarily introduced in 1980.[13] These drugs are commonly prescribed in different disease conditions because of their high efficacy, cost-benefit, ease of use, and favorite side effects, especially on the cardiovascular system.[14,15] Furthermore, SSRI-related fatality events and life-threatening complications have been shown to be the lowest when compared to other types of antidepressants.[16] Cardiac death related to administrating SSRIs has rarely been reported.[17] In this regard, the most common cardiovascular-related side effects of SSRIs include mild bradycardia and hypotension, mild QRS prolongation, and first-degree cardiac block.[18] Rarely, vasoconstriction following the administration of high-dose SSRIs may predispose the patient to myocardial infarction.[19]
Based on the previous studies, the effect of SSRIs on cardiovascular diseases is controversy and regarding the high prevalence of both cardiovascular diseases and the use of SSRIs, and the mentioned concomitant conditions, this study aimed to systematically review the published findings in the use of SSRIs and the risk for cardiac events.
MATERIALS AND METHODS
Search strategy
To find primary studies published electronically up to February 2014, we searched the databases on the Web of Science, PubMed, Scopus, Cochrane Database, CINAHL, Index Copernicus, and Google Scholar. We included articles limited to full studies in the English language. For our following raised question, “In depressed subjects using SSRIs, what would be the cardiovascular effects after the chronic use of this drug group?” we conducted our search using the keywords “cardiovascular,” “event,” “outcome,” “side effects,” and “selective serotonin reuptake inhibitors.”
Studies selection
The titles and abstracts of relevant papers were studied and extracted to an EndNote X6 by a single last year student of medicine and co-author of this study. Two independent cardiologists being as the coauthors of this study further reviewed and screened the titles and abstracts of the papers identified for their potential relevance based on the inclusion criteria and assessed the full text for including them in the review. In the case of disagreement, the discrepancy was determined in consultation with a third coauthor.
Study inclusion eligibility
We limited our findings based on the following inclusion criteria:
Articles in English,
Full-text availability,
Experimental studies.
Study exclusion eligibility
Exclusion criteria were as follows:
Editorials,
Duplicate studies,
Results of a paper that was not clear.
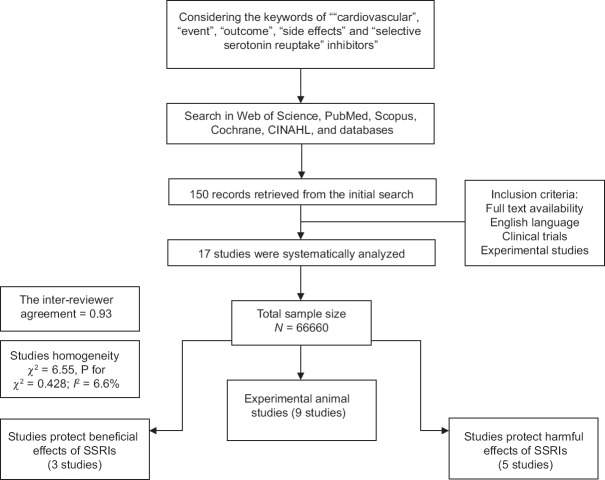
The identified abstracts were then collected and coded by each investigator. The full texts of potentially relevant studies were then pulled, and the methods and results sections were reviewed for trial design and the reporting of study end points. Each trial was accessed across the following criteria: Allocation sequence generation, allocation concealment, blinding, complete outcome data reporting, selective outcome reporting, and other apparent biases.[20,21] The main reviewed outcome was cardiovascular events following the use of SSRIs. Among 150 studies reviewed based on the included keywords, 17 met the study criteria and were finally reviewed.
The pooled relative risk (RR) was calculated for each outcome using the inverse-variance method for random effects as well as for fixed effects.[22] The data heterogeneity was assessed using the Cochrane Q-test via a Chi-square test and quantified with the I2 test.[23] We used the log RR as the dependent variable. The log RR standard error was used to measure the within-study variability, and the residual maximum likelihood method was used to estimate between the study variance. All analyses were performed using STATA version 11.0 (StataCorp; College Station, TX, USA) and CMA software version 2.2.064 (Borenstein, Hedges, Higgins, and Rothstein; Biostat; Englewood, NJ, USA).
RESULTS
Study assessment
Among 150 abstracts retrieved from the initial search, 17 studies were extracted for review based on the inclusion criteria [Figure 1]. Among the 17 studies, five articles published follow-up data for the effect of SSRIs on cardiovascular events with a range of 30-day to 1 year. Moreover, among the 17 studies, 6 were review articles [Table 1]. The inter-reviewer agreement for the study selection was high, with k = 0.93, and no obvious heterogeneity was identified among the included studies (χ2 = 6.55, P for χ2 = 0.428; I2 = 6.6%). Overall, 66,660 included patients were identified as being treated with various types of SSRIs, including fluoxetine, sertraline, citalopram, escitalopram, paroxetine, sibutramine, and benfluorex. Contradictory results were revealed regarding the beneficial or toxic effects of SSRIs on the cardiovascular system. However, the studies have claimed that both the cardioprotective and cardio-toxicity of these types of drugs led to either improved patient survival or deteriorating heart failure, respectively. Therefore, beneficial and deleterious effects of SSRIs on cardiovascular events are mentioned separately in the following sections:
Figure 1.
Flowchart of the systematic review
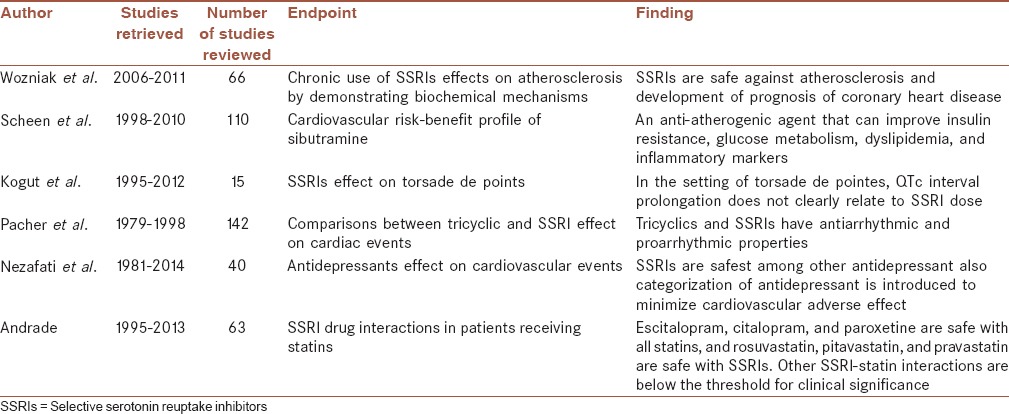
Table 1.
Review articles entered this review article
Beneficial effects of selective serotonin reuptake inhibitors
With respect to the beneficial effects of SSRIs on the cardiovascular system, one of the main protective roles of the new generations of SSRIs is related to their effects on inhibiting platelet aggregation as well as improving serotonin-related platelet abnormalities, leading to a reduction in cardiovascular mortality and morbidity.[24] In this regard, pathophysiological studies could demonstrate that the inhibitory effects of SSRIs, especially citalopram, on platelet aggregation and the progression of atherosclerosis can be related to the inhibition of collagen, thrombin, and U46619.[25,26] In addition, sibutramine, as new form of SSRI, has been shown to be an anti-atherogenic agent that can improve insulin resistance, glucose metabolism, dyslipidemia, and inflammatory markers, mostly resulting from its effect on weight loss.[27] Another beneficial effect of some forms of SSRIs, such as sertraline, is its anti-inflammatory effect as well as improving endothelial function. Some studies show that the use of sertraline effectively reduces inflammatory markers, including C-reactive protein and interleukin-6 (IL-6), after a 20-week administration of this drug led to the improvement of the endothelium-mediated dilation marker.[28] Some authors emphasized a similar role of some SSRIs, such as fluoxetine, on calcium channel blockers, so that fluoxetine could reduce [Ca2+]i and the tonicity of arteriolar smooth muscle, likely by interfering with Ca2+ entry, which can act as a therapeutic element in cardiovascular disease groups.[29,30] In a recent review article, SSRIs were concluded to be the safest antidepressants drugs, especially those with cardioprotective effects on ventricular function as well as cardiac conductive system.[31]
Deleterious effects
Along with the beneficial effects of SSRIs on the cardiovascular system, various side effects of SSRIs via different pathways have been reported to result in an increased risk for early and long-term mortality and morbidity. One of the most important cardiovascular side effects of SSRIs includes cardiac arrhythmias. Although this side effect occurs in about 4% of cardiovascular patients, it can be well tolerated in almost all patients.[32] However, in rare cases, some serious arrhythmias, such as torsade de pointes and QT prolongation, can also occur following the use of some SSRIs, such as fluoxetine, sertraline, and citalopram.[33,34]
The occurrence of other types of cardiac arrhythmias, including atrial fibrillation and bradycardia after the administration of SSRIs, particularly after a fluoxetine overdose, has also been pointed out. These arrhythmic events can be explained by inhibiting cardiac Ca2+ and Na+ channels by fluoxetine, especially in chronic treatment with this drug.[35] In this regard, the inhibition of cardiac Ca2+, Na+, and K+ and vascular Ca2+ channels by SSRI scan explain the cardiovascular side effects occasionally observed with these drugs during chronic treatment.[35]
Another important effect of SSRIs involving pathophysiological pathways in cardiovascular disorders is the interaction between some SSRIs, such as escitalopram, citalopram, and paroxetine, and some cardiovascular drugs such as statins. The main pathophysiology of this effect is related to the inhibitory effect of these drugs on the cytochrome P450 metabolic pathways, triggering statin side effects such as myopathy and rhabdomyolysis.[36] Different drug-induced behaviors have been pointed out in the occurrence of SSRI-induced syncope, for example, some authors show the protective effects of SSRIs on neurocardiogenic vasovagal syncope. In one clinical trial, after 1 month of treatment with paroxetine in patients with vasovagal syncope, spontaneous syncope was observed in 17.6% of the patients in the paroxetine group as compared to 52.9% in the placebo group.[37] In parallel with the clinically adverse consequences of administrating SSRIs, some echocardiographic assessments have focused on the structural cardiovascular changes following the use of these drugs. Some evidence has shown an association between using SSRIs and changes in the right ventricular volume and diameter. More interestingly, these changes might be different in men and women so that a higher right ventricular end-diastolic volume among women and larger right ventricular end-systolic volume in both sexes were revealed following the use of SSRIs.[38] Despite some evidence on the effects of SSRIs on the structural and functional changes of heart valves, especially mitral and aortic regurgitation, recent studies could not demonstrate morphological abnormalities in these valves.[39]
DISCUSSION
In this review, we attempted to assess the adverse effects of SSRIs on the cardiovascular system. According to the recent reports, cardiac arrhythmias are the most frequent cardiovascular events, constituting one-third of the most commonly encountered adverse reactions following the consumption of SSRIs. In this context, tachycardia, long QT syndrome, and torsade de pointes are the most common events.[40,41] In other reports, mild bradycardia, dysrhythmia, syncope, and orthostatic hypotension have been found to be associated with SSRI consumption.[35] Although arrhythmic events may occur in a notable number of patients using SSRIs, these events are usually mild and tolerable, not leading to a discontinuation of the use of that drug. Nevertheless, taking the inappropriate SSRI may lead patients to more complicated treatments as a result of the antidepressants’ complications.[42,43]
Regarding, the association between SSRI use and atherosclerotic development, it has been primarily demonstrated that there is a direct association between SSRI use and an increased serum level of inflammatory biomarkers, such as IL-6 and tumor necrosis factor-alpha. In some trials, higher levels of these markers in depressed SSRI-resistant patients, compared with healthy controls, have been found. Thus, it has been suggested that treatment with SSRIs can induce the production of these proinflammatory cytokines.[44,45,46,47,48] Moreover, a higher incidence of cardiac atherosclerotic events in those patients with a history of SSRI use has been revealed. According to the central role of inflammatory processes in developing coronary atherosclerosis, the link between the use of SSRIs and the development of atherosclerotic lesions can be predicted.
Although the initial evidence of SSRI effects on the morphological changes in heart valves was presented in the mid-1980s by Mast et al.,[49] recent reports by Maréchaux et al.[39] show no increasing risk for heart valve disease following the use of SSRIs. It has previously been demonstrated that using drugs with a high affinity for the 5-hydroxytryptamine receptor 2B (5-HT2B) receptor, such as fenfluramine, can result in drug-induced fibrotic valvular disease via the up-regulation of some genes involving the proliferation and stimulation of valvular interstitial cells. This occurs via various intracellular pathways, such as the G protein-mediated activation of protein kinase C, SRC protein, extracellularly regulated kinases 1 and 2, and transforming growth factor beta-receptor activation.[50] However, SSRIs have no 5-HT2B agonist properties. Furthermore, although stimulating the 5-HT1B receptors can induce the secretion of collagen by cardiac myofibroblasts, this receptor to SSRIs has no role in developing valvulopathy;[51] therefore, no heart valve abnormalities after the use of SSRIs can be explained.
Reviewing the literature shows significant structural changes in the right ventricular dimensions as there are increased right ventricular end-systolic and end diastolic volumes following the chronic use of SSRIs; however, these changes may not result in changes in the right ventricular ejection fraction. Thus, it seems that right ventricular hypertrophy in the right ventricular volume without any change in the ejection fraction after using SSRIs can be adaptive, such as the heart changes in athletes. In this adaptation, the right ventricular hypertrophy can cause an increase in the capillary networks and oxygen transport.[52,53] In addition, the use of SSRIs has not only no deleterious effects on the right ventricular function, they can also protect right ventricular function due to their stimulating effects on protein oxidation in the right ventricle; however, this effect could not be shown in the left ventricle.[54,55,56]
Some limitations of this study should be acknowledged. A wider range of keywords could be considered for our initial search so more articles could be extracted for analysis. In addition, some subgroup analysis such as sex-based differences due to inadequate data could not be performed. Moreover, studies with greater follow-ups would make our conclusion stronger. It is strongly recommended to conduct more studies particularly meta-analysis to study and compare other antidepressants with SSRIs for their effect on cardiovascular events.
CONCLUSION
Overall, according to our systematic review, both beneficial and adverse cardiovascular events can be discovered following the chronic use of various types of SSRIs. The use of some types of SSRIs may prevent platelet adhesion and aggregation, controlling the cardiovascular risk profile, including hypertension, insulin resistance, and body weight as well as inhibiting inflammatory processes. However, the appearance of adverse cardiac events, including cardiac arrhythmias, syncope, increased right ventricular volume, and the production of proinflammatory cytokines leading to atherosclerotic development, have also been expected by chronically using some types of SSRIs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
MHN contributed in the conception and design of the work, approval of the final version of the manuscript, and agreed for all aspects of the work
AE contributed in the conception and design of the work, approval of the final version of the manuscript, and agreed for all aspects of the work
MV conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work
SA contributed in the conception of the work, revising the draft, and agreed for all aspects of the work
PN contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
REFERENCES
- 1.Egede LE. Major depression in individuals with chronic medical disorders: Prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29:409–16. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Dickson VV, Howe A, Deal J, McCarthy MM. The relationship of work, self-care, and quality of life in a sample of older working adults with cardiovascular disease. Heart Lung. 2012;41:5–14. doi: 10.1016/j.hrtlng.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Djärv T, Wikman A, Lagergren P. Number and burden of cardiovascular diseases in relation to health-related quality of life in a cross-sectional population-based cohort study. BMJ Open. 2012;2:E001554. doi: 10.1136/bmjopen-2012-001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neil A, Stevenson CE, Williams ED, Mortimer D, Oldenburg B, Sanderson K. The health-related quality of life burden of co-morbid cardiovascular disease and major depressive disorder in Australia: Findings from a population-based, cross-sectional study. Qual Life Res. 2013;22:37–44. doi: 10.1007/s11136-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson P, Lesman-Leegte I, Lundgren J, Hillege HL, Hoes A, Sanderman R, et al. Time-course of depressive symptoms in patients with heart failure. J Psychosom Res. 2013;74:238–43. doi: 10.1016/j.jpsychores.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Cay EL, Vetter N, Philip AE, Dugard P. Psychological status during recovery from an acute heart attack. J Psychosom Res. 1972;16:425–35. doi: 10.1016/0022-3999(72)90068-2. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–56. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 10.Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415–26. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- 11.Grimsley SR, Jann MW. Paroxetine, sertraline, and fluvoxamine: New selective serotonin reuptake inhibitors. Clin Pharm. 1992;11:930–57. [PubMed] [Google Scholar]
- 12.Glassman AH. Citalopram toxicity. Lancet. 1997;350:818. doi: 10.1016/S0140-6736(05)62620-7. [DOI] [PubMed] [Google Scholar]
- 13.Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: Association with use of tricyclic agents. Am J Med. 2000;108:2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- 14.Kincaid RL, McMullin MM, Crookham SB, Rieders F. Report of a fluoxetine fatality. J Anal Toxicol. 1990;14:327–9. doi: 10.1093/jat/14.5.327. [DOI] [PubMed] [Google Scholar]
- 15.Oström M, Eriksson A, Thorson J, Spigset O. Fatal overdose with citalopram. Lancet. 1996;348:339–40. doi: 10.1016/s0140-6736(05)64513-8. [DOI] [PubMed] [Google Scholar]
- 16.Leonard BE. The comparative pharmacology of new antidepressants. J Clin Psychiatry. 1993;54(Suppl):3–15. [PubMed] [Google Scholar]
- 17.Rodriguez de la Torre B, Dreher J, Malevany I, Bagli M, Kolbinger M, Omran H, et al. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit. 2001;23:435–40. doi: 10.1097/00007691-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Glassman AH. Cardiovascular effects of antidepressant drugs: Updated. Int Clin Psychopharmacol. 1998;13(Suppl 5):S25–30. doi: 10.1097/00004850-199809005-00006. [DOI] [PubMed] [Google Scholar]
- 19.Fricchione GL, Woznicki RM, Klesmer J, Vlay SC. Vasoconstrictive effects and SSRIs. J Clin Psychiatry. 1993;54:71–2. [PubMed] [Google Scholar]
- 20.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews Of Interventions, Version 5.10 The Cochrane Collaboration. 2011. [Last updated on 2011 Mar]. Available from: http://www.cochrane-handbook.org .
- 21.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wozniak G, Toska A, Saridi M, Mouzas O. Serotonin reuptake inhibitor antidepressants (SSRIs) against atherosclerosis. Med Sci Monit. 2011;17:RA205–14. doi: 10.12659/MSM.881924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng YL, Chiang ML, Huang TF, Su KP, Lane HY, Lai YC. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb Res. 2010;126:517–23. doi: 10.1016/j.thromres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, van Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108:939–44. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 27.Scheen AJ. Cardiovascular risk-benefit profile of sibutramine. Am J Cardiovasc Drugs. 2010;10:321–34. doi: 10.2165/11584800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86:527–32. doi: 10.1038/clpt.2009.121. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z, Pacher P, Koller A. Serotonin reuptake inhibitor fluoxetine decreases arteriolar myogenic tone by reducing smooth muscle [Ca2+]i. J Cardiovasc Pharmacol. 2000;35:849–54. doi: 10.1097/00005344-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Ungvari Z, Kecskemeti V, Koller A. Serotonin reuptake inhibitor, fluoxetine, dilates isolated skeletal muscle arterioles. Possible role of altered Ca2+sensitivity. Br J Pharmacol. 1999;127:740–6. doi: 10.1038/sj.bjp.0702571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nezafati MH, Vojdanparast M, Nezafati P. Antidepressants and cardiovascular adverse events: A narrative review. ARYA Atheroscler. 2015;11:295–304. [PMC free article] [PubMed] [Google Scholar]
- 32.Kostev K, Rex J, Eith T, Heilmaier C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger Med Sci. 2014;12:Doc15. doi: 10.3205/000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kogut C, Crouse EB, Vieweg WV, Hasnain M, Baranchuk A, Digby GC, et al. Selective serotonin reuptake inhibitors and torsade de pointes: New concepts and new directions derived from a systematic review of case reports. Ther Adv Drug Saf. 2013;4:189–98. doi: 10.1177/2042098613492366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopp R, Kunitz O, Baumert JH, Rossaint R. A patient with nodal tachycardia after general anesthesia during medication with a selective serotonin reuptake inhibitor. Anasthesiol Intensivmed Notfallmed Schmerzther. 2001;36:184–7. doi: 10.1055/s-2001-11822. [DOI] [PubMed] [Google Scholar]
- 35.Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem. 1999;6:469–80. [PubMed] [Google Scholar]
- 36.Andrade C. Selective serotonin reuptake inhibitor drug interactions in patients receiving statins. J Clin Psychiatry. 2014;75:e95–9. doi: 10.4088/JCP.13f08941. [DOI] [PubMed] [Google Scholar]
- 37.Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: A randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1999;33:1227–30. doi: 10.1016/s0735-1097(98)00694-9. [DOI] [PubMed] [Google Scholar]
- 38.Ventetuolo CE, Barr RG, Bluemke DA, Jain A, Delaney JA, Hundley WG, et al. Selective serotonin reuptake inhibitor use is associated with right ventricular structure and function: The MESA-right ventricle study. PLoS One. 2012;7:e30480. doi: 10.1371/journal.pone.0030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maréchaux S, Jeu A, Jobic Y, Ederhy S, Donal E, Réant P, et al. Impact of selective serotonin reuptake inhibitor therapy on heart valves in patients exposed to benfluorex: A multicentre study. Arch Cardiovasc Dis. 2013;106:349–56. doi: 10.1016/j.acvd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Westenberg HG, Sandner C. Tolerability and safety of fluvoxamine and other antidepressants. Int J Clin Pract. 2006;60:482–91. doi: 10.1111/j.1368-5031.2006.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16:101–8. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 42.Nezafati P, Shomali A, Nezafati MH. A simple modified Bentall technique for surgical reconstruction of the aortic root – Short and long term outcomes. J Cardiothorac Surg. 2015;10:132. doi: 10.1186/s13019-015-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nezafati P, Gharipour M, Nezafati MH. Surgical ablation for atrial fibrillation: An editorial. J Thorac Dis. 2015;7:E239–42. doi: 10.3978/j.issn.2072-1439.2015.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–53. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–31. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Himmerich H, Binder EB, Künzel HE, Schuld A, Lucae S, Uhr M, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axfis. Biol Psychiatry. 2006;60:882–8. doi: 10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 47.Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–90. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Narita K, Murata T, Takahashi T, Kosaka H, Omata N, Wada Y. Plasma levels of adiponectin and tumor necrosis factor-alpha in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1159–62. doi: 10.1016/j.pnpbp.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 49.Mast ST, Gersing KR, Anstrom KJ, Krishnan KR, Califf RM, Jollis JG. Association between selective serotonin-reuptake inhibitor therapy and heart valve regurgitation. Am J Cardiol. 2001;87:989–93. doi: 10.1016/s0002-9149(01)01435-7. [DOI] [PubMed] [Google Scholar]
- 50.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 51.Rothman RB, Zolkowska D, Baumann MH. Serotonin (5-HT) transporter ligands affect plasma 5-HT in rats. Ann N Y Acad Sci. 2008;1139:268–84. doi: 10.1196/annals.1432.042. [DOI] [PubMed] [Google Scholar]
- 52.Dorn GW., 2nd The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–70. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- 53.Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res. 1983;52:57–64. doi: 10.1161/01.res.52.1.57. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Marcocci L, Wong CM, Park AM, Suzuki YJ. Serotonin-mediated protein carbonylation in the right heart. Free Radic Biol Med. 2008;45:847–54. doi: 10.1016/j.freeradbiomed.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoeper MM, Welte T. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2006;354:1091–3. doi: 10.1056/NEJMc053442. [DOI] [PubMed] [Google Scholar]
- 56.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, et al. RhoA and Rho kinase activation in human pulmonary hypertension: Role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179:1151–8. doi: 10.1164/rccm.200805-691OC. [DOI] [PubMed] [Google Scholar]