Abstract
Purpose
Wilms tumour (WT), the most common paediatric renal malignancy, is associated with mutations in a number of well-characterised genes, most notably WT1, CTNNB1, WTX and TP53. However, the majority of cases do not harbour mutations in these genes. We hypothesised that additional drivers of tumour behaviour would be contained within areas of consistent genomic copy number change, especially those associated with the WT risk groups defined by the International Society of Paediatric Oncology (SIOP).
Experimental Design
We analysed high resolution (Affymetrix 250k SNP array) genomic copy number profiles of over 100 tumours from selected risk groups treated under the SIOP protocols, further characterising genes of interest by sequencing, MLPA, or FISH.
Results
We identified FBXW7, an E3 ubiquitin ligase component, as a novel Wilms tumour gene, mutated or deleted in approximately 4% of tumours examined. Strikingly, 3/14 (21%) of tumours with epithelial type histology after neoadjuvant chemotherapy had FBXW7 aberrations, while a fourth WT patient had germline mutations in both FBXW7 and WT1. We also showed that MYCN copy number gain, detected in 9/104 (8.7%) of cases, is relatively common in WT, and significantly more so in tumours of the high risk diffuse anaplastic subtype (6/19, 32%).
Conclusions
Since MYCN is itself a target of FBXW7-mediated ubiquitination and degradation, these results suggest that a common pathway is dysregulated by different mechanisms in various WT subtypes. Emerging therapies that target MYCN, which is amplified in several other paediatric cancers, may therefore be of value in high risk Wilms tumour.
Introduction
Wilms tumour (WT) is the most common paediatric renal malignancy. Somatic or germline mutations in several genes have been identified in WT over the last two decades. WT1, which encodes a zinc finger nucleic acid binding protein with multiple roles in gene regulation and development, is mutated or deleted in around 15% of Wilms tumours (reviewed in (1)). Gain of function mutations in WNT signalling component CTNNB1 (beta catenin) are found in a similar proportion of tumours (2), usually in combination with WT1 mutations (3-6). Mutation or loss of a third gene, WTX (FAM123B), occurs in up to 30% of sporadic WTs (7), independently of other aberrations (8). Epigenetic abnormalities at 11p15, affecting expression of H19 and IGF2, are found in significant numbers of Wilms tumours, including those cases associated with Beckwith-Wiedemann syndrome (9, 10). Increased WT incidence has also been linked with a number of other rare developmental disorders, some of which have defined genetic lesions that are occasionally found in sporadic tumours (11). TP53 mutations are largely restricted to the relatively uncommon anaplastic WT subtype (12). Taken together, however, somatic mutations in known Wilms tumour genes account for fewer than half of cases, and identifying the underlying drivers of the remainder is a problem of significant biological and therapeutic interest.
We have analysed copy number aberrations in samples from the International Society of Paediatric Oncology (SIOP) 2001 Wilms tumour clinical trial and study, aiming to identify novel changes associated with particular histological subtypes and clinical risk groups, or affecting loci of general relevance to Wilms tumour. Patients in this study typically receive a short period (4-6 weeks) of neoadjuvant chemotherapy prior to surgery, with risk groups defined by histology at nephrectomy, a direct measure of in vivo chemosensitivity (13). Our analysis has now detected recurrent focal aberrations targeting two genes whose dysregulation is known to be pathogenic in other cancers. FBXW7, a ubiquitin ligase component that acts as a tumour suppressor, is frequently mutated in cholangiocarcinoma and T-cell acute lymphoblastic leukaemia, and somewhat less commonly in a range of other malignancies (14). Here we identify FBXW7 as a novel Wilms tumour gene, which is deleted or mutated in approximately 4% of the tumours examined, and in which the aberrations so far detected are restricted to tumours with intermediate risk histologies. MYCN, a MYC family transcription factor and proto-oncogene, is amplified in neuroblastoma (15), where it is an established biomarker for treatment stratification (reviewed in (16, 17)), and in several other embryonal paediatric tumours. We show that genomic gain or amplification of MYCN, previously described in only a small number of individual WT cases, is relatively common, especially in tumours of the high-risk anaplastic subtype. Since both genes are linked by a common regulatory pathway, these results may have broader implications for the mechanisms of tumourigenesis in WT.
Results
Deletion of FBXW7 in Wilms tumour
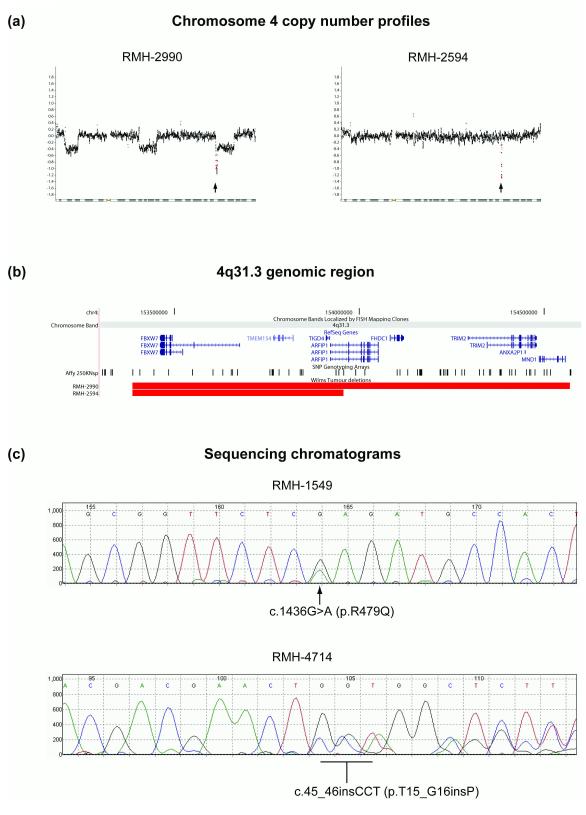
Analysis of the Affymetrix copy number data (104 tumours) revealed two samples with focal and apparently homozygous deletions on 4q31.1 (Figure 1a). In both samples, loss of 1p and gain of 1q were also observed. In one case (RMH-2594) the 4q31.1 deletion was the sole additional major abnormality detected in the genome at the resolution of the 250k Nsp array. In the second (RMH-2990), three further less focal aberrations consistent with heterozygous deletions were detected on chromosome 4, but no other major genomic changes were apparent. The focal 4q31.1 deletions in both samples contained the complete sequence of the FBXW7 gene. Although the focal aberration in RMH-2990 encompassed seven other RefSeq genes (Figure 1b), the more limited deletion in RMH-2594 (Figure 1b) included only two complete genes (TMEM154, TIGD4) and part of a third (ARFIP1) in addition to FBXW7. As FBXW7 is a well-characterised tumour suppressor gene (14), while TMEM154, TIGD4 and ARFIP1 have no established roles in cancer, we prioritised FBXW7 for further evaluation.
Figure 1.
FBXW7 mutations in Wilms tumour. (a) Chromosome 4 copy number profiles (log2 scale) for WT samples RMH-2990 and RMH-2594. The position of the FBXW7 gene is indicated with an arrow; FBXW7-specific probe positions are in red. (b) Extent of the 4q31.3 deletions relative to NCBI RefSeq genes and Affymetrix 250k SNP probe positions6. (c) Sequence chromatograms for FBXW7 mutations detected in WT samples RMH-1549 and RMH-4714.
Point mutation of FBXW7 in Wilms tumour
To determine if FBXW7 in WT is subject to point mutations or other abnormalities below the resolution of the Affymetrix 250k array, we sequenced the coding sequence of this gene in 88 samples, 77 of which had also been analysed on the array. All sequences were complete except in 7 cases, where data were not obtained for 1 or 2 of the 13 coding exons – see Supplementary Data. Two further mutants were detected (Figure 1c), in both cases in tumours with neutral copy number profiles (no major changes detected, not shown). In RMH-1549, a heterozygous G to A transition (c.1436G>A, relative to the alpha isoform) results in the substitution of Arg-479 with Gln (p.R479Q). Arg-479 is the second most common mutational hotspot in FBXW7 across a range of tumour types, and retention of the wild-type allele is typical of primary tumours harbouring such mutations (14). In RMH-4714, a heterozygous 3 base insertion (c.45_46insCCT, Figure 1c) introduces a proline at residue 16 (T15_G16insP) of the predominant FBXW7 alpha isoform, a mutation previously observed in prostate cancer (14).
Sequencing FBXW7 in matched peripheral blood DNA from the patients with tumours RMH-1549 and RMH-4714 revealed that the mutation in RMH-1549 was confined to the tumour, whereas the proline insertion in RMH-4714 was also present in the germline. It is also notable that both tumours with FBXW7 deletions detected on the array (RMH-2594 and RMH-2990) and the sample with the Arg-479 hotspot mutation (RMH-1549) were of the relatively uncommon epithelial sub-type, found in only 10% of the WTs classified according to the SIOP criteria for tumours resected after chemotherapy (13). This subtype is considered ‘intermediate risk’. Since only 14/77 of the jointly arrayed and sequenced samples were epithelial type, this result suggests that FBXW7 mutations exhibit specificity to this subtype (p = 0.018, Fisher’s exact test). The germline proline insertion (RMH-4714) was in a patient with stromal type histology, an intermediate risk subtype frequently associated with WT1 and CTNNB1 mutations (18-21). No FBXW7 aberrations were detected in tumours with high risk histologies, or in the mixed or regressive intermediate risk subtypes.
MYCN gain in Wilms tumour
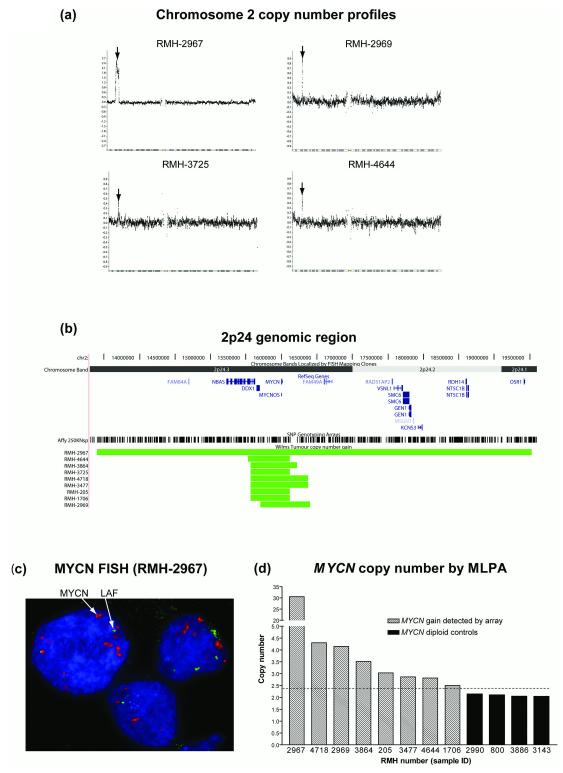
Nine samples had evidence of MYCN gain (examples in Figure 2a, extent of gain in Figure 2b). In six of these tumours, this was a focal (typically ~0.25Mb) low copy number event (typically consistent with single copy gain). In two cases (RMH-2969, RMH-4718) a somewhat higher level, but still focal, gain was observed. In the remaining sample (RMH-2967) a broader, high level amplification (>10 copies) was detected, and confirmed by FISH in 90/94 assessed nuclei of cells from a frozen tumour touch preparation (Figure 2c). The level of gain in eight of the nine samples was assessed by MLPA, which confirmed the array results (Figure 2d). Six of the nine samples, including those with the highest levels of gain, were of the high risk diffuse anaplastic subtype. One other (RMH-3725) was also classed as a high risk histology (blastemal type). The remaining samples (RMH-205, RMH-4644) were of intermediate risk mixed histology. Since only 19/104 of the tumours profiled were of the diffuse anaplastic subtype, and 6 of these (32%) had MYCN gain, these results indicate a significant association between anaplasia and this aberration (p = 0.001, Fisher’s exact test).
Figure 2.
MYCN gain in Wilms tumour. (a) Chromosome 2 copy number profiles (log2 scale) for four examples of WT with gain at the MCYN locus. The position of the MYCN gene is indicated with an arrow. (b) Extent of the 2p24.3 gains relative to NCBI RefSeq genes and Affymetrix 250k SNP probe positions6. (c) MYCN amplification (red) detected by FISH with reference to LAF copy number control on 2q (green). (d) MYCN copy number measured by MLPA (mean of two different MYCN-specific probes, linear scale) for eight samples with gain detected by the Affymetrix array, and four control samples without 2p24.3 aberrations in their array profiles. A copy number of 2.4 (1.2x diploid) was taken as the threshold of gain (dashed line).
Status of known WT genes in samples with FBXW7 or MYCN aberrations
To determine if other known WT genes are mutated in the samples with FBXW7 or MYCN aberrations, we examined the coding sequences of WT1 and WTX, and the exon 3 sequence of CTNNB1, in all four samples with FBXW7 deletion or mutation, and in 8 of the 9 samples with MYCN gain; sequence data were not available for RMH-3725 (see Supplementary Data). The SNP data for all 13 samples of interest were also examined for inferred loss of heterozygosity (tumour only, without reference to matched normal, (22)) at 11p13 and 11p15.5.
RMH-4714, the sample with a germline FBXW7 mutation, was found to have mutations in both WT1 and CTNNB1; no other WT1, CTNNB1 or WTX mutations were detected in this sample series. Two heterozygous WT1 mutations were found in RMH-4714: c.948_949insG, a single base insertion that introduces a frameshift in exon 2, and c.1568C>T (p.R390X, relative to the longest WT1 isoform translated from the ATG initiation codon at c.401), a single base substitution that introduces a stop codon in exon 9, terminating the protein in its third zinc finger and eliminating the fourth and final zinc finger. The c.1568C>T (p.R390X) mutation was also found in the germline, but c.948_949insG was confined to the tumour, potentially representing a second hit in the other allele (allelic specificity was not determined). Two CTNNB1 nucleotides were heterozygously mutated in RMH-4714 (tumour only), substituting adjacent amino acid residues: c.398C>G (p.P44A) and c.401T>C (p.S45P). Ser-45 is a mutational hotspot in WT and other tumours, while simultaneous P44A and S45P substitutions have been reported in WT and hepatocellular carcinoma (23, 24). RMH-1549 had evidence of loss of heterozygosity at 11p15.5, but not 11p13; none of the other 12 samples had inferred LOH in either of these regions.
Since the patient with tumour RMH-4714 carried germline mutations in both FBXW7 and WT1, we reviewed the clinical data for this case. Although not diagnosed with any known WT-associated syndrome or genitourinary abnormalities, the patient (a phenotypic and genetic XX female) had a younger than average age at diagnosis (14 months), and presented with bilateral renal masses, both features suggestive of genetic predisposition. The larger mass (11cm diameter) proved to be a Wilms tumour (RMH-4714). The smaller mass (2 cm diameter) in the contralateral kidney showed pronounced cystic change after chemotherapy, hence it could not be confirmed whether this may have been a WT precursor lesion (nephrogenic rest).
Histology of samples with FBXW7 or MYCN aberrations
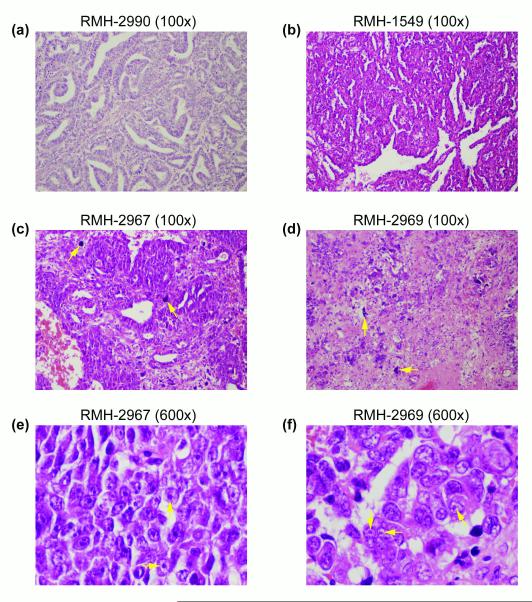
Since FBXW7 mutations were associated with epithelial histology, and MYCN gain was associated with diffuse anaplasia, we reviewed the pathology reports and (where available) formalin-fixed paraffin-embedded sections of the UK (CCLG) samples with aberrations in either gene to determine if they had specific distinguishing features in addition to those that defined their histological subtypes (see Supplementary Data). All three samples with FBXW7 aberrations, but no mutations in other WT genes (RMH-1549, RMH-2594, RMH-2990), had unambiguous epithelial-type histology (e.g. Figure 3a, 3b), consisting of 90-100% epithelial elements (tubules with a range of differentiation and tubulo-papillary structures). No specific features beyond those normally associated with epithelial-type histology were observed, though RMH-1549, the sample with a mutation at the R479Q hotspot, was more highly differentiated than the other two tumours. RMH-4714, the sample with mutations in WT1, CTNNB1 and FBXW7, was clearly of stromal type histology (~85% stromal elements), though some epithelial elements (~12.5%) were detectable (not shown). All 6 tumours with MYCN gain and diffuse anaplasia were confirmed to have the characteristic features of anaplastic WT, including nuclear enlargement, hyperchromasia and atypical mitotic figures (Figure 3c, 3d). However, the tumour with the highest level of MYCN gain, RMH-2967, also had some areas of rhabdoid-like features, including vesicular nuclei, very prominent nucleoli, and non-cohesive cells (Figure 3c, 3e). Similar but less prominent features were also evident in three of the remaining five MYCN+ anaplastic WTs (e.g. Figure 3d, 3f), but in only one of six UK anaplastic tumours without MYCN gain.
Figure 3.
Haematoxylin and eosin stained paraffin-embedded sections showing histology of tumours with FBXW7 or MYCN aberrations. (a) RMH-2990 (100x magnification). Epithelial type WT showing poorly and moderately differentiated tubules with some papillary structures. (b) RMH-1549 (100x). Epithelial type WT showing highly differentiated tubules. (c) RMH-2967 (100x). Diffuse anaplastic WT; examples of anaplastic cells are indicated with arrows. (d) RMH-2969 (100x) Diffuse anaplastic WT; examples of anaplastic cells are indicated with arrows. (e) RMH-2967 (600x). Diffuse anaplastic WT with areas of rhabdoid-like histology including prominent nucleoli (indicated with arrows). (f) RMH-2969 (600x). Diffuse anaplastic WT with areas of rhabdoid-like histology including prominent nucleoli (indicated with arrows).
Discussion
FBXW7 mutations have not previously been described in Wilms tumour. However, both point mutations detected here have been observed in other tumours. Arg-479 is a key surface residue in the FBXW7 WD40 repeat III, a component of the beta-propeller structure responsible for substrate recognition, and a frequent mutational hotspot in multiple tumour types (14). As in our cases, primary tumours with FBXW7 point mutations typically retain the wild-type allele (14). Such heterozygous mutants, in which substrate binding is disrupted while other interactions remain unaffected, are believed to act via haploinsufficient (25) or dominant negative (26) mechanisms, and there is direct evidence that R479Q has dominant negative activity (14).
The T15_G16insP insertion has previously been reported in a single prostate tumour, functionally tested in an embryonic kidney cell line system, and found to abolish nuclear localisation of the protein and its binding to a known substrate, CCNE1 (Cyclin E) (14). Though the potential dominant negative activity of the mutant protein was not assessed, it retained the ability to interact with known co-factors, a property consistent with a dominant negative mechanism of action. The minor beta and gamma FBXW7 isoforms would not be affected by this insertion, but neither has the ability to localise to the nucleoplasm and interact with nuclear substrates like Cyclin E even in the wild type form, and so could not compensate for the mutation. While T15_G16insP in RMH-4714 is a germline mutation, the functional data, together with the evolutionary conservation of adjacent T15 and G16 residues in most vertebrate species from primates to Fugu (not shown), strongly suggest a deleterious mutation rather than a rare polymorphism; no insertions at this position have been reported in NCBI dbSNP (build 130).
FBXW7, as an important tumour suppressor gene, is likely to be the primary, and potentially sole significant, target of the focal homozygous deletions in RMH-2594 and RMH-2990. Similar aberrations are found in T-cell acute lymphoblastic leukaemia (T-ALL), a tumour type with a high frequency of FBXW7 point mutations. Our analysis of a published series of 50 T-ALL Affymetrix SNP arrays (NCBI GEO accession GSE9113, (27)) revealed four tumours with deletions that included FBXW7, two of which were less focal than those we detected in WT (not shown), while a recent report (28) also describes ‘discrete’ deletions in T-All samples at this locus. These results suggest that FBXW7 is targeted by both point mutations and deletions in tumour types where it is commonly inactivated. No such deletions were detected when we examined publicly available data (GEO accession GSE12494, (29), not shown) from a large SNP array series derived from neuroblastoma, a tumour in which FBXW7 point mutations have not been described. Nevertheless, we have not yet ruled out the potential contribution to the tumour phenotype of deletion of the (2-7) immediately adjacent genes in our samples. Other loci on 4q may also be significant in WT. An earlier study (30) has reported loss of heterozygosity on 4q, including a region on 4q24-25 that does not overlap with FBXW7, while our own data on multiple genomic array platforms (not shown) suggest that loss of the entire 4q chromosome arm is relatively frequent in WT.
The potential association between FBXW7 status and histological subtype we observed is intriguing. All three cases that had FBXW7 aberrations but no mutations in the three other Wilms tumour genes examined belonged to the relatively uncommon epithelial subtype, and the association between histology and FBXW7 status was significant. It may be relevant that deletion of a single copy of Fbxw7 in a Tp53-hemizygous mouse model results in a shift of the spectrum of observed malignancies towards tumours of epithelial tissues (25). The single tumour with mutations in FBXW7, WT1, and CTNNB1 had stromal-type histology at nephrectomy. Mutations in both WT1 and CTNNB1 (frequently together, as here) have previously been associated with this histology (18-21). We may speculate that in situations where both the WT1/CTNNB1 and FBXW7 axes are disrupted, the stromal phenotype associated with the former is dominant, overriding any tendency to develop epithelial predominance mediated by the latter. We also note that simultaneous mutation of WT1 and FBXW7 has recently been described in T-ALL (28), though the functional significance of this result is unclear, as the mutation frequencies of the two genes appear to be unrelated. The presence of a germline FBXW7 mutation in the patient with aberrations in all three genes, and an abnormality in the contralateral kidney, raises the possibility that disruption of FBXW7 may contribute to tumour predisposition. However, since one of the two WT1 mutations in the tumour is also present in the germline, it is not possible to separate the potential role of contribution of FBXW7 from the known role of WT1.
Earlier studies have reported MYCN gain or amplification in a small number of individual cases (31-33), though the data were too limited to test for potential associations with subtype. The present study is the first to analyse a WT series including a relatively large number of anaplastic tumours on a high resolution SNP platform. Our finding of an association between MYCN gain and a high risk histology in Wilms tumour is in keeping with emerging evidence that elevated MYCN copy number, originally described in neuroblastoma (15) and used in risk stratification (16, 17), is associated with adverse outcome in other childhood tumours, including rhabdomyosarcoma (34) and medulloblastoma (35, 36). MYCN amplification is also observed in retinoblastoma (37), though existing data do not demonstrate a clinical correlation (38). Six of the nine patients in whose WT samples we detected MYCN gain suffered a relapse, and five died. Two had intermediate risk mixed histology tumours and four had high risk histology, including one blastemal type and three anaplastic tumours. Samples from these three anaplastic cases had the highest levels of MYCN gain in the series; the three remaining anaplastic cases with MYCN aberrations had lower levels of gain and did not relapse. On histological review, the three samples with the highest level of gain were found to have areas containing rhabdoid-like features on a background of diffuse anaplasia. When the detailed histology of all twelve UK anaplastic samples was reviewed, rhabdoid-like features were detected in two additional samples, one (with MYCN gain) from a patient who had not relapsed, and the other (without MYCN gain) from a patient who had relapsed and died. A much larger WT series would be required to determine if MYCN gain in combination with anaplasia is associated with a significantly worse outcome than anaplasia alone, or if the rhabdoid-like features that appeared more common in the subset of anaplastic tumours with MYCN gain are prognostically significant.
The current data for both genes are largely confined to European nephrectomy samples from patients treated under current SIOP protocols. These tumours have therefore been exposed to pre-operative chemotherapy, and represent histological risk groups defined by their in vivo chemosensitivity (13). These groups have no direct equivalents in North American WT series from patients treated under Childhood Oncology Group (COG) protocols, who are typically chemo-naïve at resection.
However, features such as anaplasia and the predominance of particular cell types (e.g. ‘epithelial predominance’) are recognised in individual immediate nephrectomy samples from COG patients. It would be interesting to conduct a similar analysis in samples drawn from a chemo-naïve series, to determine if the apparent subtype-specificity we have observed is restricted to classes defined by initial chemotherapy (where additional mechanisms of selection for subclones carrying the aberrations we detected may operate), or is more broadly applicable to tumour classes characterised by specific histological features, irrespective of chemotherapy.
Though genomic aberrations in FBXW7 or MYCN are present in only a minority of tumours, other evidence suggests that mechanisms that affect pathways involving these genes are of more general importance in WT. Overexpression of MYCN is a recognised feature of WT (39-41), and its relative expression level has been identified as a significant clinical prognostic marker (42, 43). FBXW7 is a component of an SCF (SKP1/CUL1/FBXW7) E3 ubiquitin ligase complex, which targets a number of key dominant proto-oncogene products for ubiquitination and subsequent proteasome-mediated degradation (26), with mutations leading to accumulation of its targets. It is therefore suggestive that the protein level of one of these targets, Cyclin E, has been shown to correlate with WT aggressiveness and metastasis (44). Intriguingly, MYCN itself was recently reported to be a direct target of the FBXW7 SCF complex (45). This implies that loss of FBXW7 or gain of MYCN could have overlapping downstream effects, though the association of these alterations with different histological risk groups suggests a more complex picture. MYCN is only one of several known targets of FBXW7, so that disruption of the latter could impact on multiple pathways unaffected by copy number changes in the former, and it is unclear if dysregulation of MYCN levels by inactivation of FBXW7 would affect MYCN downstream targets to the same degree as direct copy number changes at the MYCN locus. The timing of mutational events and the broader pattern of concomitant genomic changes may also be significant. We detected FBXW7 aberrations in tumours with relatively stable genomes and in one case identified a germline change, features that suggest that disruption of this gene is a relatively early event. In contrast, the majority of MYCN gains were observed in anaplastic tumours with a background of widespread genomic disruption, features suggestive of a relatively late stage of tumour development.
Our results also have implications for the development of future therapeutic strategies. Although WT in general has an excellent response to treatment, anaplastic tumours that are metastatic at diagnosis or that recur remain largely intractable to current therapies. Genomic gain of a biomarker significantly associated with this high risk histology, and with known oncogenic activity, therefore presents a potentially attractive target for novel treatment agents. Emerging therapies directed at MYCN function in other tumours, particularly neuroblastoma (46), could therefore be of significant clinical value. The link between the FBXW7 and MYCN pathways suggests further possibilities for intervention. For example, the finding that AURKA, itself a known FBXW7 substrate, plays a critical role in sequestering MYCN from FBXW7-mediated degradation (45), highlights one class of interactions that could be targetable. As knowledge of these pathways continues to develop, so should the potential for more effective treatment of the Wilms tumour patients with the poorest prognosis.
Materials and Methods
Frozen tumour obtained from post-chemotherapy nephrectomy specimens was collected from 104 patients treated in the SIOP WT 2001 clinical trial at UK (n = 85) and German (n = 19) centres. Since the availability of suitable frozen material that meets quality control standards for array analysis depends on multiple factors, including the pathology practices at each clinical centre, samples were not consecutive within the trial or by histological risk group. A pragmatic approach to sample selection was taken, based on ensuring that approximately equal numbers of tumours in each of the major histological groups of the SIOP 2001 classification (13) were represented (see Supplementary Data), resulting in a sample series enriched in some of the rarer subtypes. These samples were supplemented by 5 tumours from other sources in the FBXW7 sequencing analysis only. Patient consent and ethical approval were obtained for the use of all samples. A section from each sample was reviewed by a paediatric pathologist to confirm tumour content without significant contamination by normal tissue. DNA was extracted from frozen material by standard protocols, and profiled at UCL Genomics on the Affymetrix Human Mapping 250k Nsp SNP array according to the manufacturer’s instructions. Data were processed for copy number estimation using the Affymetrix CNAT 4.0 package, with quantile normalisation and a 0.1Mb Gaussian smoothing window. Normal control data from the 30 Caucasian CEPH trio mothers1 was obtained from the HapMap consortium2, and used as the baseline for copy number comparisons. The copy number data set was processed and annotated for downstream analysis with custom Perl scripts3 and visualised in CGH Explorer (47)4. Data were processed for inferred LOH analysis with dChip (22)5.
The FBXW7, WT1, and WTX coding sequences, and the CTNNB1 exon 3 sequence, were amplified from tumour samples by standard methods (see Supplementary Data for primers). Unidirectional sequencing using one primer from each pair and the Applied Biosystems BigDye system was used for initial mutation detection; all candidate mutations were confirmed by bidirectional sequencing.
The Kreatech Poseidon MYCN (2p24) & LAF (2q11) fluorescence in situ hybridization (FISH) probe set (Kreatech Diagnostics, Amsterdam) was used to detect MYCN copy number with reference to the LAF locus (control) according to the manufacturer’s instructions. The P252-NB Multiplex Ligation-dependent Probe Amplification (MLPA) kit (MRC-Holland, Amsterdam) was used to measure MYCN copy number by the NHS Northern Genetics Service, Newcastle upon Tyne.
For detailed examination of histology, formalin-fixed, paraffin-embedded tissue sections were prepared and stained with haematoxylin and eosin by standard methods.
Supplementary Material
Translational relevance.
Wilms tumours treated with pre-operative chemotherapy according to SIOP protocols are classified by histological subtype at resection. This reflects in vivo chemosensitivity and predicts subsequent prognosis but has a poorly defined molecular basis. We have identified FBXW7 as a new WT gene, mutations of which are associated with epithelial type (intermediate risk) tumour histology, and have demonstrated that copy number gain of MYCN is associated with anaplastic (high risk) histology. FBXW7 mediates degradation of MYCN, whose overexpression at the protein level is linked with poor outcome in WT. Hence, these results implicate a common oncogenic pathway that could be targeted by new therapeutic agents, such as those being developed to inhibit MYCN function in neuroblastoma. Since anaplastic WT, in its metastatic or recurrent form, remains largely intractable to current therapies, the identification of a potential target associated with this histology is of high clinical relevance.
Acknowledgements
Grant support: Cancer Research UK grant C1188/A4614.
We thank the families, clinicians and pathologists who contributed the samples used in this study to the Children’s Cancer and Leukaemia Group Tumour Bank, UK, and the Paediatric Tumour Cell Bank of the Society of Paediatric Oncology and Haematology, Germany. We thank Clare Bedwell and Nick Bown, NHS Northern Genetics Service, Newcastle upon Tyne, for the MLPA analysis. This work was supported by Cancer Research UK and the Royal Marsden Hospital Children’s Fund. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
browser tracks from http://genome.ucsc.edu
References
- 1.Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- 2.Koesters R, Ridder R, Kopp-Schneider A, et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms’ tumors. Cancer Res. 1999;59:3880–2. [PubMed] [Google Scholar]
- 3.Li CM, Kim CE, Margolin AA, et al. CTNNB1 mutations and overexpression of Wnt/beta-catenin target genes in WT1-mutant Wilms’ tumors. Am J Pathol. 2004;165:1943–53. doi: 10.1016/s0002-9440(10)63246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiti S, Alam R, Amos CI, Huff V. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res. 2000;60:6288–92. [PubMed] [Google Scholar]
- 5.Royer-Pokora B, Weirich A, Schumacher V, et al. Clinical relevance of mutations in the Wilms tumor suppressor 1 gene WT1 and the cadherin-associated protein beta1 gene CTNNB1 for patients with Wilms tumors: results of long-term surveillance of 71 patients from International Society of Pediatric Oncology Study 9/Society for Pediatric Oncology. Cancer. 2008;113:1080–9. doi: 10.1002/cncr.23672. [DOI] [PubMed] [Google Scholar]
- 6.Ruteshouser EC, Hendrickson BW, Colella S, Krahe R, Pinto L, Huff V. Genome-wide loss of heterozygosity analysis of WT1-wild-type and WT1-mutant Wilms tumors. Genes Chromosomes Cancer. 2005;43:172–80. doi: 10.1002/gcc.20169. [DOI] [PubMed] [Google Scholar]
- 7.Rivera MN, Kim WJ, Wells J, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642–5. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- 8.Ruteshouser EC, Robinson SM, Huff V. Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer. 2008;47:461–70. doi: 10.1002/gcc.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa O, Eccles MR, Szeto J, et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature. 1993;362:749–51. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- 10.Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–9. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 11.Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43:705–15. doi: 10.1136/jmg.2006.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardeesy N, Falkoff D, Petruzzi MJ, et al. Anaplastic Wilms’ tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994;7:91–7. doi: 10.1038/ng0594-91. [DOI] [PubMed] [Google Scholar]
- 13.Vujanic GM, Sandstedt B, Harms D, Kelsey A, Leuschner I, de Kraker J. Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med Pediatr Oncol. 2002;38:79–82. doi: 10.1002/mpo.1276. [DOI] [PubMed] [Google Scholar]
- 14.Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–12. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 15.Schwab M, Alitalo K, Klempnauer KH, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305:245–8. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Spitz R, Oberthur A, Westermann F, Berthold F. Risk estimation of neuroblastoma patients using molecular markers. Klin Padiatr. 2008;220:137–46. doi: 10.1055/s-2008-1065345. [DOI] [PubMed] [Google Scholar]
- 17.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- 18.Corbin M, de Reynies A, Rickman DS, et al. WNT/beta-catenin pathway activation in Wilms tumors: a unifying mechanism with multiple entries? Genes Chromosomes Cancer. 2009;48:816–27. doi: 10.1002/gcc.20686. [DOI] [PubMed] [Google Scholar]
- 19.Fukuzawa R, Heathcott RW, Sano M, Morison IM, Yun K, Reeve AE. Myogenesis in Wilms’ tumors is associated with mutations of the WT1 gene and activation of Bcl-2 and the Wnt signaling pathway. Pediatr Dev Pathol. 2004;7:125–37. doi: 10.1007/s10024-003-3023-8. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher V, Schneider S, Figge A, et al. Correlation of germ-line mutations and two-hit inactivation of the WT1 gene with Wilms tumors of stromal-predominant histology. Proc Natl Acad Sci U S A. 1997;94:3972–7. doi: 10.1073/pnas.94.8.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher V, Schuhen S, Sonner S, et al. Two molecular subgroups of Wilms’ tumors with or without WT1 mutations. Clin Cancer Res. 2003;9:2005–14. [PubMed] [Google Scholar]
- 22.Beroukhim R, Lin M, Park Y, et al. Inferring loss-of-heterozygosity from unpaired tumors using high-density oligonucleotide SNP arrays. PLoS Comput Biol. 2006;2:e41. doi: 10.1371/journal.pcbi.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austinat M, Dunsch R, Wittekind C, Tannapfel A, Gebhardt R, Gaunitz F. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol Cancer. 2008;7:21. doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haruta M, Arai Y, Sugawara W, et al. Duplication of paternal IGF2 or loss of maternal IGF2 imprinting occurs in half of Wilms tumors with various structural WT1 abnormalities. Genes Chromosomes Cancer. 2008;47:712–27. doi: 10.1002/gcc.20572. [DOI] [PubMed] [Google Scholar]
- 25.Mao JH, Perez-Losada J, Wu D, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–9. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 26.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 27.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 28.Tosello V, Mansour MR, Barnes K, et al. WT1 mutations in T-ALL. Blood. 2009;114:1038–45. doi: 10.1182/blood-2008-12-192039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 30.Yuan E, Li CM, Yamashiro DJ, et al. Genomic profiling maps loss of heterozygosity and defines the timing and stage dependence of epigenetic and genetic events in Wilms’ tumors. Mol Cancer Res. 2005;3:493–502. doi: 10.1158/1541-7786.MCR-05-0082. [DOI] [PubMed] [Google Scholar]
- 31.McQuaid S, O’Meara A. N-myc oncogene amplification in paediatric tumours. Ir J Med Sci. 1990;159:172–4. doi: 10.1007/BF02937236. [DOI] [PubMed] [Google Scholar]
- 32.Norris MD, Brian MJ, Vowels MR, Stewart BW. N-myc amplification in Wilms’ tumor. Cancer Genet Cytogenet. 1988;30:187–9. doi: 10.1016/0165-4608(88)90112-4. [DOI] [PubMed] [Google Scholar]
- 33.Schaub R, Burger A, Bausch D, Niggli FK, Schafer BW, Betts DR. Array comparative genomic hybridization reveals unbalanced gain of the MYCN region in Wilms tumors. Cancer Genet Cytogenet. 2007;172:61–5. doi: 10.1016/j.cancergencyto.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Williamson D, Lu YJ, Gordon T, et al. Relationship between MYCN copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J Clin Oncol. 2005;23:880–8. doi: 10.1200/JCO.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 35.Aldosari N, Bigner SH, Burger PC, et al. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch Pathol Lab Med. 2002;126:540–4. doi: 10.5858/2002-126-0540-MAMOAI. [DOI] [PubMed] [Google Scholar]
- 36.Pfister S, Remke M, Benner A, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–36. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 37.Lee WH, Murphree AL, Benedict WF. Expression and amplification of the N-myc gene in primary retinoblastoma. Nature. 1984;309:458–60. doi: 10.1038/309458a0. [DOI] [PubMed] [Google Scholar]
- 38.Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46:617–34. doi: 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- 39.Nisen PD, Rich MA, Gloster E, et al. N-myc oncogene expression in histopathologically unrelated bilateral pediatric renal tumors. Cancer. 1988;61:1821–6. doi: 10.1002/1097-0142(19880501)61:9<1821::aid-cncr2820610917>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Shaw AP, Poirier V, Tyler S, Mott M, Berry J, Maitland NJ. Expression of the N-myc oncogene in Wilms’ tumour and related tissues. Oncogene. 1988;3:143–9. [PubMed] [Google Scholar]
- 41.Williams RD, Hing SN, Greer BT, et al. Prognostic classification of relapsing favorable histology Wilms tumor using cDNA microarray expression profiling and support vector machines. Genes Chromosomes Cancer. 2004;41:65–79. doi: 10.1002/gcc.20060. [DOI] [PubMed] [Google Scholar]
- 42.Wittmann S, Wunder C, Zirn B, et al. New prognostic markers revealed by evaluation of genes correlated with clinical parameters in Wilms tumors. Genes Chromosomes Cancer. 2008;47:386–95. doi: 10.1002/gcc.20544. [DOI] [PubMed] [Google Scholar]
- 43.Zirn B, Hartmann O, Samans B, et al. Expression profiling of Wilms tumors reveals new candidate genes for different clinical parameters. Int J Cancer. 2006;118:1954–62. doi: 10.1002/ijc.21564. [DOI] [PubMed] [Google Scholar]
- 44.Berrebi D, Leclerc J, Schleiermacher G, et al. High cyclin E staining index in blastemal, stromal or epithelial cells is correlated with tumor aggressiveness in patients with nephroblastoma. PLoS ONE. 2008;3:e2216. doi: 10.1371/journal.pone.0002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Tonini GP, Pistoia V. Molecularly guided therapy of neuroblastoma: a review of different approaches. Curr Pharm Des. 2006;12:2303–17. doi: 10.2174/138161206777585193. [DOI] [PubMed] [Google Scholar]
- 47.Lingjaerde OC, Baumbusch LO, Liestol K, Glad IK, Borresen-Dale AL. CGH-Explorer: a program for analysis of array-CGH data. Bioinformatics. 2005;21:821–2. doi: 10.1093/bioinformatics/bti113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.