Abstract
Importance
Cognitive delay is the most common form of impairment among children born very preterm (VPT) at 32 weeks or less or with very low birth weight (VLBW) of 1250 g or less. It is important to identify factors that are robust predictors of long-term outcome because the ability to predict future prognosis will assist in health care and educational service planning and provision.
Objective
To identify prognostic factors for poor cognitive development in children born VPT or with VLBW.
Evidence Review
A systematic review was conducted using MEDLINE, EMBASE, and PyscINFO databases to identify studies published between January 1, 1990, and June 1, 2014, reporting multivariable prediction models for neurodevelopment in VPT or VLBW children. Thirty-one studies comprising 98 risk factor models for cognitive outcome were identified. Two independent reviewers extracted key information on study design, outcome definition, risk factor selection, model development, and reporting and conducted a risk-of-bias assessment.
Findings
There was evidence that male sex, nonwhite race/ethnicity, lower level of parental education, and lower birth weight were predictive of global cognitive impairment in children younger than 5 years. In older children, only the influence of parental education was sustained. Male sex was also predictive of language impairment in early infancy, but not in middle childhood. Gestational age was a poor predictor of cognitive outcome, probably because of a reduced discriminatory power in cohorts restricted to a narrow gestational age range. The prognostic value of neonatal brain injury was unclear; however, studies adopted mixed strategies for managing children with physical or neurosensory disability.
Conclusions and Relevance
The influence of perinatal risk factors on cognitive development of VPT or VLBW children appears to diminish over time as environmental factors become more important. It is difficult to isolate cognitive outcomes from motor and neurosensory impairment, and the strategy for dealing with untestable children has implications for risk prediction.
This is the first article from a comprehensive systematic review of risk factor analyses for poor neurodevelopmental outcomes in very preterm (VPT) (≤32 weeks) or very low birth weight (VLBW) (≤1250 g) survivors. The objective of this comprehensive review was to consolidate the evidence on the risk of impairment in the domains of cognition, motor function, behavior, hearing, and vision, to inform future prognostic research. The focus of this first article is to identify risk factors that are robust predictors of impaired cognitive function, including language skills, executive function, and academic attainment, as well as global IQ.
Prematurity has a pervasive effect on all neurodevelopmental domains. However, while cerebral palsy (CP) and neurosensory disorders such as deafness and blindness can have a severe effect on development, cognitive impairments are by far the most prevalent sequelae in the VPT or VLBW population. Cognitive delay has been reported to be as high as 40% at school age among extremely preterm (EPT) children born at less than 28 weeks’ gestation.1–3 The IQ scores at school age of preterm children without severe disability have consistently been found to be lower than those of their term control subjects and related to gestational age (GA) at birth.4
In addition to being at increased risk of global cognitive impairment, VPT or VLBW children are more likely to perform less well on tests of attention and executive function compared with their full-term peers,5 even after adjusting for IQ.6–8 They also have a higher rate of language problems in both the expressive and receptive domains that persists into middle childhood.9 Problems with cognitive and language development mean that many VPT or VLBW survivors are at high risk of poor academic attainment and reduced lifelong earning potential and life chances. A significant proportion require full-time specialist education, and most of those in mainstream education require specialist academic, health, or behavioral support services to aid their transition through school.10
There is likely to be a complex relationship between cognitive function, biological and environmental factors, and clinical events during and after the perinatal period of a VPT birth. To help promote optimal development, the contribution of all these factors to risk needs to be determined. The objective of this review article was to summarize published multivariable outcome prediction models that aim to identify the combination of factors most strongly associated with cognitive impairment in early infancy and later childhood.
Methods
The methods for the overall systematic review of poor neurodevelopment have been previously published in a review protocol, available at http://www.crd.york.ac.uk/PROSPERO. The registration number is CRD42014006943.
Search Strategy
Three electronic search strategies were devised in MEDLINE, EMBASE, and PsycINFO databases (eBoxes 1, 2, and 3 in the Supplement) using the National Institutes of Health Medical Subject Headings. The searches identified any journal articles published between January 1, 1990, and June 1, 2014, reporting a multivariable risk prediction model for a neurodevelopmental outcome assessed after age 18 months in VPT or VLBW children. No language restrictions were made. The bibliographies of all articles included for data extraction were hand searched for further eligible articles.
Eligibility Criteria
Articles were included in the review if they satisfied the following eligibility criteria: (1) they contained original data; (2) the study population was born after January 1, 1990; (3) the study population was 32 weeks’ GA or younger or with birth weight of 1250 g or less and not a highly select group (based on other clinical criteria); and (4) one objective was to perform a multivariable risk factor analysis (>2 variables) of a neurodevelopmental outcome assessed after 18 months of age. Explanatory prognostic factor studies that investigated the causal pathway between a single prognostic factor and an outcome to estimate effect size were not included in the review. Current guidelines recommend not combining these 2 distinct types of study because their objectives and model-building strategies differ and could lead to biased results if synthesized.11,12
Data Extraction
All articles identified by the search strategies were screened on title and abstract for definite exclusions and duplicates (screen 1). For the remaining articles, the full text was retrieved, and the inclusion criteria were applied (screen 2). The 2 screens were performed by the first author (L.L.) in the first instance, but if there was uncertainty about the eligibility of an article, it was screened independently by the second author (R.M.). If a decision could not be reached, the article was referred to the rest of the author review team (J.M., J.J.K., and N.M.). Non–English-language articles included in the review were fully translated. Multiple articles based on the same cohort of children underwent a panel review (by L.L., R.M., and N.M.). Articles reporting the same outcome domain (cognition, motor function, behavior, hearing, and vision) at the same age at assessment (<5 years and ≥5 years) were assessed on relevance to the review, and only one article was selected for data extraction. For all articles eligible for inclusion, both reviewers (L.L. and R.M.) independently completed a full data extraction form and risk-of-bias assessment on a customized database (Access 2010; Microsoft Corporation). These were cross-validated for discrepancies and were referred to the rest of the author review team if agreement could not be reached.
Risk-of-Bias Assessment
Overwhelming evidence shows that the conduct and reporting of published articles describing the development or validation prediction models are poor,13 which has led to the creation of quality assessment tools specific for these types of studies. In this review, the quality of studies was assessed according to a modified version of the Quality in Prognosis Studies tool,14 which is a standardized set of criteria recommended for use in reviews of prognosis (eTable 1 in the Supplement). The tool focuses on the following 6 areas of potential bias pertinent to studies of prognosis: study participation, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and statistical analysis. Studies were graded as yes, partly, or no for each bias domain and were classified as having a low to moderate risk of bias if they were graded as yes or partly in all 6 bias domains and moderate to high risk of bias otherwise.
Data Synthesis and Reporting
The results were presented in accord with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.15 Risk factors that were statistically significant (P < .05) in the final model were reported for each study. Studies were grouped according to the type of outcome studied (global cognitive function measured by a general cognitive test or IQ score, language, executive function, and academic attainment) and according to the age at assessment (<5 years and ≥5 years). This is because assessments in early infancy can be unreliable and are more crude measurements of cognitive development that rely to some extent on motor function, whereas assessments in later childhood measure higher-order cognitive functioning; therefore, risk factors may differ.A risk factor was presented graphically if it was statistically significant in the final model of at least 1 study with low to moderate risk of bias and was included in the final model of at least 2 other studies (including studies with moderate to high risk of bias) within the same outcome domain. The plots display the number and quality of all studies that entered each risk factor into the final model and whether the risk factor was reported as a significant predictor or as nonsignificant. Because no clear conclusions could be made about risk factors considered in the final model of only 1 or 2 studies, the graphs were truncated at this point because they become noninformative.
Results
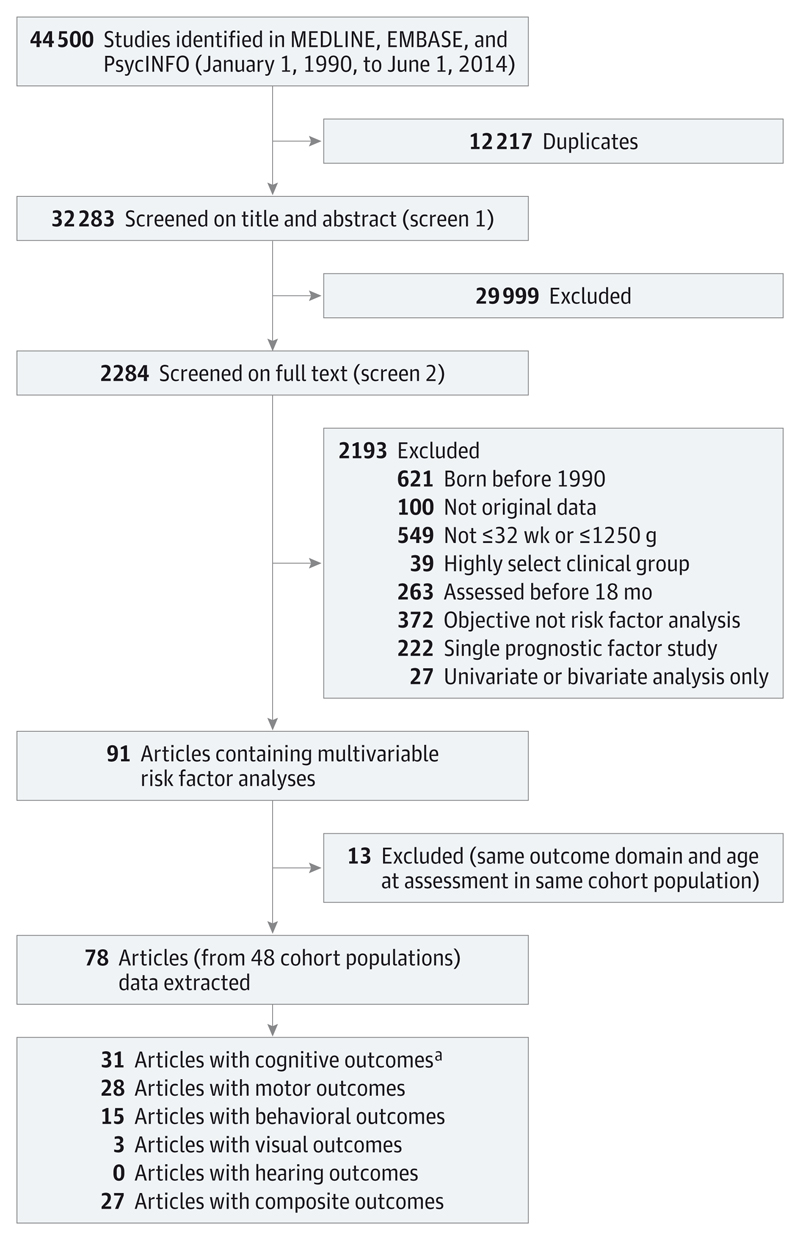
The search strategy for the comprehensive systematic review retrieved 44 500 articles, and after removing duplicates, the first screen on title and abstract was performed on 32 283 articles (Figure 1). For 29 999, the title or abstract clearly indicated that the topic of the article was not relevant to the review question or did not satisfy one of the inclusion criteria. The remaining 2284 articles were screened on full text, applying the full set of eligibility criteria. Eligibility was unclear in 136 (6%), and were reviewed by the second independent reviewer (R.M.), or the author was contacted (if uncertainty was because of missing information). After applying the eligibility criteria, 91 articles (from 48 cohort populations) containing multivariable risk factor analyses were eligible for inclusion. Following panel review, a further 13 articles were excluded because they reported the same outcome domain at the same age at assessment in the same cohort as another article with a more relevant objective. Five of the articles excluded because of cohort overlap were based on cognitive outcomes.8,16–19 The remaining 78 articles were included in the data extraction for the comprehensive systematic review.No further articles were identified in the hand search of bibliographies. This review article summarizes the results of the 31 studies20–50 reporting risk factor analyses for cognitive outcomes. These 31 studies were based on 21 independent cohort populations and reported a total of 98 risk factor models.
Figure 1. Flow Diagram.
a Reviewed in this article.
Study Characteristics
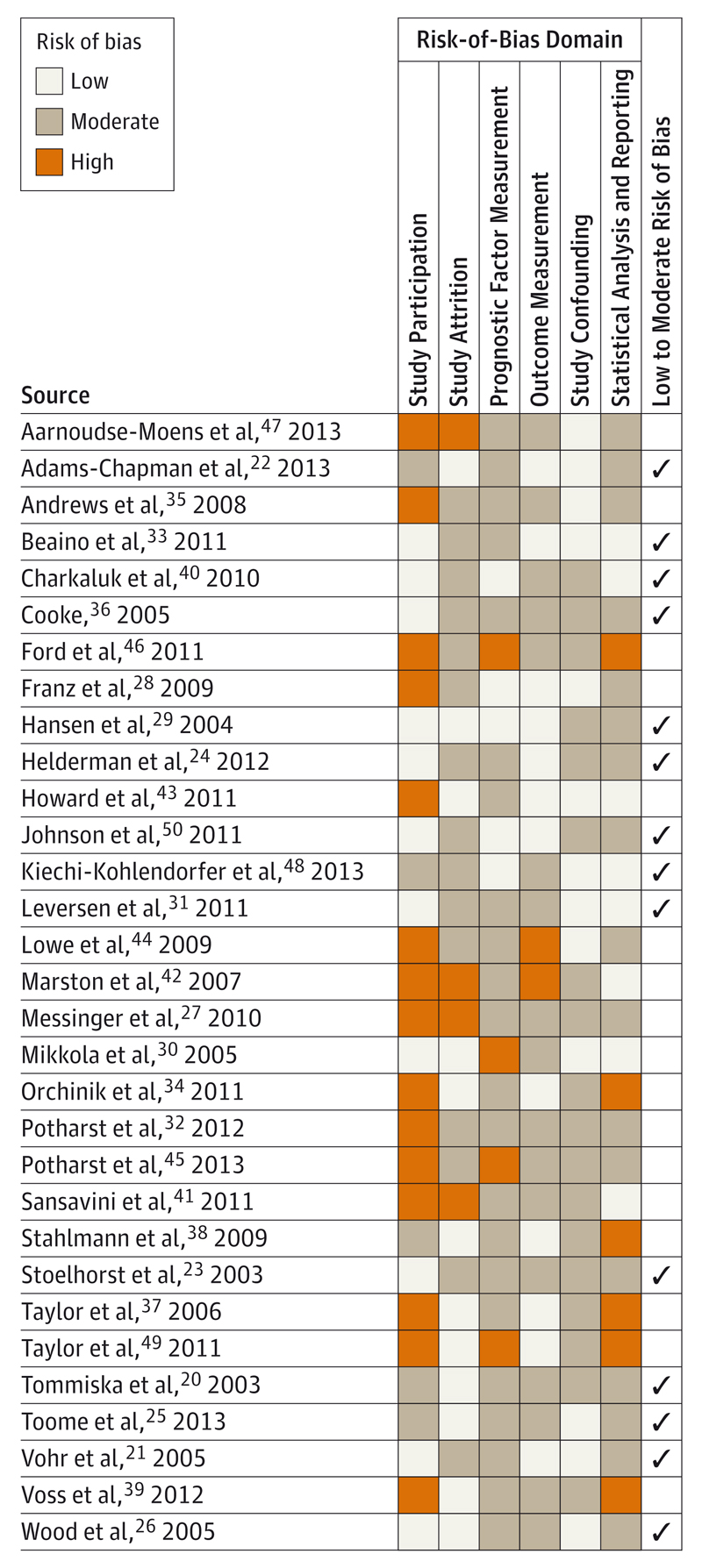
The main study design was prospective cohort(n = 27). There was also one cross-sectional study46 and 3 randomized clinical trial populations.27,42,44 Of the 27 prospective cohorts, 12 were ascertained from all live births in a geographically defined region,* and 10 were recruited from a single-center neonatal intensive care unit.† Studies were conducted in 12 countries, including the United States (n = 9), United Kingdom (n = 4), Netherlands (n = 4), Germany (n = 3), Australia (n = 2), Finland (n = 2), and France (n = 2) and 1 study each from Austria, Denmark, Estonia, Italy, and Norway. The median sample size was 219 (range, 45-3785), and 3 studies21,22,33 had more than 1000 participants. Four studies24,26,38,50 were restricted to EPT children, and 3 studies35,44,47 excluded multiple births. The risk-of-bias assessment classified 14 studies (45%) as low to moderate risk of bias and 17 studies (55%) as moderate to high risk of bias (Figure 2).
Figure 2. Risk-of-Bias Assessment.
Shown are 31 studies comprising 98 risk factor models for cognitive outcome.
Prognostic Factors for Global Cognitive Impairment
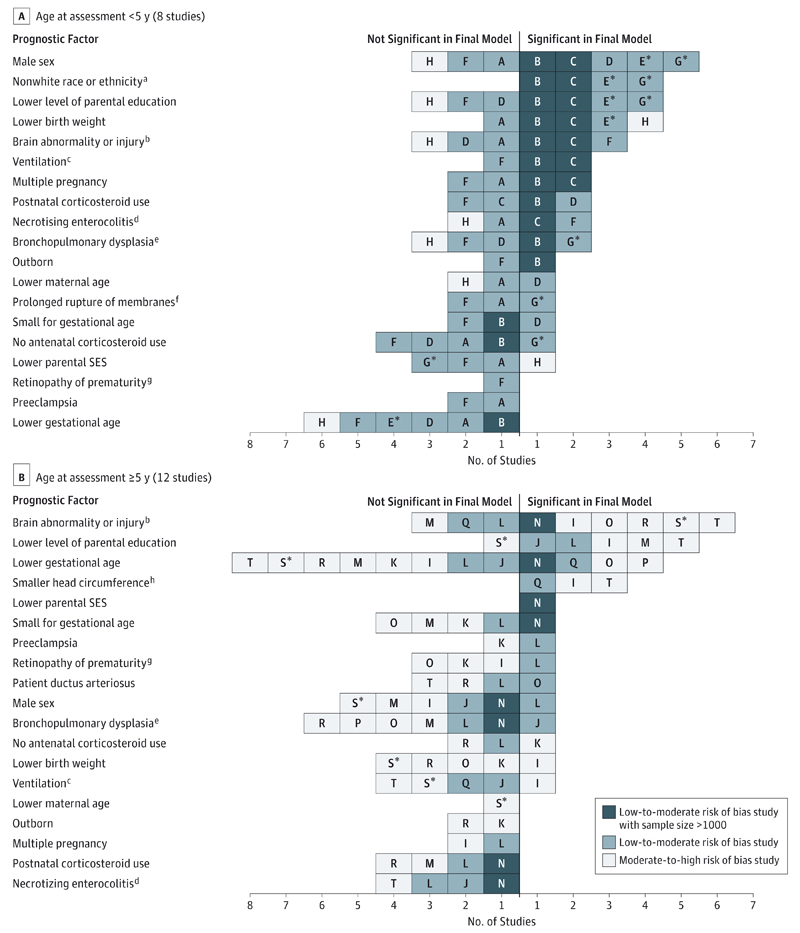
Twenty studies contained a risk factor analysis for general cognitive function or IQ (Table 1 and Table 2). Eight studies20–27 assessed outcome between ages 1.5 and 2.5 years and 12 studies28–39 between ages 5 and 13 years. The most common assessment used before age 5 years was the Mental Development Index from the Bayley Scales of Infant Development version II51 or the Cognitive Composite Score from version III52 in the more recent studies. The Mental Development Index assesses cognition through evaluation of sensory perception, knowledge, memory, problem solving, and early language. The more recent version splits cognitive and language skills into separate domains. There was more variety in measurement scales used in assessments at age 5 years and older, with the most common being the Mental Processing Composite Score from the Kaufman Assessment Battery for Children53 and the full-scale IQ from Wechsler’s Preschool and Primary Scale of Intelligence–Revised.54 Risk factors that were found to be significant in the final model of at least 1 study with low to moderate risk of bias and examined in the final model of at least 2 other studies are shown in Figure 3A (for children <5 years) and Figure 3B (for children ≥5 years).
Table 1. Summary of Studies Reporting Risk Factor Analyses for Global Cognitive Impairment in Children Born Very Preterm or With Very Low Birth Weight Assessed at Younger Than 5 Years.
| Source (Study Identifier) | Country and Recruitment Period | Age at Assessment, y | GA, wk/BW, g | Design and Participants | Survivors Assessed, No. (%)a | Outcome Measure, Continuous Unless Otherwise Specified | Method for Dealing With Untestable Children | Significant Risk Factors for Poorer Outcome at P < .05 in Final Model |
|---|---|---|---|---|---|---|---|---|
| Tommiska et al,20 2003 (A) | Finland 1996-1997 | 1.5 | <1000 g | PC of infants born and treated in a single-center NICU (Helsinki) and enrolled for the national routine FUP | 78 (94) | MDI score from BSID-II | Excluded if severe developmental problem (n = 3) or exhaustion (n = 2) | No. of days from January 1, 1996, to birth |
| Vohr et al,21 2005 (B) | United States 1993-1998 | 1.5-1.8 | <33 wk and <1000 g | PC of infants admitted to the NICU of 12 centers participating in the multicenter NICHD NRN routine FUP | 3785 (80) | MDI score from BSID-II (<70 vs ≥70), blinded assessment | Excluded if test not completed (n = 118) | Birth epoch (1993-1994 vs 1995-1996), lower BW, BPD, any high-frequency ventilation, IVH 3-4, male sex, lower maternal education, no private insurance, multiple pregnancy, nonwhite race/ethnicity, outborn,b PVL, PN corticosteroid use |
| Adams-Chapman et al,22 2013 (C) | United States 2006-2008 | 1.5-1.8 | <1000 g | PC of infants admitted to the NICU of 20 centers participating in the multicenter NICHD NRN routine FUP | 1477 (91) | Cognitive Composite Score from BSID-III, blinded assessment | Assigned a score of 54 if severely delayed (n = 39) | Lower BW, black race/ethnicity, dysfunctional feeding, GMFCS≥2, non–English speaking, male sex, lower maternal education, MV days, multiple pregnancy, NEC 2-3, no private insurance, IVH 3-4 or PVL |
| Stoelhorst et al,23 2003 (D)c | Netherlands 1996-1997 | 2 | <32wk | PC of all live births in 3 Dutch health regions comprising 9% of the population | 146 (62) | MDI score from BSID-I | Assigned a score of 50 if severely disabled (n = 3), otherwise excluded (n = 5) | Male sex, lower maternal age, non-Dutch, PN corticosteroid use, SGA |
| Helderman et al,24 2012 (E) | United States 2002-2004 | 2 | <28wk | PC of all live births in 14 centers in 5 states (ELGAN study) | 921 (77) | MDI score from BSID-II (<55 and 55-69 vs ≥70), blinded assessment | Excluded if GMFCS ≥1 (n = 83) | BW <− 2 SDs, BMI >30, male sex, lower maternal education, nonwhite race/ethnicity |
| Toome et al,25 2013 (F) | Estonia 2007 | 2 | <32wk | PC of all live births in Estonia enrolled in the national neonatal research routine FUP | 155 (99) | Cognitive Composite Score from BSID-III (<70 vs ≥70) | Assigned a score of −4 SDs below the mean (No. not reported) | IVH 3-4 or PVL 2-4, NEC 2-3 |
| Wood et al,26 2005 (G) | United Kingdom and Republic of Ireland 1995 | 2.5 | <26wk | PC of all live births in the United Kingdom and Republic of Ireland (EPICure study) | 196 (64) | MDI score from BSID-II | Excluded if MDI <55 or functional motor disability (n = 52) | Afro-Caribbean race/ethnicity, no AN corticosteroid use, BPD, male sex, lowermaternal education, PROM |
| Messinger et al,27 2010 (H)d | United States 1999-2001 | 2.5 | <1000 g | Infants admitted to the NICU of 12 centers participating in the multicenter NICHD NRN routine FUP and enrolled in a glutamine supplementation RCT | 539 (47) | MDI score from BSID-II | Excluded if test uncompleted (No. not reported) | BW ≤750 g, higher maternal income, higher MDI at 18 mo |
Abbreviations: AN, antenatal; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPD, bronchopulmonary dysplasia; BSID, Bayley Scales of Infant Development51; BW, birth weight; ELGAN, Extremely Low Gestational Age Newborns; FUP, follow-up; GA, gestational age; GMFCS, Gross Motor Functional Classification System68; IVH, intraventricular hemorrhage; MDI, Mental Developmental Index from the BSID; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NICHD NRN, National Institute of Child Health and Human Development Neonatal Research Network; PC, prospective cohort; PN, postnatal; PROM, prolonged rupture of membranes; PVL, periventricular leukomalacia; RCT, randomized clinical trial; SGA, small for gestational age.
Percentage of survivors assessed for outcome measure specified.
Born outside of the hospital where they were admitted to the NICU.
Two models for motor skills reported, with the model based on 2-year outcome included and the model based on 1.5-year outcome not included.
Several models for cognitive function fitted, with the full model adjusting for 18-month MDI and Behavior Rating Scale total score included.
Table 2. Summary of Studies Reporting Risk Factor Analyses for Global Cognitive Impairment in Children Born Very Preterm or With Very Low Birth Weight Assessed at 5 Years and Older.
| Source (Study Identifier) | Country and Recruitment Period | Age at Assessment, y | GA, wk/BW, g | Design and Participants | Survivors Assessed, No. (%)a | Outcome Measure, Continuous Unless Otherwise Specified | Method for Dealing With Untestable Children | Significant Risk Factors for Poorer Outcome at P < .05 in Final Model |
|---|---|---|---|---|---|---|---|---|
| Franz et al,28 2009 (I)b | Germany 1996-1999 | 4.6-7 | <30 wk and <1500g | PC study of infants admitted to a single-center level 3 NICU (Ulm University) | 219 (83) | MPC from KABC, blinded assessment | Assigned a score of 30 if minimal speech and a score of 20 if minimal sensory or motor achievements elicited | Lower BW SDS, smaller HC SDS gain (discharge to 5 y), IVH or PVH ≥3, lower maternal education, MV days, PVL |
| Hansen et al,29 2004 (J)c | Denmark 1994-1995 | 5 | <28 wk or <1000 g | PC of all live births in Denmark ascertained from all 18 neonatal care units and the Danish Medical Birth Register (ETFOL Study) | 247 (94) | FIQ from WPPSI-R (continuous score and <70 vs ≥70), blinded assessment | Excluded if test not completed (n = 5); children with CP, visual disability, first language not Dutch, or >27 wk GA excluded from analysis of continuous score (n = 110) | Model 1 (continuous score): BPD, lower parental education; Model 2 (<70 vs ≥70): BPD, IVH 3-4, or PVL |
| Mikkola et al,30 2005 (K)c | Finland 1996-1997 | 5 | <1000 g | PC of all live births in Finland enrolled for the national routine FUP | 172 (83) | FIQ from WPPSI-R (continuous score and <70 vs ≥70) | Excluded if cognitively impaired and unable to cooperate (n = 9), excluded if test not completed (n = 12) | Model 1 (continuous score): no AN corticosteroid use, IVH 3-4, male sex, multiparity, multiple pregnancy, lower parental SES, vaginal delivery; Model 2 (<70 vs ≥70): no AN corticosteroid use, BPD, hospital area, perforated NEC |
| Leversen et al,31 2011 (L)d | Norway 1999-2000 | 5 | <28 wk or <1000 g | PC of all live births in Norway | 248 (67) | FIQ from WPPSI-R | Excluded if CP, blind, deaf, or autistic (n = 33); excluded if test not completed (n = 25) | Model 1 (GA <28 wk): lower maternal education, preeclampsia, ROP >2; Model 2 (GA ≥28 wk): male sex |
| Potharst et al,32 2012 (M)e | Netherlands 2002-2004 | 5 | <30 wk or <1000 g | PC study of infants admitted to a single-center NICU (Amsterdam) | 102 (68) | FIQ from WPPSI-III | Excluded if too disabled to be tested (n = 4) | Behavior problems at 2 y, lower MDI at 2 y, lower parental education, parental foreign country of birth, sepsis, or meningitis |
| Beaino et al,33 2011 (N) | France 1997 | 5 | <33wk | PC of all live births in 9 French regions comprising one-third of all births (EPIPAGE study) | 1503 (62) | MPC from KABC (<70 and 70-84 vs ≥85), blinded assessment | Excluded if moderate to severe neurosensory disability (n = 70), excluded if test not completed (n = 239) | Breastfed at discharge, cystic PVL or IPH, GA ≤28 wk, IVH grade 3/echodensities/VD, lower parental SES, SGA, ≥3 siblings |
| Orchinik et al,34 2011 (O)f | United States 2001-2003 | 5 | <28 wk or <1000 g | PC of infants admitted to a single-center NICU (Ohio) participating in the multicenter NICHD NRN routine FUP | 142 (72) | WJ-III COG Brief Intellectual Ability <10th centile, blinded assessment | Assigned a score of 40 if too low functioning to comply with test demands | GA <25 wk, infection (sepsis, NEC, or meningitis), IVH 3-4/PVL/ VD, neurosensory disorder or MDI <70 at 20 mo |
| Andrews et al,35 2008 (P)c | United States 1996-1999 | 5-8 | <32wk | PC of infants admitted to a single-center NICU (Alabama) participating in the multicenter NICHD NRN routine FUP, multiple births excluded | 259 (69) | WISC-IV or DAS if <6 y or unable to complete the WISC-IV (continuous score and <70 vs ≥70) | Excluded if test not completed (n = 2) | Model 1 (continuous score): younger GA, PVL; Model 2 (<70 vs ≥70): no history of PROM, younger GA |
| Cooke,36 2005 (Q) | England 1991-1992 | 7 | <32wk | PC of all live births in all 8 hospitals in the Liverpool postal district | 280 (77) | WISC-III (<89 vs ≥89, mean of the group) | Excluded if not free of major disability and not attending mainstream school (n = 29) | Younger GA, smaller HC at 7 y, PDA |
| Taylor et al,37 2006 (R)c,g | United States 1992-1995 | 8 | <1000 g | PC of infants admitted to a single-center NICU (Ohio) participating in the multicenter NICHD NRN routine FUP | 204 (86) | MPC from KABC (continuous and <1 SD below mean of control group), blinded assessment | Excluded if untestable because of severe developmental impairments (n = 10) | Model 1 (continuous score): longer neonatal hospital stay, NEC, NRI >3, PVL, VD; Model 2 (<70 vs ≥70): longer neonatal hospital stay, NRI >3, PVL, VD |
| Stahlmann et al,38 2009 (S) | Germany 1997-1999 | 7-9 | <27wk | PC of all live births in all 8 perinatal centers in Schleswig-Holstein | 75 (82) | MPC from KABC or equivalent (<70 vs ≥70), blinded assessmenth | Assigned a score of <70 if untestable because of extremely limited capacities | IVH 3-4/PVL |
| Voss et al,39 2012 (T) | Germany 1993-1998 | 10-13 | <1000 g | PC study of infants admitted to a single-center NICU (Hannover) | 148 (87) | HAWIK-III composite IQ score | Assigned a score of 39 (40 is the lowest possible score) | HC increase <6 mm per wk, IVH 3-4/PVL, immigrant status, parenteral nutrition >41 d |
Abbreviations: AN, antenatal; BPD, bronchopulmonary dysplasia; BSID, Bayley Scales of Infant Development51; BW, birth weight; CP, cerebral palsy; DAS, Differential Ability Scales69; EPIPAGE, Etude Epidemiologique sur les Petits Ages Gestationnels; ETFOL, Ekstrem Tidlig Født Og Lavvægtig (Danish National Study in Infants With Extremely Low Gestational Age and Birth Weight; FIQ, Full-scale IQ from WPPSI-R; FUP, follow-up; GA, gestational age; HAWIK, Hamburg Wechsler Intelligence Test for Children70; HC, head circumference; IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage; KABC, Kaufman Assessment Battery for Children53; MDI, Mental Developmental Index from the BSID; MPC, Mental Processing Composite Score from the KABC; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NICHD NRN, National Institute of Child Health and Human Development Neonatal Research Network; NICU, neonatal intensive care unit; NRI, Neonatal Risk Index; PC, prospective cohort; PROM, prolonged rupture of membranes; PVH, periventricular hemorrhage; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; SDS, standard deviation score; SES, socioeconomic status; SGA, small for gestational age; VD, ventricular dilatation; WISC, Wechsler Intelligence Scale for Children71; WJ-III COG, Woodcock-Johnson Tests of Cognitive Abilities, Third Edition72; WPPSI-R, Wechsler’s Preschool and Primary Scale of Intelligence–Revised.54
Percentage of survivors assessed for outcome measure specified.
Two models for cognitive function reported; the same perinatal factors fitted with change in weight variables added to the first model and change in head circumference variables added to the second model. Perinatal factors are included in Figure 3B as significant if P < .05 in both models and nonsignificant if P ≥ .05 in both models and otherwise are not included.
Two models for cognitive function reported, with one based on dichotomous outcome and the other based on continuous outcome. Risk factors are included in Figure 3B as significant if P < .05 in both models and nonsignificant if P ≥ .05 in both models and otherwise are not included.
Two models for cognitive function reported for each gestational age group (<28 weeks and ≥28 weeks). Risk factor was considered significant if P < .05 in either model.
Two models for FIQ at 5 years reported, with one including 2-year developmental assessments and the other including 3-year developmental assessments. The former model is reported as 2-year assessments, which are more routine in general practice.
Each risk factor was fitted separately and adjusted for sex, race/ethnicity, parental SES, and months in school at testing (the article did not report the results for the adjustment factors).
Each risk factor was fitted separately and adjusted for sex, race/ethnicity, parental SES, family stressors, and family resources (the article did not report the results for the adjustment factors).
Seven children were tested with an equivalent instrument (HAWIK, Snijders-Oomen Nonverbal Intelligence Test, or the Culture Fair Intelligence Tests).
Figure 3. Evidence Synthesis of Risk Factors for Global Cognitive Impairment in Children Born Very Preterm or With Very LowBirthWeight.
Prognostic factors are presented if significant (P < .05) in the final model of at least 1 study with low-to-moderate risk of bias and entered into the final model of at least 3 studies (across all ages). A through T indicate study identifiers listed in Table 1 and Table 2 (* denotes an extremely preterm cohort); SES, socioeconomic status.
a Nonwhite (B and E), black (C), or Afro-Caribbean (G).
b Intraventricular hemorrhage or periventricular leukomalacia (B, C, D, F, H, I, L, M, O, S, and T), periventricular leukomalacia or ventricular dilatation (R), intraventricular hemorrhage grades 2 to 4 (A), parenchymal lesion (Q), intraventricular hemorrhage grades 1 to 3, echodensities, ventricular dilatation, cystic periventricular leukomalacia, or intraparenchymal hemorrhage (N).
c Any high-frequency (B), any mechanical ventilation (J), or mechanical ventilation days (C, F, I, Q, S, and T).
d Perforated necrotizing enterocolitis (A), necrotizing enterocolitis stages 2 to 3 (C and F), surgical or radiograph diagnosed (J), bowel perforation or necrotizing enterocolitis (T), or not specified (H, L, and N).
e Oxygen requirement at 36 weeks’ gestational age (B, D, F, G, J, L, M, N, O, and R) or not specified (H and P).
f More than 24 hours before labor (G) or not specified (A and F).
g Stage 3 to 4 (I, K, and L), at least stage 3 with laser therapy (F), or stage 4 to 5 or treatment with cryotherapy or laser therapy (O).
h Increase in head circumference from discharge to 5 years (I), occipitofrontal circumference 7-year centile (Q), or increase in head circumference less than 6 mm per week (T).
Among studies in which the age at assessment was younger than 5 years (Figure 3A), the 2 largest studies21,22 with low to moderate risk of bias and at least 1 other study with low to moderate risk of bias found the following factors to be predictive of poorer cognitive development: male sex, nonwhite race/ethnicity, lower level of parental education, lower birth weight, and brain injury during the neonatal period. However, the other studies20,23,25,27 that also examined these risk factors sometimes contradicted these findings, with the exception of race/ethnicity. There was also some evidence that the absence of antenatal corticosteroid use and lower GA were not predictive of poorer cognitive function in children younger than 5 years.
Most of the studies examining cognitive function at 5 years and older had moderate to high risk of bias (Figure 3B). The association between level of parental education and cognitive impairment was also evident in this age group, but the association with male sex was greatly diminished. Race/ethnicity was not entered into the final model in any of the studies among older children (or was not reported when it was used as an adjustment factor in 2 studies34,37). Therefore, it was not possible to determine whether the influence of this factor prevailed into middle childhood. Most studies in this age group also found that younger GA had little prognostic value in a multivariable prediction model.
Prognostic Factors for Impaired Language Development
Risk factor analyses for language development were conducted in 8 studies (eTable 2 in the Supplement). Five studies22,25,40–42 assessed outcome between ages 1.5 and 3 years, and 3 studies34,37,43 with moderate to high risk of bias assessed outcome between ages 5 and 8 years. There was more heterogeneity in the types of tests used to measure language skills compared with cognition. The eFigure in the Supplement shows the risk factors that were found to be significant in the final model of at least 1 study with low to moderate risk of bias and entered into the final model of at least 2 other studies.
All 5 studies22,25,40–42 conducted in children younger than 5 years included male sex in the final model and reported that this variable was predictive of poor language development. It was not possible to comment on the effect of male sex in middle childhood because 2 studies34,37 among 3 conducted at age 5 years and older adjusted for it but did not report the results for adjustment factors while the third study43 did not enter sex into the final model because it was not significant in the univariate analysis. Three studies22,40,43 reported that lower level of parental education was associated with poor language development, and 2 studies25,41 reported no such association. There were also mixed findings for the prognostic value of children being small for GA. It was not possible to draw any conclusions about neonatal brain injury as a prognostic factor for language impairment, possibly because studies used different strategies to deal with children with severe neurosensory impairment for whom standard assessments could not be used, with some imputing the lowest possible score and others excluding this group completely. As with cognition, there was evidence that GA was not a strong predictor of language development in a multivariable model.
Prognostic Factors for Impaired Executive Function
Seven studies32,34,37,44–47 with moderate to high risk of bias presented risk factor analyses for different aspects of executive function (eTable 3 in the Supplement), with all except one based on age at assessment of 5 to 12 years. The median number of tests administered within each study was 5, and the maximum was 13. The risk factors listed in eTable 3 in the Supplement were significant in at least 1 of the final models. It was difficult to combine these results in any meaningful way because of the small number of studies using a wide variety of tests to measure interrelated cognitive processes.
Prognostic Factors for Poor Academic Attainment
Four studies (2 studies48,50 with low to moderate risk of bias and 2 studies37,49 with moderate to high risk of bias) performed risk factor analyses for academic attainment (eTable 4 in the Supplement), all based on age at assessment between 5 and 12 years. All 4 studies presented a model on mathematical ability, 2 studies presented a model on letter and word identification, and 1 study presented a model on reading scores. Again, there were too few studies and insufficient overlap in the risk factors entered into the final models to combine the results and draw any meaningful conclusions.
Discussion
For the VPT or VLBW population, there was fairly strong evidence that male sex was a prognostic factor for poorer cognitive development and language skills in early infancy, a finding supported by other studies55–57 that have focused exclusively on the association of infant sex with cognitive function. However, in the studies conducted later in childhood that were included in this review, the influence of sex on general cognition was largely diminished. We were unable to comment on whether this finding was also true for language development because of the lack of studies assessing children at 5 years and older. There were similar findings for nonwhite race/ethnicity and lower birth weight in relation to cognitive impairment. Both factors were clearly prognostic in early infancy, but no evidence was available in middle childhood for race/ethnicity, with a lack of association in later years for birth weight. There was evidence that a lower level of parental education was predictive of cognitive impairment, supported by a recent study58 in an EPT population that focused solely on this hypothesis. Unlike factors related to infant characteristics, the influence of parental education appeared to persist into middle childhood. Evidence for the prognostic value of parental education in relation to language development was weak.
Research has shown links among nonwhite race/ethnicity, lower birth weight, and parental education or socioeconomic status (SES),59,60 so it is notable that these factors were independent predictors in the final models of the 4 studies21,22,24,26 in the age group younger than 5 years. Other studies61,62 have found that the effect of race/ethnicity is strongly mediated by markers of deprivation. In the present review, level of parental education emerged as a prognostic factor of cognitive outcome, whereas parental SES did not. This finding may be because of multicollinearity, or possibly a single marker of parental SES such as income or occupation (as used in most studies in the review) is insufficient to capture an accurate measure of social disadvantage. Combining a range of social markers into a composite score may be a more effective modeling strategy.
Many studies that have focused exclusively on the relationship between brain injury diagnosed in the neonatal period and subsequent cognitive function have reported strong linear trends with grade of severity.63–66 However, the prognostic value of brain injury in the multivariable models reported in this review was mixed. This result is possibly because cognitive and language development is multifactorial, unlike a diagnosis of CP, which is more directly related to focal brain injury, so that the influence of perinatal factors becomes less pronounced when other variables are entered into a model. The unclear findings may also reflect the different modeling strategies adopted by the studies. Some studies excluded children with CP or other neurosensory impairment, some imputed lowest scores, and others adjusted for motor disability.
There was strong evidence that GA was not a robust predictor of cognitive and language development in infancy or in middle childhood in the VPT or VLBW population. Although the relationship between older GA and improved cognition is well established across the whole spectrum of GA from 25 to 40 weeks,4 it does not emerge as an important predictor in individual studies with preterm subgroups defined by restricted GA. Although a strong positive relationship with GA is seen when survival without neurodevelopmental impairment is calculated as a function of all live births, the association weakens when the denominator is survivors at discharge, as with all the studies included in this review. This occurs because the proportion of surviving children rises steeply with GA, while the proportion of impaired survivors does not.
Our study has strengths and limitations. We used a broad search filter with no language restriction to capture all studies with exploratory risk factor analyses, which is recommended in this type of review.67 No further articles were identified in the hand-search of bibliographies of all studies included, so it is unlikely that there were any major omissions. The study cohorts spanned an 18-year period; hence, some of the factors affecting outcome in the early 1990s may not be so relevant to current preterm populations. They also represent diverse international populations, with differing methods of ascertainment and clinical practices, which may explain the unclear pattern of the results for some factors. Also, studies did not all consider the same sets of candidate factors. Multiple models based on the same cohort population were a major issue, particularly studies on executive function, which often performed a whole battery of tests. Using standard rules, we selected studies and models for inclusion before data synthesis was conducted, although it was difficult to apply a strict set of criteria for each case. Another difficulty in this review was the sheer variety of assessments used, particularly among children 5 years and older.
Conclusions
In conclusion, there was evidence that male sex, nonwhite race/ethnicity, lower level of parental education, and lower birth weight were significant predictors of global cognitive impairment in children 18 to 30 months old who were born VPT or with VLBW. After age 5 years, the effect of infant sex and birth weight diminished, level of parental education was still influential, and there was no evidence on the lasting effect of race/ethnicity. It is unlikely that race/ethnicity itself is a causal factor for cognitive impairment because other research has demonstrated a strong correlation between race/ethnicity, poverty, and social disadvantage. There was evidence that male sex was predictive of language development in early infancy, but no evidence that this result was sustained into childhood. There were mixed findings on the prognostic value of brain injury during the neonatal period on language and cognition, which may reflect the heterogeneous selection criteria and methods of dealing with missing data related to severe disability across the studies. There was evidence that within the VPT or VLBW population GA had little value as a prognostic factor in multivariable models predicting the risk of cognitive or language development at any age older than 18 months. The findings of this review lend support to the view that the effect of perinatal risk factors diminishes over time as other environmental and social factors become more influential.
Supplementary Material
At a Glance.
The objective of this systematic review was to identify risk factors that are robust predictors of cognitive impairment in children born very preterm (VPT) or with very low birth weight (VLBW).
There was evidence that male sex, nonwhite race/ethnicity, lower level of parental education, and lower birth weight were predictive of global cognitive impairment in VPT or VLBW children younger than 5 years.
In VPT or VLBW children 5 years and older, only the influence of parental education was sustained, suggesting that the influence of perinatal risk factors diminishes over time and that environmental and social factors become more important.
Male sex was also predictive of language impairment in VPT or VLBW infants younger than 5 years, but there was no evidence of an association beyond this age.
There is a need for good-quality, well-conducted studies of prognosis in the VPT or VLBW population, particularly in older children, among whom the evidence base is weak.
Acknowledgments
Funding/Support: This study was funded by Doctoral Research Fellowship NIHR-DRF-2012-05-206 from the National Institute for Health Research.
Role of the Funder/Sponsor: The sponsor and the funding organization had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Linsell had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Linsell, Morris, Kurinczuk, Marlow.
Acquisition, analysis, or interpretation of data: Linsell, Malouf,
Drafting of the manuscript: Linsell, Morris, Kurinczuk, Marlow.
Critical revision of the manuscript for important intellectual content: Linsell, Morris, Kurinczuk, Marlow.
Administrative, technical, or material support: All authors.
Additional Contributions: Nia Wyn Roberts, MSc(Econ) (Health Care Libraries, Bodleian Libraries, University of Oxford) provided input and expertise during the database search phase of the review. No compensation was provided.
Conflict of Interest Disclosures: None reported.
Disclaimer: This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Contributor Information
Louise Linsell, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, England.
Reem Malouf, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, England.
Joan Morris, Centre for Environmental and Preventive Medicine, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, England.
Jennifer J. Kurinczuk, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, England.
Neil Marlow, Institute of Women’s Health, University College London, London, England.
References
- 1.Farooqi A, Hägglöf B, Sedin G, Gothefors L, Serenius F. Chronic conditions, functional limitations, and special health care needs in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2006;118(5):e1466–e1477. doi: 10.1542/peds.2006-1070. [DOI] [PubMed] [Google Scholar]
- 2.Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P, Doyle LW, Victorian Infant Collaborative Study Group Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 5.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 6.Marlow N, Hennessy EM, Bracewell MA, Wolke D, EPICure Study Group Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120(4):793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HG, Klein N, Minich NM, Hack M. Middle-school-age outcomes in children with very low birthweight. Child Dev. 2000;71(6):1495–1511. doi: 10.1111/1467-8624.00242. [DOI] [PubMed] [Google Scholar]
- 8.Aarnoudse-Moens CS, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. Executive function in very preterm children at early school age. J Abnorm Child Psychol. 2009;37(7):981–993. doi: 10.1007/s10802-009-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158(5):766–774.e1. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F283–F289. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg T, Heymans MW, Leone SS, et al. Overview of data-synthesis in systematic reviews of studies on outcome prediction models. BMC Med Res Methodol. 2013;13(42):42. doi: 10.1186/1471-2288-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley RD, Hayden JA, Steyerberg EW, et al. PROGRESS Group Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10(2):e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68(2):134–143. doi: 10.1016/j.jclinepi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992-1995. Arch Pediatr Adolesc Med. 2000;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 17.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B, NICHD Neonatal Network Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 18.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. 2000;105(6):1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 19.Vohr BR, Wright LL, Dusick AM, et al. Neonatal Research Network Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 20.Tommiska V, Heinonen K, Kero P, et al. A national two year follow up study of extremely low birthweight infants born in 1996-1997. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F29–F35. doi: 10.1136/fn.88.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–643. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 22.Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J Pediatr. 2013;163(3):680–5.e1, 3. doi: 10.1016/j.jpeds.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoelhorst GM, Rijken M, Martens SE, et al. Leiden Follow-up Project on Prematurity Developmental outcome at 18 and 24 months of age in very preterm children: a cohort study from 1996 to 1997. Early Hum Dev. 2003;72(2):83–95. doi: 10.1016/s0378-3782(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 24.Helderman JB, O’Shea TM, Kuban KC, et al. ELGAN Study Investigators Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129(3):494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toome L, Varendi H, Männamaa M, Vals MA, Tänavsuu T, Kolk A. Follow-up study of 2-year-olds born at very low gestational age in Estonia. Acta Paediatr. 2013;102(3):300–307. doi: 10.1111/apa.12091. [DOI] [PubMed] [Google Scholar]
- 26.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, EPICure Study Group The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messinger D, Lambert B, Bauer CR, Bann CM, Hamlin-Smith K, Das A. The relationship between behavior ratings and concurrent and subsequent mental and motor performance in toddlers born at extremely low birth weight. J Early Interv. 2010;32(3):214–233. doi: 10.1177/1053815110380917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123(1):e101–e109. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 29.Hansen BM, Hoff B, Uldall P, et al. Danish ETFOL Group Perinatal risk factors of adverse outcome in very preterm children: a role of initial treatment of respiratory insufficiency? Acta Paediatr. 2004;93(2):185–189. doi: 10.1080/08035250310008230. [DOI] [PubMed] [Google Scholar]
- 30.Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996-1997. Pediatrics. 2005;116(6):1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 31.Leversen KT, Sommerfelt K, Rønnestad A, et al. Prediction of neurodevelopmental and sensory outcome at 5 years in Norwegian children born extremely preterm. Pediatrics. 2011;127(3):e630–e638. doi: 10.1542/peds.2010-1001. [DOI] [PubMed] [Google Scholar]
- 32.Potharst ES, Houtzager BA, van Sonderen L, et al. Prediction of cognitive abilities at the age of 5 years using developmental follow-up assessments at the age of 2 and 3 years in very preterm children. Dev Med Child Neurol. 2012;54(3):240–246. doi: 10.1111/j.1469-8749.2011.04181.x. [DOI] [PubMed] [Google Scholar]
- 33.Beaino G, Khoshnood B, Kaminski M, et al. EPIPAGE Study Group Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011;100(3):370–378. doi: 10.1111/j.1651-2227.2010.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 2011;17(6):1067–1079. doi: 10.1017/S135561771100107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198(4):466.e1–466.e11. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke RW. Perinatal and postnatal factors in very preterm infants and subsequent cognitive and motor abilities. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F60–F63. doi: 10.1136/adc.2004.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27(6):459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Stahlmann N, Rapp M, Herting E, Thyen U. Outcome of extremely premature infants at early school age: health-related quality of life and neurosensory, cognitive, and behavioral outcomes in a population-based sample in northern Germany. Neuropediatrics. 2009;40(3):112–119. doi: 10.1055/s-0029-1243166. [DOI] [PubMed] [Google Scholar]
- 39.Voss W, Jungmann T, Wachtendorf M, Neubauer AP. Long-term cognitive outcomes of extremely low-birth-weight infants: the influence of the maternal educational background. Acta Paediatr. 2012;101(6):569–573. doi: 10.1111/j.1651-2227.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- 40.Charkaluk ML, Truffert P, Fily A, Ancel PY, Pierrat V, Epipage study group Neurodevelopment of children born very preterm and free of severe disabilities: the Nord-Pas de Calais Epipage cohort study. Acta Paediatr. 2010;99(5):684–689. doi: 10.1111/j.0803-5253.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sansavini A, Guarini A, Savini S. Linguistic and cognitive delays in very preterm infants at 2 years: general or specific delays? Rev Logop Fon Audiol. 2011;31(3):133–147. [Google Scholar]
- 42.Marston L, Peacock JL, Calvert SA, Greenough A, Marlow N. Factors affecting vocabulary acquisition at age 2 in children born between 23 and 28 weeks’ gestation. Dev Med Child Neurol. 2007;49(8):591–596. doi: 10.1111/j.1469-8749.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 43.Howard K, Roberts G, Lim J, et al. Biological and environmental factors as predictors of language skills in very preterm children at 5 years of age. J Dev Behav Pediatr. 2011;32(3):239–249. doi: 10.1097/DBP.0b013e31820b7882. [DOI] [PubMed] [Google Scholar]
- 44.Lowe J, MacLean PC, Shaffer ML, Watterberg K. Early working memory in children born with extremely low birth weight: assessed by object permanence. J Child Neurol. 2009;24(4):410–415. doi: 10.1177/0883073808324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potharst ES, van Wassenaer-Leemhuis AG, Houtzager BA, et al. Perinatal risk factors for neurocognitive impairments in preschool children born very preterm. Dev Med Child Neurol. 2013;55(2):178–184. doi: 10.1111/dmcn.12018. [DOI] [PubMed] [Google Scholar]
- 46.Ford RM, Neulinger K, O’Callaghan M, Mohay H, Gray P, Shum D. Executive function in 7–9-year-old children born extremely preterm or with extremely low birth weight: effects of biomedical history, age at assessment, and socioeconomic status. Arch Clin Neuropsychol. 2011;26(7):632–644. doi: 10.1093/arclin/acr061. [DOI] [PubMed] [Google Scholar]
- 47.Aarnoudse-Moens CS, Weisglas-Kuperus N, Duivenvoorden HJ, Oosterlaan J, van Goudoever JB. Neonatal and parental predictors of executive function in very preterm children. Acta Paediatr. 2013;102(3):282–286. doi: 10.1111/apa.12101. [DOI] [PubMed] [Google Scholar]
- 48.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Pehboeck-Walser N, Fussenegger B. Early risk predictors for impaired numerical skills in 5-year-old children born before 32 weeks of gestation. Acta Paediatr. 2013;102(1):66–71. doi: 10.1111/apa.12036. [DOI] [PubMed] [Google Scholar]
- 49.Taylor HG, Klein N, Anselmo MG, Minich N, Espy KA, Hack M. Learning problems in kindergarten students with extremely preterm birth. Arch Pediatr Adolesc Med. 2011;165(9):819–825. doi: 10.1001/archpediatrics.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol. 2011;36(1):74–95. doi: 10.1080/87565641.2011.540541. [DOI] [PubMed] [Google Scholar]
- 51.Bayley N. Bayley Scales of Infant Development–II. 2nd ed. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- 52.Bayley N. Bayley Scales of Infant Development–III. 3rd ed. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 53.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- 54.Wechsler D. Wechsler’s Preschool and Primary Scale of Intelligence–Revised. New York, NY: Psychological Corp Harcourt Brace, Inc; 1989. [Google Scholar]
- 55.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD, Nichd Neonatal Research Network Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–1248. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 56.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–310. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 57.Skiöld B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Adén U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164(5):1012–1018. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 58.Ko G, Shah P, Lee SK, Asztalos E. Impact of maternal education on cognitive and language scores at 18 to 24 months among extremely preterm neonates. Am J Perinatol. 2013;30(9):723–730. doi: 10.1055/s-0032-1331034. [DOI] [PubMed] [Google Scholar]
- 59.Kelly Y, Panico L, Bartley M, Marmot M, Nazroo J, Sacker A. Why does birthweight vary among ethnic groups in the UK? findings from the Millennium Cohort Study. J Public Health (Oxf) 2009;31(1):131–137. doi: 10.1093/pubmed/fdn057. [DOI] [PubMed] [Google Scholar]
- 60.Nepomnyaschy L. Socioeconomic gradients in infant health across race and ethnicity. Matern Child Health J. 2009;13(6):720–731. doi: 10.1007/s10995-009-0490-1. [DOI] [PubMed] [Google Scholar]
- 61.Duncan AF, Watterberg KL, Nolen TL, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Effect of ethnicity and race on cognitive and language testing at age 18-22 months in extremely preterm infants. J Pediatr. 2012;160(6):966–971.e2. doi: 10.1016/j.jpeds.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooks-Gunn J, Klebanov PK, Duncan GJ. Ethnic differences in children’s intelligence test scores: role of economic deprivation, home environment, and maternal characteristics. Child Dev. 1996;67(2):396–408. [PubMed] [Google Scholar]
- 63.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, NICHD Research Network Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167–e1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marret S, Marchand-Martin L, Picaud JC, et al. EPIPAGE Study Group Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8(5):e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012;7(12):e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherlock RL, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81(11):909–916. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS One. 2012;7(2):e32844. doi: 10.1371/journal.pone.0032844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 69.Eliot CD. Differential Ability Scales. San Antonio, TX: Psychological Corp; 1990. [Google Scholar]
- 70.Tewes U, Rossmann R, Schallberger U. Der Hamburg-Wechsler-Intelligenztest fur Kinder (HAWIK-III) Bern, Switzerland: Huber-Verlag; 1999. [Google Scholar]
- 71.Wechsler D. WISC-IV Administration and Scoring Manual. San Antonio, TX: Psychological Corp; 2003. [Google Scholar]
- 72.Woodcock RC, McGrew KS, Mather S. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.