Abstract
Objective
In addition to slowing diabetes development among participants in the Diabetes Prevention Program (DPP), intensive lifestyle change and metformin raised HDL-cholesterol (HDL-C) compared to placebo treatment. We investigated the lifestyle and metabolic determinants as well as effects of biomarkers of inflammation, endothelial dysfunction and coagulation and their changes resulting from lifestyle and metformin interventions on the increase in HDL-C in the DPP.
Methods
The effects of a 1 year period of intensive lifestyle change aimed at achieving 7% weight loss or metformin 850 mg twice daily versus placebo on HDL-C was assessed in 3070 participants with impaired glucose tolerance, and on HDL particle concentration (HDL-P) and size in a subgroup of 1645 individuals. Treatment-associated changes in lifestyle and metabolic factors as well as in novel biomarkers were investigated for their associations with change in HDL-C using multiple regression analysis.
Results
After adjusting for BMI, waist circumference, insulin resistance, glycemia, dietary saturated fat, alcohol intake, physical activity and nine different biomarkers, only adiponectin accounted for the effect of intensive lifestyle change on HDL-C via an increase in large HDL-P. By contrast baseline and change in BMI and tissue plasminogen activator levels attenuated the effect of metformin on HDL-C, with adiponectin having no specific effect.
Conclusion
While both lifestyle and metformin interventions used to prevent diabetes increase HDL-C, the mechanisms involved differ between the two treatments and may have consequences for future risk of cardiovascular disease.
Key terms: Adiponectin, HDL, Lifestyle change, Metformin
INTRODUCTION
Dyslipidemia, characterized by elevated triglyceride-rich lipoproteins, small dense LDL, and reduced HDL cholesterol (HDL-C), is a major cardiovascular disease (CVD) risk factor in type 2 diabetes (1) and is also common in subjects at high risk for diabetes (2) providing an opportunity for intervention to prevent CVD early in the development of diabetes. Furthermore since diabetes and CVD are believed to arise from “common soil” (3), interventions that retard diabetes development may also slow atherosclerosis. In the Diabetes Prevention Program (DPP), intensive lifestyle change (ILS) and metformin treatment in a population with impaired glucose tolerance (IGT) reduced the incidence of diabetes and ameliorated CVD risk factors (4). In particular, a reduction in large triglyceride rich particles was shown to occur with ILS, while both metformin and ILS decreased small dense LDL and increased the concentration of large HDL particles (5). While the mechanisms underlying the changes in triglyceride-rich and LDL have been well studied (6, 7) the basis for the effects of these interventions on changes in HDL are less well understood.
Adiponectin, an insulin-sensitizing adipokine, associates strongly with HDL-C (8, 9) and we recently demonstrated that adiponectin was linked to changes in lipoproteins in the DPP (5). Here we have explored in detail the nature of the associations between factors known to influence HDL such as weight, waist circumference, insulin resistance, glycemia, dietary factors and adiponectin, in order to evaluate the basis for the effect of these two interventions on HDL. In addition to measuring HDL-C, HDL subfractions were assessed to better understand the effects of the interventions on HDL. Furthermore since HDL has been shown to be influenced by inflammatory and endothelial factors, as well as having antithrombotic effects (10) and since both lifestyle change and metformin treatment may influence these processes, we also investigated whether the changes in HDL resulting from these interventions in DPP were associated with changes in biomarkers of these pathways. Finally, since we have shown that lifestyle and metformin alter HDL and its subfractions in different ways, we have examined whether the determinants of the HDL changes induced by these two interventions differ, since this could have therapeutic relevance.
MATERIAL AND METHODS
Study participants and procedures
The eligibility criteria, design, and methods of the DPP have been reported elsewhere (4). In brief, selection criteria included: age ≥25 years, BMI ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), fasting plasma glucose of 95–125 mg/dl and 2-hour post-load glucose of 140–199 mg/dl. Persons taking medications known to alter glucose tolerance, having experienced a CVD event in the prior 6 months or having illnesses that could interfere with participation in the trial were excluded. Participants were randomly assigned to one of three interventions: metformin 850 mg twice daily, placebo twice daily, or an intensive program of lifestyle modification (ILS) (Fig 1). Treatment assignments were stratified according to clinical center and double blinded for the metformin and placebo groups. The goals of the ILS were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a low-calorie, low-fat diet and to engage in moderate physical activity for at least 150 min/week. Diabetes was diagnosed on the basis of an annual OGTT or a semiannual fasting plasma glucose test according to American Diabetes Association criteria (11). The diagnosis required confirmation by a second test, usually within six weeks. Written informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
Figure 1.
CONSORT Flow Diagram
Clinical and metabolic variables
Standardized interviewer-administered questionnaires were used to obtain demographic and clinical data. Blood pressure, height, weight and waist circumference were measured using standard techniques (4). Dietary information was collected by interview at baseline and at year 1 using a modified Block food-frequency questionnaire (12). Total MET (metabolic equivalent) hours per week of physical activity were assessed by the 1-year recall using the Modifiable Activity Questionnaire (13). Glucose, insulin and lipid profile measurements and biomarker assays were performed at the Northwest Lipid Research Laboratories, University of Washington, Seattle as previously reported (4). For the HDL-C measurement, apoB-containing lipoproteins were removed from plasma by precipitation with dextran sulfate, and cholesterol in HDL particles was measured using Roche reagent on a Roche Modular P autoanalyzer. The accuracy of the method is monitored by the Center for Disease Control (CDC) reference method and the inter-assay CV is consistently <2%. Insulin sensitivity was assessed by the surrogate measurement 1/fasting insulin (1/FI) (14).
Biomarker assays
Total circulating adiponectin was measured using a latex particle-enhanced turbidimetric assay (Otsuka Pharmaceutical, Tokyo, Japan). The within-run and between-run coefficients of variation (CV) for this assay are 6.21% and 9.25% respectively. Plasma high-sensitivity C-reactive protein (CRP) and fibrinogen were measured immunochemically using Dade-Behring reagent on the Behring Nephelometer autoanalyzer (BNII), and the two CVs were 2.10% and 3.10% for CRP and 2.70% and 2.60% for fibrinogen respectively. Tissue plasminogen activator (tPA) levels were measured in citrated plasma using an ELISA assay (Asserachrom tPA; Diagnostica Stago), which measures total tPA antigen, with CVs of 6.45% and 6.70% respectively. Soluble E-selectin (sE-selectin) intercellular adhesion molecule 1 (sICAM-1), interleukin 6 (IL-6), and monocyte chemotactic protein 1 (MCP-1) were measured by ELISA assays from R&D Systems (Minneapolis MN). Within-run and between-run CVs for these ELISA assays were respectively 5.80% and 7.90% for sE-selectin, 4.6% and 5.5% for sICAM-1, 7.4% and 7.7% for IL-6 and 5.8% and 5.7% for MCP-1.
Lipoprotein NMR measurements
HDL subclass particle concentrations (P) and average HDL-P size were measured by NMR spectroscopy using the LipoProfile-3 algorithm at LipoScience, Inc, (now LabCorp, Raleigh, NC) (15) on a subgroup of the main cohort who had blood samples collected in heparin stored at −70° C until analysis. 1986 baseline samples and 1645 paired samples were available for analysis of change from baseline at 1 year. Although there were differences in HDL-C, adiponectin, and BMI at baseline there were no differences in changes of these variables in those with versus without NMR measurements at baseline and 1 year. The HDL-P size subclass categories investigated included large HDL-P (8.8 to 13 nm), medium-sized HDL-P (8.2 to 8.8 nm), and small HDL-P (7.3 to 8.2 nm) expressed in micromoles per liter. Weighted-average HDL-P size (in nanometer diameter units) was computed as the sum of the diameter of each subclass multiplied by its relative mass percentage as estimated from the amplitude of its NMR signal. HDL subclass distributions determined by NMR and gradient gel electrophoresis are highly correlated (15, 16). Inter-assay reproducibility (coefficient of variation), determined from 80 replicate analyses of 8 plasma pools over 20 days, was 6% for HDL-P, 0.7% for HDL size and 9, 14, and 6% for large, medium, and small HDL-P, respectively.
Data Analysis
Differences among treatment groups were assessed by the chi-square test for categorical covariates and ANOVA or median test (as appropriate) for continuous covariates with the significance level set at 0.01 using unadjusted p-values. Spearman correlations were used to describe the bivariate relationships among the lipoprotein measures, anthropometric and metabolic variables. The analyses of adjusted treatment effect on changes in HDL-C were assessed using analysis of covariance with adjustment for baseline value, age at randomization, sex, race/ethnicity plus baseline and changes in metabolic variables. The p-values for the treatment effects were adjusted for the 3 pairwise comparisons. Multiple regression models were used separately for each treatment group to examine which changes in key metabolic, anthropometric, and lifestyle variables accompanied the change in HDL-C from baseline to year 1. Change in HDL-C was the dependent variable with the following independent covariates: treatment assignment, age at baseline, sex, self-reported race, and baseline and change in metabolic variables with log transformations applied as appropriate and coefficients expressed per 1 sd unit.
RESULTS
Baseline characteristics
Table 1 demonstrates lifestyle, clinical and metabolic characteristics, HDL-C and HDL subfractions and novel biomarkers, stratified by sex. There were significant differences between men and women for most variables. In particular, values of HDL-C, large and medium HDL particle concentrations (P), total HDL-P, HDL size, BMI, CRP, IL-6, sICAM-1, fibrinogen, adiponectin and use of lipid lowering medications were greater in women, and triglyceride, waist circumference, small HDL-P, saturated fat intake, tPA, and sE-selectin were higher in men. The overall use of lipid lowering medications in this cohort was limited to 4.9% of participants. On average, all groups had been weight stable for the previous six months (4).
Table 1.
Baseline clinical and metabolic characteristics of the participants
| All | Women | Men | p-value | ||
|---|---|---|---|---|---|
| N* | 3070 | 2072 | 998 | ||
| Age (years) | 50.0 [43.3, 57.9] | 48.6 [42.4, 55.7] | 53.3 [46.4, 62.0] | <0.001 | |
| Phys Activity (met-hrs/wk) | 9.9 [3.9, 20.7] | 8.1 [3.2, 17.0] | 14.9 [5.8, 28.4] | <0.001 | |
| Alcohol | <0.001 | ||||
| % None | 1682(55.8%) | 1274 (62.7%) | 408 (41.6%) | ||
| % <1 drink/wk | 573(19.0%) | 390 (19.2%) | 183 (18.7%) | ||
| % 1 drink /wk to <1/day | 613(20.3%) | 310 (15.2%) | 303 (30.9%) | ||
| % ≥1 drink/day | 146(4.8%) | 59 (2.9%) | 87 (8.9%) | ||
| Diet saturated fat (g) | 23.9 [16.1, 35.1] | 23.0 [15.6, 34.0] | 25.8 [17.1, 37.8] | <0.001 | |
| BMI (kg/m2) | 32.8 [29.0, 37.4] | 33.8 [29.6, 38.7] | 30.9 [28.0, 34.4] | <0.001 | |
| Waist (cm) | 103.9 [94.8, 113.6] | 102.5 [93.0, 112.7] | 106.0 [98.8, 115.3] | <0.001 | |
| HbA1c (%) | 5.9 [5.6, 6.2] | 5.9 [5.6, 6.2] | 5.9 [5.6, 6.2] | 0.55 | |
| Log 1/FI (pmol/L) | −5.1 [−5.4, −4.7] | −5.1 [−5.5, −4.8] | −5.1 [−5.4, −4.7] | 0.435 | |
| Statin use (%) | 4.2% | 5.8% | 3.5% | 0.002 | |
| Lipid-lowering meds (%) | 4.9% | 7.0% | 4.0% | 0.002 | |
| Triglyceride (mmol/L) | 1.6 [1.1, 2.3] | 1.6 [1.1, 2.2] | 1.7 [1.2, 2.4] | <0.001 | |
| HDL-C (mmol/L) | 1.1 [1.0, 1.3] | 1.2 [1.0, 1.4] | 1.0 [0.9, 1.2] | <0.001 | |
| Large HDL-P (µmol/L)* | 3.4 [2.2, 5.4] | 4.2 [2.8, 6.1] | 2.4 [1.7, 3.5] | <0.001 | |
| Medium HDL-P (µmol/L)* | 11 [8.1, 14.7] | 12.3 [8.9, 16.5] | 9.2 [6.5, 12] | <0.001 | |
| Small HDL-P (µmol/L)* | 19.1 [16.1, 22.3] | 18.5 [15.4, 21.9] | 20.1 [17.3, 22.9] | <0.001 | |
| Total HDL-P (µmol/L)* | 34.4 [30.8, 38.8] | 35.9 [32.0, 40.2] | 32.3 [29.1, 35.5] | <0.001 | |
| HDL size (nm)* | 8.8 [8.6, 9.1] | 8.8 [8.7, 9.2 | 8.6 [8.5, 8.8] | <0.001 | |
| Adiponectin (ng/ml) | 7.3 [1.7, 7.6] | 7.8 [6.0, 10.3] | 6.3 [4.9, 8.0] | <0.001 | |
| tPA (ng/ml) | 10.9 [8.7, 13.4] | 10.4 [8.3, 12.7] | 12.0 [9.9, 14.6] | <0.001 | |
| Fibrinogen (mg/dL) | 374.0 [328.0, 432.0] | 388.0 [340.0, 448.0] | 348.0 [307.0, 397.0] | <0.001 | |
| CRP (mg/L) | 3.7 [1.7, 7.6] | 5.0 [2.5, 9.2] | 2.0 [0.9, 3.8] | <0.001 | |
| IL6 (pg/ml) | 1.9 [1.3, 3.0] | 2.1 [1.4, 3.2] | 1.6 [1.1, 2.5] | <0.001 | |
| MCP1 (pg/ml) | 138.0 [115.7, 167.1] | 135.3 [113.2, 165.4] | 143.7 [121.0, 169.6] | <0.001 | |
| sICAM (ng/ml) | 247.0 [209.6, 288.8] | 251.4 [212.0, 293.4] | 239.8 [203.6, 278.3] | <0.001 | |
| sE selectin (ng/ml) | 44.0 [32.9, 56.7] | 43.7 [32.3, 56.2] | 44.5 [34.2, 57.7] | 0.046 | |
Data are expressed in median [Interquartile range] or number (%).
HDL subfractions measured in a subset of 716 men and 1269 women.
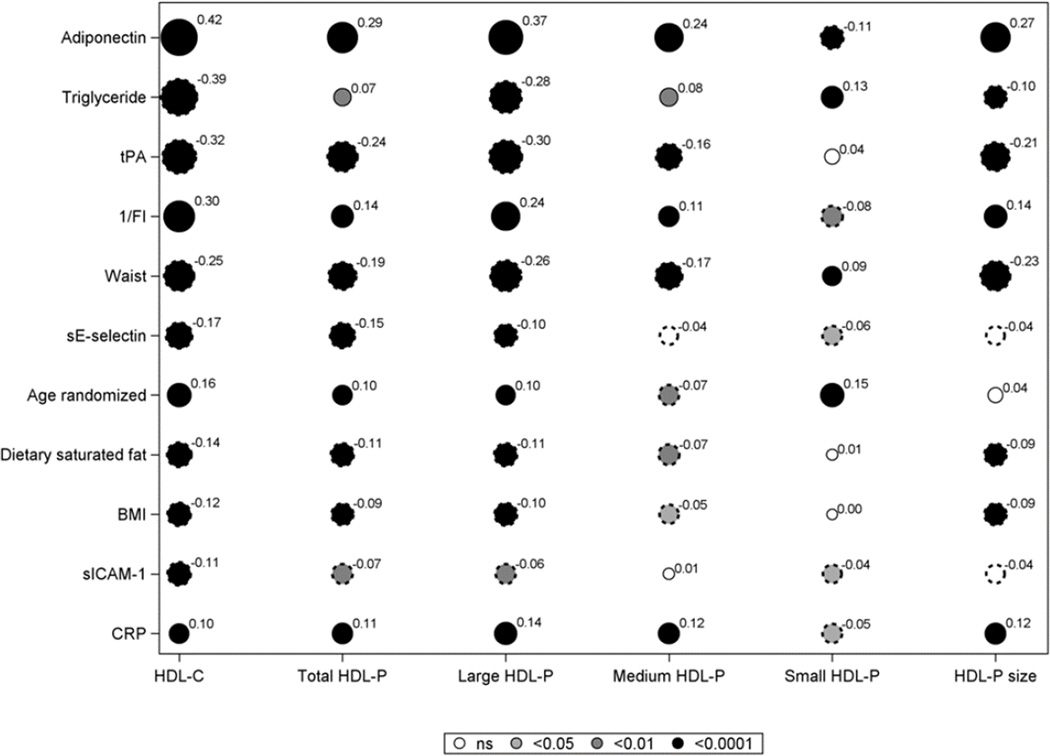
Fig. 2 demonstrates the bivariate correlations between baseline factors known to influence HDL-C concentrations as well as measured biomarkers, and HDL-C, HDL P and HDL size, ranked by r value on HDL-C. IL-6, MCP-1, fibrinogen, HbA1c, physical activity and alcohol intake had correlations of <0.10 with HDL-C and are not shown nor considered for further analysis except for HbA1c to assess the role of glycemia. Adiponectin and 1/FI had strong positive correlations with HDL-C while triglyceride, waist and tPA were inversely related to HDL-C. Similar associations were noted for these variables with total, large and medium HDL-P and HDL size. Small HDL-P was less strongly and inversely related to adiponectin and 1/FI, and directly associated with triglyceride, waist and age. There were more modest inverse associations for sE-selectin, BMI and saturated fat and direct associations for CRP with HDL-C and large and total HDL-P.
Figure 2.
Spearman correlation coefficients for baseline values of lifestyle, metabolic factors, and biomarkers with HDL-C, total HDL-P and HDL-P subfractions, and HDL size.
Solid circles indicate a positive correlation and scalloped circles a negative correlation. The diameter of each circle is proportional to the absolute value of the correlation shown as a superscript. The shading reflects the p-value: white for p>0.05; light grey for p<0.05 and medium grey for p<0.01 and black for p<0.0001. Variables with correlation of less than 0.1 with HDL-C are not shown.
Effects of interventions at 1 year
Table 2 depicts the 1 year changes in variables by intervention arm and for selected variables by sex. There were significant interactions by sex for waist, triglyceride, adiponectin, tPA, sE-selectin, saturated fat and HbA1c, and these are shown in the lower part of Table 2. For these variables the change in ILS was greater compared to both placebo and metformin groups in men and women. None of these variables changed in the metformin group versus placebo except waist and HbA1c in both sexes and tPA in men. For the remaining variables there were significant treatment differences without sex interactions. The effect of interventions on changes in HDL measures were similar in men and women except for changes in HDL size and large HDL-P in the metformin group, and in total HDL-P in both placebo and ILS groups which were greater in women. HDL-C, large HDL-P and HDL size increased with ILS, while small HDL-P decreased compared to placebo. In the case of metformin treatment similar though smaller increases were noted for HDL-C, large HDL-P and HDL size, and in addition small HDL-P increased compared to placebo. Large HDL-P, medium HDL-P and HDL-size increased more in the ILS versus the metformin group, whereas total and small HDL-P were higher with metformin treatment.
Table 2.
Characteristics after one year by treatment group
| Placebo | Metformin | Lifestyle | p-value | |
|---|---|---|---|---|
| BMI (kg/m2) | −0.16 (−0.26, −0.05) | −0.97 (−1.07, −0.87) | −2.42 (−2.57, −2.27) | <0.001*†‡ |
| Log 1/FI (pmol/L) | −0.03 (−0.06, 0.01) | 0.16 (0.13, 0.19) | 0.25 (0.21, 0.28) | <0.001* † ‡ |
| HDL-C (mmol/L) | 0 00(−0.01, 0.01) | 0.02 (0.01, 0.03) | 0.03 (0.02, 0.04) | <0.001* † |
| Large HDL-P (µmol/L) | 0.22 (0.09, 0.34) | 0.54 (0.42, 0.67) | 0.97 (0.82, 1.12) | <0.001* † ‡ |
| Medium HDL-P (µmol/L) | 0.26 (−0.15, 0.67) | −0.55 (−0.98, −0.11) | 0.65 (0.19, 1.11) | 0.0005*‡ |
| Small HDL-P (µmol/L) | 0.19 (−0.20, 0.58) | 1.04 (0.62, 1.46) | −1.35 (−1.79, −0.90) | <0.001* † ‡ |
| Total HDL-P (µmol/L) | 0.67 (0.31, 1.02) | 1.03 (0.67, 1.40) | 0.28 (−0.12, 0.67) | 0.019‡ |
| HDL size (nm) | 0.02 (−0.01, 0.04) | 0.05 (0.03–0.08) | 0.13 (0.18, 0.34) | <0.001† ‡ |
| Log CRP (mg/L) | −0.02 (−0.07, 0.02) | −0.14 (−0.19, −0.10) | −0.39 (−0.44, −0.35) | <0.001* † ‡ |
| sICAM (ng/ml) | −4.67 (−7.40, −1.93) | −14.84 (−17.88, −11.80) | −19.87 (−22.75, −16.99) | <0.001* † ‡ |
| MAQ physical activity (met-hr/wk) | 1.1 [−0.8, 3.0] | 1.4 [−0.5, 3.3] | 7.3 [5.8, 8.7} | <0.001† ‡ |
| % Statin use | 9.2% | 7.8% | 5.8% | 0.01† |
| % Lipid lowering meds | 10% | 8.6% | 6.5% | 0.02† |
| Waist (cm) | ||||
| Men | −0.55 (−1.05, −0.04) | −2.15 (−2.65, −1.65) | −7.63 (−8.37, −6.89) | <0.001* † ‡ |
| Women | −0.78 (−3.75, 2.60) | −2.24 (−5.55, 1.07) | −5.77 (−9.75, −1.40) | <0.001* † ‡ |
| Log Triglyceride (mmol/L) | ||||
| Men | −0.06 (−0.10, −0.02) | −0.04 (−0.08, 0) | −0.23 (−0.28, −0.19) | <0.001† ‡ |
| Women | −0.07 (−0.10,−0.04) | −0.05 (−0.08, −0.02) | −0.13 (−0.16, −0.11) | <0.001† ‡ |
| Adiponectin (ng/ml) $ | ||||
| Men | 0.15 (0.02, 0.28) | 0.12 (−0.02, 0.25) | 1.06 (0.85, 1.27) | <0.001† ‡ |
| Women | 0.08 (−0.02, 0.18) | 0.29 (0.16, 0.41) | 0.71 (0.58, 0.84) | <0.001† ‡ |
| tPA (ng/ml) $ | ||||
| Men | −0.86 (−1.20, −0.53) | −2.40 (−2.78, −2.03) | −3.26 (−3.69, −2.84) | <0.001* † ‡ |
| Women | −0.68 (−0.93, −0.44) | −1.89 (−2.13, −1.66) | −2.18 (−2.43, −1.93) | <0.001† ‡ |
| sE selectin (ng/ml) $ | ||||
| Men | 1.41 (0.07, 2.76) | 0.27 (−0.88, 1.41) | −5.64 (−7.03, −4.25) | <0.001† ‡ |
| Women | −0.88 (−1.82, 0.05) | −0.50 (−1.32, 0.32) | −4.10 (−4.79, −3.42) | <0.001† ‡ |
| Dietary saturated fat (g) $ | ||||
| Men | −5.02 (−6.79, −3.25) | −3.61 (−4.82, −2.40) | −12.57 (−14.30, −10.84) | <0.001† ‡ |
| Women | −4.17 (−5.31, −3.03) | −5.62 (−6.65, −4.58) | −11.01 (−12.09, −9.93) | <0.001† ‡ |
| HbA1c $ | ||||
| Men | 0.11 (0.07, 0.15) | −0.01 (−0.04, 0.02) | −0.14 (−0.17, −0.10) | <0.001* † ‡ |
| Women | 0.08 (0.05, 0.11) | 0.01 (−0.01, 0.03) | −0.07 (−0.09, −0.05) | <0.001* † ‡ |
Data are presented as mean change (95%CI) or percent.
p<0.05 for interaction of treatment group×sex;
p<0.05 for Placebo vs Metformin;
p<0.05 for Placebo vs Lifestyle;
p<0.05 for Metformin vs Lifestyle.
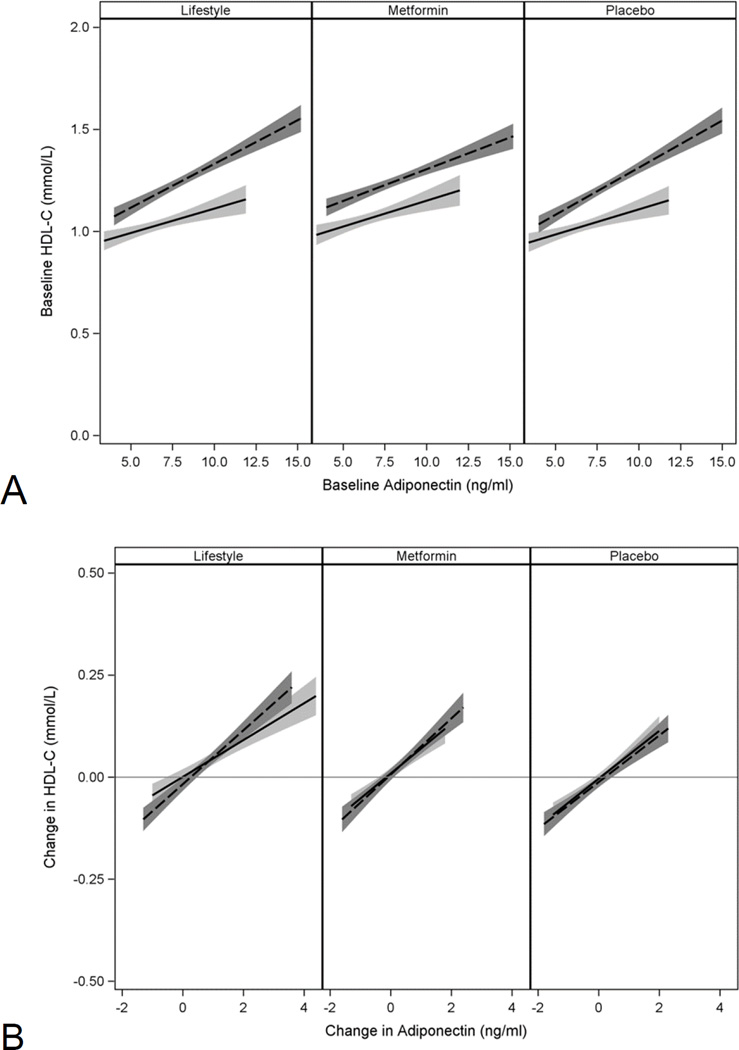
All biomarker values were reduced by ILS compared to placebo and metformin groups except adiponectin which was increased. CRP, sICAM-1 and tPA in men fell in the metformin group versus placebo. Because of the sex interaction for adiponectin we assessed the relationship between baseline and changes in adiponectin and HDL-C separately by sex. Women as expected had higher baseline HDL-C, and the association between baseline HDL-C and adiponectin was stronger for women than men (p<0.001) but similar in the two sexes for the changes at 1 year (Fig 3, Panels A and B).
Figure 3.
Relationship between baseline HDL-C and adiponectin levels (Panel A) and between 1 year changes in HDL-C and adiponectin (Panel B) according to treatment group.
Dashed lines for women, solid lines for men, shaded areas indicate 5–95 CI.
Determinants of the effects of interventions on HDL-C
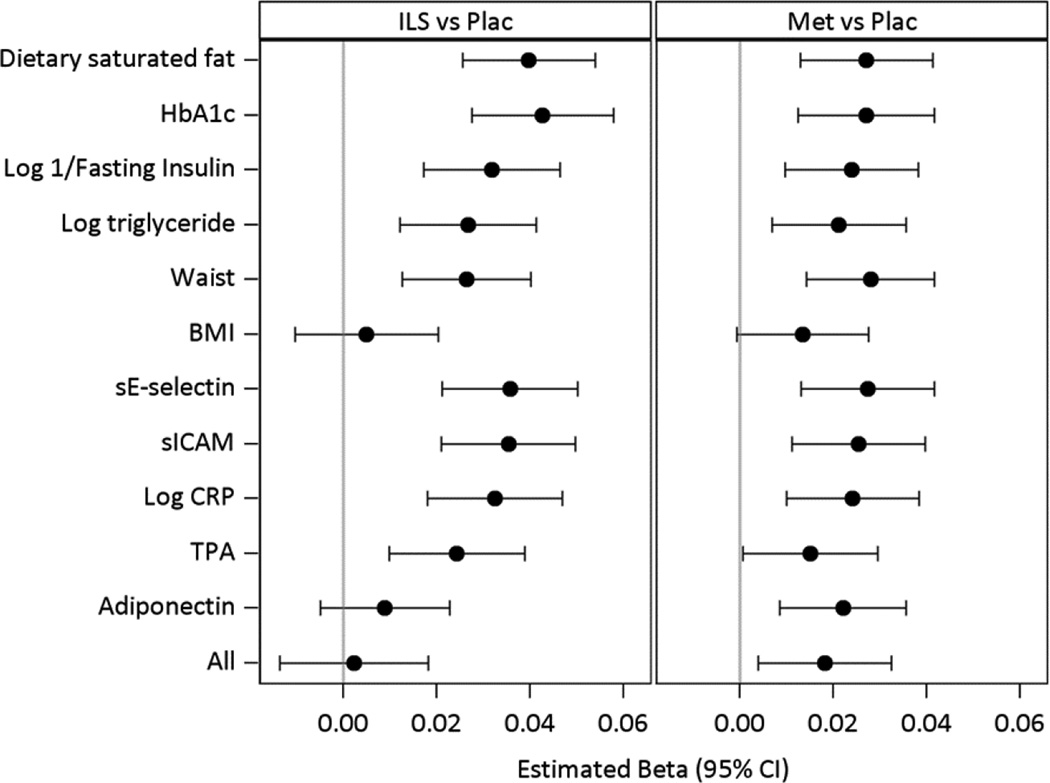
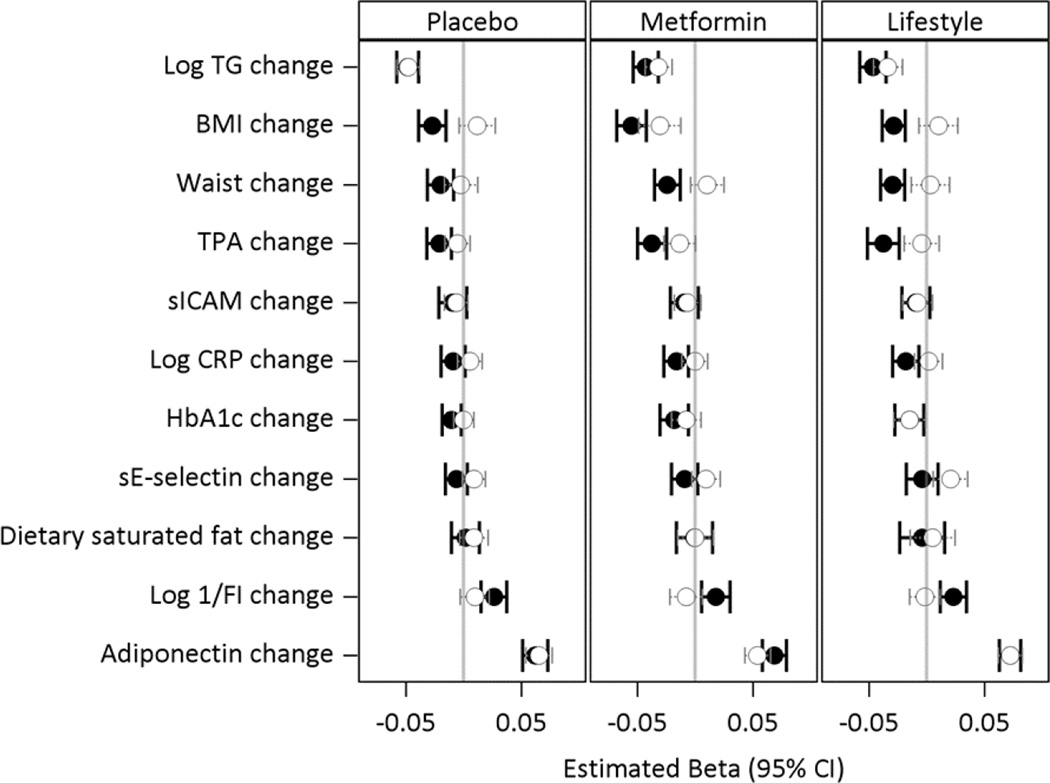
Fig 4 presents the results of models testing whether the differences in HDL-C changes accompanying ILS (left hand panel) and metformin treatment (right hand panel) compared to the placebo are accounted for by saturated fat intake, waist, BMI and metabolic factors known to influence HDL-C levels as well as selected biomarkers. The effect of the intervention adjusted for demographic variables only is the reference. Each subsequent model shows the effect of the standardized change and baseline value of the given variable added to the reference model on the HDL-C change adjusted for baseline HDL-C. The full model includes adjustment for all of the variables (All). Both lifestyle and metformin effects were diminished with adjustment for BMI. The only other measure that had significant influence in accounting for the effect of ILS was adiponectin. Each of BMI and adiponectin accounted for the ILS effect individually but only change in adiponectin and not change in BMI remained associated with the HDL-C in the full model (Fig 5). The metformin effect to increase HDL-C was partly explained by baseline and changes in BMI (metformin versus placebo p=0.06) and attenuated after adjustment for tPA (metformin versus placebo p=0.047).
Figure 4.
Adjusted effects of ILS and metformin on changes in HDL-C.
The effect of each variable (baseline and change after one year) is tested after adjustment for demographic factors in individual models as well as their combination in a full model (All) for their effects in accounting for the difference in the HDL-C change between the intensive lifestyle (ILS) and the placebo group in the left hand panel, and for the difference in HDL-C change between metformin and the placebo group in the right hand panel.
Figure 5.
Adjusted association of changes in risk factors on the change in HDL-C after 1 year of intervention by treatment group.
In the regression model adjusted for baseline age, sex, race-ethnic groups, and baseline value of the risk factor, the coefficients are expressed per 1 SD change in each variable shown. Panel A, Placebo group; Panel B, Metformin group; and Panel C Lifestyle (ILS) group. Each variable is tested in an individual model after adjustment for demographic factors and baseline level (dashed line) and in a full model which includes the effects of the changes and baseline values in all other variables (solid line). The standard deviations of the changes used in the models are Log TG (0.38), BMI (2.21), tPA (3.41), sICAM (47), log CRP (0.76), HbA1c (0.34), sE-selectin (11.4), dietary saturated fat (14.6), log 1/FI (0.52), and adiponectin (1.53).
Although triglyceride, waist, CRP, 1/FI and HbA1c did not influence the specific ILS and metformin-associated change in HDL-C, they were associated with 1-year changes in HDL-C in all three groups (CRP associated only in the metformin and ILS groups), but these associations were lost after adjustment for all co-variates including BMI, adiponectin and tPA, except for triglyceride in all 3 groups and HbA1c in the ILS group only (Fig 5).
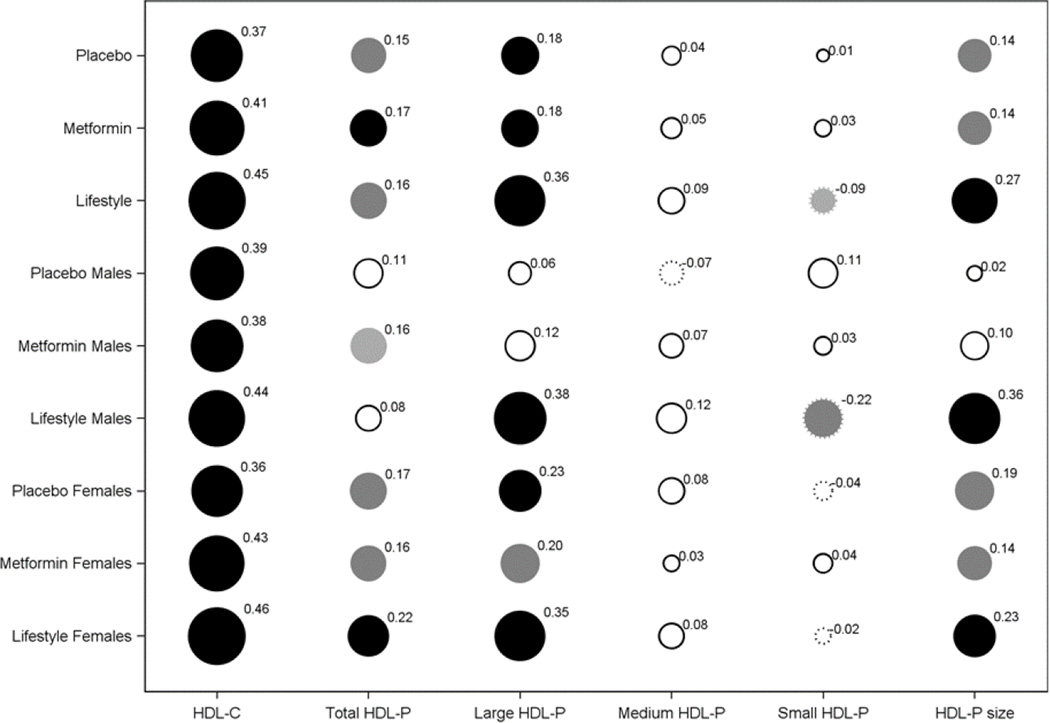
Associations between adiponectin change and change in HDL-C, HDL-P and HDL size
Fig 6 demonstrates associations between change in adiponectin and in HDL-C and HDL-P concentrations and size by sex and treatment group. Change in adiponectin correlated strongly with changes in HDL-C in both sexes within each intervention group. Adiponectin change was also positively associated with large HDL-P and HDL size in a similar manner to its pattern of association with HDL-C except in men from the placebo and metformin groups. There was no association between adiponectin change and small HDL-P except for an inverse correlation for men in the ILS, nor with medium HDL-P. There were moderate positive associations between adiponectin and total HDL-P changes in all three groups mainly in women.
Figure 6.
Spearman correlation coefficient for change in adiponectin by treatment group and sex and change in HDL-C, total HDL-P and HDL-P subfractions, and HDL size.
Solid circles indicate a positive correlation and scalloped circles a negative correlation. The diameter of each circle is proportional to the absolute value of the correlation shown by the superscript. The shadingr reflects the p-value: white for p>0.05; light grey for p<0.05 and medium grey for p<0.01 and black for p<0.0001.
DISCUSSION
In addition to slowing development of diabetes in high risk subjects, ILS and metformin treatment also improve cardiovascular risk factors such as low HDL-C. As previously reported in the DPP (5) although their effects on HDL-C were modest, they were associated with substantial but differing changes in HDL subfractions and size. In the ILS, the HDL-C increase was accompanied by increases of 40.4% and 7.1% respectively in large and medium-sized HDL-P and a 6.7% decrease in small HDL-P, resulting in an overall increase in HDL size but no net change in total HDL-P. By contrast in the metformin group both large and small HDL-P increased by 12.8% and 5.6% respectively with a 4.4% fall in medium-sized HDL-P, resulting in an increase in total HDL-P compared to ILS, but with no net change in HDL size.
To understand the determinants of these changes in HDL we first examined baseline associations between lifestyle and metabolic factors known to influence HDL-C as well as a group of biomarkers of inflammation, endothelial function and coagulation that could also be related to HDL change. Triglyceride, the index of insulin sensitivity 1/FI, age, dietary saturated fat, waist and BMI correlated with HDL-C levels. Neither alcohol intake nor physical activity which are both known to influence HDL-C levels were associated, although alcohol intake in this cohort is low and the measure used for physical activity has limitations. Among biomarkers, adiponectin and tPA - a surrogate measure of plasminogen activator inhibitor 1 (PAI-1) (17), were strongly associated with HDL-C, with more modest associations noted for the endothelial dysfunction markers sE-selectin and sICAM-1, and for CRP. Adiponectin was directly and strongly correlated with large, medium and total HDL-P and HDL size, but inversely with small HDL-P as previously reported (5). 1/FI had a similar but somewhat weaker effect. This pattern of association between adiponectin and HDL-P subfractions and size is consistent with an effect of adiponectin to shift the distribution of HDL particles from smaller to larger particles which would be expected to lead to a subsequent slowing in the fractional catabolic rate of HDL (18), combined possibly with an increase in HDL synthesis (19) leading to an increase in total HDL-P. Triglyceride had the opposite association, consistent with evidence that triglyceride elevation impairs the maturation of small HDL into larger particles (18). tPA was inversely associated with total, large and medium HDL-P and HDL size but was not related to small HDL-P. This finding agrees with previous work demonstrating an independent association between PAI-1 and HDL-C (20). Although the basis for this association is not known, it may reflect opposite effects of the inflammatory response associated with prediabetes on PAI-1 and HDL. Similar though weaker effects were noted with sE-selectin and sICAM-1 which may be related to the action of HDL to suppress adhesion molecule expression (21). The basis for the associations between insulin sensitivity are thought to be secondary to known effects of insulin resistance on triglyceride-rich lipoprotein metabolism, but could also be linked to HDL through adiponectin, an insulin sensitizer.
At 1 year, ILS increased physical activity and adiponectin levels, and reduced waist, BMI, insulin resistance, and saturated fat as well as CRP, IL-6, tPA, fibrinogen, sE-selectin, sICAM-1 and fibrinogen compared to the placebo group. When the changes in these variables were regressed on the difference in the HDL-C change in the ILS compared to the placebo groups, change in adiponectin and BMI but not waist circumference fully accounted for the ILS effect on HDL-C, with BMI dependent upon the adiponectin change. The discordance between the association of adiponectin- dependent BMI change on HDL-C and the absence of an effect of waist circumference, could reflect the more modest effect of visceral versus subcutaneous effect on adiponectin production (22). Changes in other variables such as triglyceride and 1/FI accounted little for the effect of ILS on change in HDL-C. The apparent independence of the association between changes in adiponectin and HDL-C from alterations in weight parallels our observation of a weight-independent association between adiponectin and prediction of diabetes development (23). These findings thus indicate that the increase in adiponectin resulting from ILS is linked to benefits that are independent of the accompanying weight change or change in waist circumference.
As was shown in the baseline analysis, change in adiponectin in the ILS was also strongly associated with changes in HDL-C, large HDL-P and HDL size in both sexes and with change in total HDL-P in women only. The overall similarity in the associations between adiponectin and HDL measures in the static baseline state and the more dynamic ILS-induced 1 year changes in these parameters, strengthens support for the concept that adiponectin enhances HDL maturation from smaller to larger particles. These findings could result from an effect of adiponectin to increase lipoprotein lipase (24) which would elevate the number of large HDL particles relative to small particles, enlarge HDL particle size and raise HDL-C by enhancing triglyceride-rich lipoprotein hydrolysis leading to increased transfer of cholesterol-rich lipoprotein surface material into HDL (25). As discussed, an effect of adiponectin on HDL production has also been noted (19) and it is also possible that causality is reversed and that the increase in HDL may lead to a rise in adiponectin levels (26).
In the course of preparing this work, a similar finding was reported from the Look AHEAD study, demonstrating that with intensive lifestyle change in subjects with type 2 diabetes, the rise in HDL-C could be accounted for by the increase in adiponectin after adjustment for weight, glycemia, triglyceride and physical fitness (27). Our results in prediabetic subjects confirm their findings. These authors did not measure HDL subfractions or size but they showed that both higher molecular weight and lower molecular weight forms of adiponectin contributed independently to the HDL-C change. Although total adiponectin levels were measured in our report, the Look AHEAD study demonstrated that together the two adiponection subfractions accounted for the same variance in HDL-C change as did total adiponectin.
In contrast to the findings in the ILS group, adiponectin changes did not account for the change in HDL-C associated with metformin treatment, that is, the effect of metformin to increase HDL-C is adiponectin-independent. Despite this, there remained an association between the HDL-C and adiponectin change in the metformin and placebo groups. Furthermore as in the ILS group, the change in adiponectin in the metformin and placebo groups also correlated directly with changes in large HDL-P and HDL size although this was not evident in the men. Overall, these findings attest to the robustness of the association between changes in adiponectin and HDL since it was evident even in circumstances where a major adiponectin-independent effect of metformin on HDL occurred. Furthermore unlike in the ILS, where adiponectin change was inversely related to changes in small HDL-P in men, there was instead a significant adiponectin-independent increase in small HDL-P in the metformin group that occurred in the absence of triglyceride change, suggesting a direct effect of metformin, perhaps on the generation of small HDL-P. A further difference between the effects of the two active interventions on HDL was the fact that change in BMI but not waist circumference continued to be associated with the increase in HDL-C even after adjustment for adiponectin, triglyceride and other variables in the metformin but not the ILS group. This indicates that the effects of the two interventions on weight have different metabolic consequences.
ILS and to a lesser extent metformin had favorable effects on levels of biomarkers of inflammation (CRP, IL-6), endothelial dysfunction (sE-selectin, sICAM-1), and coagulation (fibrinogen, tPA) in addition to adiponectin. Activation of these pathways have been shown to have deleterious effects on HDL concentration or function (87) and tPA, sE-selectin, sICAM-1 associated inversely with HDL-C at baseline. We therefore tested whether favorable intervention-related changes in the pathways reflected by these biomarkers were related to changes in HDL-C. Only sICAM and tPA associated with a change in HDL-C, although these were accounted for changes in other factors such as triglyceride, BMI, insulin sensitivity and adiponectin. However the change in tPA did attenuate the specific metformin-associated increase in HDL-C. Several previous studies have demonstrated that metformin lowers tPA levels (28), although the mechanism is not understood. The finding that the decrease in tPA levels in metformin treated subjects appeared to account for part of the metformin effect on HDL-C after adjustment for familiar factors such as triglyceride, BMI and insulin sensitivity is of interest and deserves further study.
In summary, the adiponectin increase was the major determinant of the HDL-C rise in the ILS group independent of the effects of favorable changes in weight, waist, insulin resistance, physical activity, triglyceride and saturated fat intake, as well as markers of inflammation, endothelial dysfunction and coagulation. The increase in adiponectin was strongly related to the increase in large HDL particles suggesting that changes in adiponectin increased HDL-C by enhancing the maturation of HDL. In contrast, although adiponectin change was related to HDL-C and large HDL-P change among participants treated with metformin it did not account for the specific metformin effect on HDL. Instead several adiponectin-independent effects were related to the rise in HDL-C after metformin but not ILS interventions, namely an effect operating via a change in BMI, an attenuating influence of tPA, and an increase in small HDL-P. Currently both United States and European guidelines for prevention of type 2 diabetes recommend lifestyle modifications aimed at weight reduction as a first approach, with metformin treatment as a secondary option (29, 30). Investigations such as this that identify differences in the effects of these interventions on metabolic outcomes in subjects at high risk for diabetes, highlight the importance of longer-term studies to evaluate the clinical significance of these findings.
Supplementary Material
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS. During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
ABBREVIATIONS
- DPP
Diabetes Prevention Program
- HDL-C
high density lipoprotein cholesterol
- HDL-P
high density lipoprotein particle concentration
- MET
metabolic equivalent
- 1/FI
1/fasting insulin
- CRP
high sensitivity C reactive protein
- tPA
tissue plasminogen activator
- sE-selectin
soluble E-selectin
- sICAM-1
soluble intercellular adhesion molecule
- IL-6
interleukin 6
- MCP-1
monocyte chemoattractant protein 1
- NMR
nuclear magnetic resonance
- ILS
intensive lifestyle change
- PAI-1
plasminogen activator inhibitor 1
Footnotes
Clinical Trials Registration: NCT00004992
Conflicts of interest: none
Authorship: All authors should have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
A complete list of Centers, investigators, and staff can be found in the Appendix.
REFERENCES
- 1.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316(7134):823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetes Prevention Program Research Group. Lipid, lipoproteins, C-reactive protein, and hemostatic factors at baseline in the Diabetes Prevention Program. Diabetes Care. 2005;28(10):2472–2479. doi: 10.2337/diacare.28.10.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner SM, Stern MP. Hazuda Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–2898. doi: 10.1001/jama.263.21.2893. 263. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–340. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, et al. Lifestyle and metformin treatment favorably influence lipoprotein sub-fraction distribution in the Diabetes Prevention Program. J Clin End Metab. 2013;98(10):3989–3998. doi: 10.1210/jc.2013-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 7.Packard CJ. Small dense low-density lipoprotein and its role as an independent predictor of cardiovascular disease. Curr Opin Lipidol. 2006;17(4):412–417. doi: 10.1097/01.mol.0000236367.42755.c1. [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 9.Weiss R, Otvos JD, Flyvbjerg A, Miserez AR, Frystyk J, Sinnreich R, Kark JD. Adiponectin and lipoprotein particle size. Diabetes Care. 2009;32(7):1317–1319. doi: 10.2337/dc09-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrougui H, Momo CN, Khalil A. Health benefits of high-density lipoproteins in preventing cardiovascular diseases. J Clin Lipidol. 2012;6(6):524–533. doi: 10.1016/j.jacl.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, et al. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 13.Kriska AM, Caspersen CJ. Introduction to the collection of physical activity questionnaires in a collection of physical activity questionnaires for health-related research. Kriska and Caspersen (Eds.). Centers for Disease Control and Prevention. Med Sci Sports Exerc. 1997;29(Supp):S5–S9. [PubMed] [Google Scholar]
- 14.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151(12):190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 15.Otvos JD, Jeyarajah EJ, Bennett, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38(9):1632–1638. [PubMed] [Google Scholar]
- 16.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Lowe GD, Danesh J, Lewington S, Walker M, Lennon L, Thomson A, et al. Tissue plasminogen activator antigen and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2004;25(3):252–259. doi: 10.1016/j.ehj.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Chan DC, Barrett PH, Ooi EM, Ji J, Chan DT, Watts GF. Very low density lipoprotein metabolism and plasma adiponectin as predictors of high-density lipoprotein apolipoprotein A-I kinetics in obese and nonobese men. J Clin Endocrinol Metab. 2009;94(3):989–997. doi: 10.1210/jc.2008-1457. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura F, Oku H, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun. 2007;358(4):1091–1095. doi: 10.1016/j.bbrc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Raiko JR, Oikonen M, Wendelin-Saarenhovi M, Siitonen N, Kähönen M, Lehtimäki T, et al. Plasminogen activator inhitor-1 associates with cardiovascular risk factors in healthy young adults in the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012;224(1):208–212. doi: 10.1016/j.atherosclerosis.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 21.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 22.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 219(1–2):9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Mather K, Funahashi T, Matsuzawa Y, Edelstein S, Bray G, Kahn S, et al. for the DPP Research Group. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57(4):980–986. doi: 10.2337/db07-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganguly R, Schram K, Fang X, Kim M, Rodrigues B, Thong FS, et al. Adiponectin increases LPL activity via RhoA/ROCK-mediated actin remodelling in adult rat cardiomyocytes. Endocrinology. 2011;152(1):247–54. doi: 10.1210/en.2010-0530. [DOI] [PubMed] [Google Scholar]
- 25.Taskinen MR, Nikkilä EA. High density lipoprotein subfractions in relation to lipoprotein lipase activity of tissues in man--evidence for reciprocal regulation of HDL2 and HDL3 levels by lipoprotein lipase. Clin Chim Acta. 1981;112(3):325–332. doi: 10.1016/0009-8981(81)90455-1. [DOI] [PubMed] [Google Scholar]
- 26.Van Linthout S, Foryst-Ludwig A, Spillmann F, Peng J, Feng Y, Meloni M, et al. Impact of HDL on adipose tissue metabolism and adiponectin expression. Atherosclerosis. 2010;210(2):438–444. doi: 10.1016/j.atherosclerosis.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi-Sunyer FX, Schwenke DC, et al. Look AHEAD Research Group. Adiponectin and the mediation of HDL-cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res. 2012;53(12):2726–2733. doi: 10.1194/jlr.M030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012 Jun;222(2):390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadigan C, Meigs JB, Rabe J, D'Agostino RB, Wilson PW, Lipinska I, et al. Framingham Heart Study. Increased PAI-1 and tPA antigen levels are reduced with metformin therapy in HIV-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab. 2001;86(2):939–943. doi: 10.1210/jcem.86.2.7410. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Position Statement. Prevention or Delay of Type 2 Diabetes Diabetes Care. 2015;38(Suppl. 1):S31–S32. doi: 10.2337/dc15-S008. [DOI] [PubMed] [Google Scholar]
- 31.Paulweber B, Valensi P, Lindström J, Lalic NM, Greaves CJ, McKee M, Kissimova-Skarbek K, Liatis S, Cosson E, Szendroedi J, Sheppard KE, Charlesworth K, Felton A-M, Hall M, Rissanen A, Tuomilehto J, Schwarz PE, Roden M for the Writing Group on behalf of the IMAGE Study Group. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res. 2010;42(Suppl. 1):S3–S36. doi: 10.1055/s-0029-1240928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.