Abstract
Identifying biological mechanisms through which the experience of adversity emerges as individual risk for mental illness is an important step towards developing strategies for personalized treatment and, ultimately, prevention. Preclinical studies have identified epigenetic modification of gene expression as one such mechanism. Recent clinical studies have suggested that epigenetic modification, particularly methylation of gene regulatory regions, also acts to shape human brain function associated with risk for mental illness. However, it is not yet clear if differential gene methylation as a function of adversity contributes to the emergence of individual risk for mental illness. Using prospective longitudinal epigenetic, neuroimaging, and behavioral data from 132 adolescents, we demonstrate that changes in gene methylation associated with lower socioeconomic status predict changes in risk-related brain function. Specifically, we find that lower socioeconomic status during adolescence is associated with an increase in methylation of the proximal promoter of the serotonin transporter gene, which predicts greater increases in threat-related amygdala reactivity. We subsequently demonstrate that greater increases in amygdala reactivity moderate the association between a positive family history for depression and the later manifestation of depressive symptoms. These initial results suggest a specific biological mechanism through which adversity contributes to altered brain function, which in turn moderates the emergence of general liability as individual risk for mental illness. If replicated, this prospective pathway may represent a novel target biomarker for intervention and prevention amongst high-risk individuals.
Introduction
While specific stressors including exposure to traumatic events or the experience of childhood maltreatment and neglect have been associated with increased risk for mental illness,1 non-specific, relatively common risk factors, such as low socioeconomic status, account for a large proportion of psychiatric morbidity in the population.2 Low socioeconomic status may confer risk through a variety of mechanisms, including higher levels of perceived and objective stress and cumulative environmental risk such as poor housing quality, noise pollution, and exposure to violence.3 Lower socioeconomic status (SES) has been associated with a host of negative outcomes including poorer general health and increased risk for mental illness including depression, anxiety, and addiction.2,4,5 Given the insidious nature of low SES, it is at best difficult to directly mitigate these associated risks. An alternative strategy is to identify specific biological processes mediating the association between lower SES and individual risk thereby creating possible targets through which risk may be buffered.
One candidate biological process through which the environment may be exerting its influence is epigenetics, which encompasses molecular mechanisms mediating the effects of extrinsic factors on intrinsic functions through the modulation of gene expression.6 In particular, experience-dependent methylation of gene regulatory regions such as proximal promoter sites has been proposed as one epigenetic mechanism through which stress may drive risk for future mental illness.6 Generally, relatively increased DNA methylation of gene promoters, which is most often associated with decreased gene expression, has been associated with exposure to both specific7 and non-specific stressors including lower SES.8 These epigenetic changes in gene expression subsequently impact risk-related brain function and behavior.9
Recent clinical studies have specifically implicated DNA methylation of the proximal promoter region of the serotonin transporter gene (SLC6A4) as a potential epigenetic mechanism of increased risk for mental illness. For example, the experience of specific (i.e., child abuse)10 and non-specific (i.e., lower SES)11 stressors is associated with increased methylation of multiple CpG sites within SLC6A4, including the proximal promoter, and these patterns of methylation have been associated with higher depressive symptoms.12 Moreover, we have demonstrated that relatively increased SLC6A4 proximal promoter methylation is associated with increased threat-related amygdala reactivity, which not only plays a central role in stress responsiveness but also is implicated in the etiology and pathophysiology of stress-related disorders including depression.13,14 Collectively these epigenetic findings are consistent with the important modulatory role of the serotonin transporter on stress physiology, amygdala function, and mood.15
Despite these findings, cross-sectional data collected at one point in time do not allow for examination of dynamic changes in DNA methylation. Thus, it is unclear if the experience of stress is associated with a change in SLC6A4 methylation and, if so, how this manifests as a change in risk-related brain function. Here we use longitudinal epigenetic, neuroimaging, and behavioral data to construct a prospective indirect effects model examining whether lower socioeconomic status predicts increased future risk for depression via increased SLC6A4 proximal promoter methylation and resulting sensitization of threat-related amygdala reactivity. We test this model in adolescents at high and low risk for depression due to presence or absence of a family history of depression, respectively.
In prior analyses of this cohort, we have found that either a positive family history of depression or exposure to stressful life events predict greater increases in the reactivity of the left amygdala to fearful facial expressions from Wave 1 to Wave 2.16 Thus, we first examined if adversity-related changes in SLC6A4 proximal promoter methylation may, in part, explain this sensitization of amygdala reactivity. We modeled the effect of three types of stressors previously associated with risk for depression, including childhood maltreatment, stressful life events, and low socioeconomic status, on changes in methylation. We hypothesized that exposure to these stressors would be associated with an increase in SLC6A4 proximal promoter methylation from Wave 1 to Wave 2, and that this increase would be associated with sensitized amygdala reactivity to threat over the same time period.
Although a positive family history is one of the strongest predictors of the future development of depression, not all individuals with this risk factor will become depressed.17,18 Thus, we next examined the extent to which greater increases in threat-related amygdala reactivity associated with differential SLC6A4 methylation predicted individual risk for depression in adolescents and whether this risk was specific for youth with a positive family history of depression. Prior studies have found that threat-related amygdala reactivity predicts greater psychological vulnerability to future trauma,19,20 and we recently demonstrated in a large sample of young adults that a baseline measure of threat-related amygdala reactivity predicts the likelihood of experiencing anxiety and depression symptoms following subsequent exposure to stressful life events several years later.14 Accordingly, we hypothesized that increases in amygdala reactivity from Wave 1 to Wave 2 would predict greater increases in depressive symptoms from Wave 2 to Wave 3, approximately one year later.
Materials and Methods
Participants
In line with our prior work,13 analyses focused on the subset of 183 Caucasian, non-Hispanic participants from the Teen Alcohol Outcomes Study (TAOS)16 in order to avoid potential population stratification due to differences in ancestry (see Supplementary Table 1 for full sample demographics). We used genetic ancestry21 to confirm self-report of race/ethnicity (see Supplemental Methods).
Participants were 11 to 15 years old at Wave 1, 13 to 18 years old at Wave 2, and 14 to 19 years old at Wave 3. After complete description of the study to the participants, parents provided written informed consent and participants provided assent following procedures approved by the Institutional Review Board at the University of Texas Health Sciences Center San Antonio. Twenty participants (19 positive for a family history of depression [FH+] and 1 negative for a family history [FH−]) had an anxiety disorder diagnosis at study entry or developed an anxiety disorder between Waves 1 and 2. Anxiety diagnosis (dummy-coded) was included as a covariate in analyses run in the full sample, but removed as a covariate in multi-group analyses given the single diagnosis in the FH− group. Seven FH+ participants developed a diagnosis of major depression between Waves 1 and 2 and 2 FH+ participants developed a diagnosis between Waves 2 and 3. We did not include depression diagnosis as a covariate since depressive symptoms at Wave 2 were included as a covariate in all analyses. There were 21 pairs of siblings included in this sample. Recruitment procedures, attrition, and exclusion of participants for fMRI quality control at Waves 1 and 2 have been reported previously.16 We also examined characteristics of attrition from Wave 1 to 3. Fewer FH+ participants (n=33) completed the Wave 3 follow-up compared to FH− participants (n=46), χ2(1)=5.12, p=.024. There were no differences in attrition based on gender, age, CTQ emotional neglect scores, stressful life events, SES, or depressive symptoms assessed at Wave 1.
Environmental risk factors
Consistent with our prior research,22 emotional neglect was assessed at Wave 1 with the emotional neglect subscale of the Childhood Trauma Questionnaire.23 To assess SES at Wave 1, parents reported education levels and income for themselves and their spouses (if married). Consistent with prior research,24 a composite SES indicator was calculated by averaging the standardized values of these variables (Supplementary Table 2). Stressful life events occurring the year prior to the first fMRI scan were assessed at Wave 1 with the Stressful Life Events Schedule.25 As reported in our prior research,16 objective threat ratings for each life event were squared, summed, and averaged to obtain a mean level of objectively-rated stressful life events occurring the year prior to the first wave.
SLC6A4 methylation
Details regarding acquisition and analysis of SLC6A4 methylation have been reported previously for the first wave of the study.13 Procedures were identical for obtaining methylation levels at the second wave of the study. In line with prior research,13 data from the 20 CpG sites proximal to the transcription start site of SLC6A4 measured at Wave 1 and Wave 2 were entered into a principal components analysis. Consistent with our prior research conducted with Wave 1 data,13 the principal component analysis including Wave 1 and Wave 2 data resulted in 5 components with eigenvalues greater than 1, and scores for the first component (representing 24.1% of the total variance) were extracted for each individual (Supplementary Table 3). Similar to our prior work in this sample,16 residualized change scores were calculated to assess changes greater or less than expected at Wave 2 based on Wave 1 values. Methylation data were available for 178 participants at Wave 1 and 136 participants at Wave 2; residualized change scores (i.e., for those participants that provided valid data at both Wave 1 and Wave 2) were available in 132 participants.
Amygdala reactivity
Details regarding acquisition, pre-processing, and analysis of fMRI data have been reported previously.16 All analyses were conducted with mean parameter estimates extracted from functional clusters significant at p<.05 family-wise error corrected within amygdala regions of interest. We focused analyses on values extracted from the left amygdala for the contrast of Fearful Faces > Shapes, based on our prior finding that left amygdala reactivity to fearful faces increased from Wave 1 to Wave 2 in adolescents at higher risk for depression.16 In order to examine specificity of effects to the basolateral or centromedial amygdala, we also extracted values for the contrast of Fearful Faces > Shapes using probabilistic regions of interest based on cytoarchitectonic mapping26 and corrected for multiple comparisons by applying a Bonferroni-corrected threshold of p<.025. Similar to the procedure with SLC6A4 methylation, we calculated a residualized change score. Imaging data were available for 139 participants at Wave 1 and 107 participants at Wave 2; change scores were available in 87 participants.
Depressive symptoms
Adolescents completed the Youth Self Report27 at each wave of the study. To assess depressive symptoms, we used Affective Problems scores from the DSM-oriented scales,28 which have been shown to correspond to DSM-IV major depressive disorder symptoms.29 Residualized change scores were calculated to assess changes in symptoms from Wave 2 to 3. Symptom scores were available in 136 participants at Wave 2 and 79 participants at Wave 3; residualized change scores were available in 79 participants.
Covariates
We included the following as covariates on all paths of the model: age at Wave 1, time (in years) between waves, gender (dummy-coded: 0 = Male, 1 = Female), anxiety diagnosis (except in multi-group models), and the risk factors (CTQ emotional neglect, SES, and stressful life events at Wave 1). Note that the risk factors were predictors of interest in the first path and that they were then carried forward as covariates on all other paths of the model. Family history (dummy-coded: 0 = negative family history, 1 = positive family history) was also included as a covariate in analyses conducted in the full sample; this was removed as a covariate when conducting multi-group analyses to test for moderation by family history. As described in detail elsewhere,13 a subset of participants (n=103) were genotyped for the serotonin transporter-linked polymorphic region (5-HTTLPR) and rs25531. Genotype (dummy-coded: 0 = LALA; 1 = LA/S, LA/LG, S/S, LG/LG, S/LG) was included as a covariate on all paths. Genotype distribution did not deviate from Hardy-Weinberg equilibrium for the 5-HTTLPR, χ2(1)=.001, p=.97, or rs25531, χ2(1)=.44, p=.51, genotypes. Finally, to ensure that changes in methylation and amygdala reactivity between Wave 1 and Wave 2 emerged prior to the onset of depressive symptoms, we also included YSR Affective Problems scores at Wave 2 as a covariate in all analyses. The one exception was in the final path (predicting residualized change in Affective Problems scores from Wave 2 to 3) because YSR Affective Symptoms at Wave 2 are implicitly controlled for using residualized change as an outcome. We also examined gender as a moderator of all paths of the analysis (reported in Supplemental Information).
Statistical Analyses
All statistical analyses were conducted using MPlus v7 software with full information maximum likelihood estimation, which provides unbiased estimates in the presence of missing data. Each path was first estimated in the full sample including all covariates described above. All predictors were mean centered to aid in interpretation of the results and the variance and covariance of all predictors was modeled. Next, multi-group analyses were conducted to test for moderation by family history. Moderation was assessed using χ2 difference tests; if constraining parameter estimates to be equal between groups leads to a significant decrease in model fit, then one can conclude the effect is significantly moderated by family history. Additional tests to examine the robustness of these effects are reported in the Supplemental Information.
After testing each path individually, we then constructed an indirect effects model to test the indirect effect of environmental risk factors on changes in depressive symptoms, mediated by changes in SLC6A4 methylation and amygdala reactivity to fear. Based on results from testing paths individually, we constrained the paths from environmental risk to SLC6A4 methylation and from SLC6A4 methylation to amygdala reactivity to be equal across family history groups, but allowed the final path (change in amygdala reactivity to change in symptoms) to be free across the positive family history and negative family history groups given evidence for moderation. We also freed each parameter in the model individually and tested whether allowing parameter estimates for any of the covariates or variances to vary by family history improved the fit of the model. None of the covariates were significantly moderated by family history; therefore, all parameters except that path were constrained to be equal in the model. The 5-HTTLPR genotype covariate was removed from this model due to poor model fit; removing this covariate from each path resulted in a significant improvement in model fit, Δχ2(4)=50.22, p<.001. To provide a measure of general effect size for the indirect effect, we report the product of coefficients αβ statistic. We also provide bootstrapped confidence intervals, which use a Monte Carlo simulation (1,000 draws), which do not assume normality of the distribution of indirect effects to assess significance.
Results
Lower socioeconomic status at Wave 1 predicts greater increases in SLC6A4 proximal promoter methylation at Wave 2
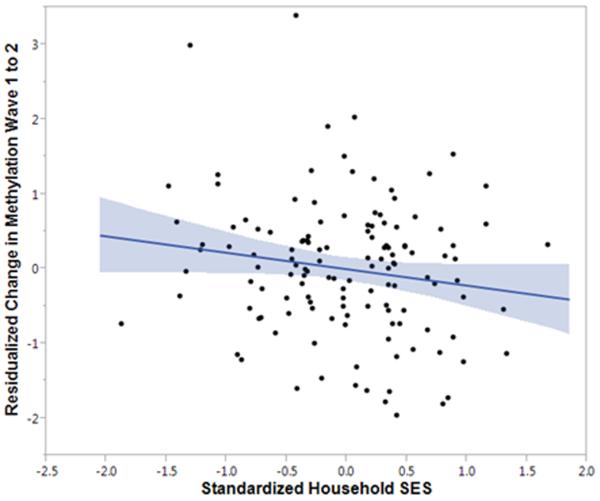
We first tested whether stressors previously associated with risk for depression predicted changes in SLC6A4 methylation. Consistent with our hypothesis, lower socioeconomic status measured at Wave 1 prospectively predicted greater increases in SLC6A4 proximal promoter methylation two years later at Wave 2, B=−.33, SE=.1, Beta=−.24, p=.02, Δr2=.05 (Figure 1). In fact, SES was the only measure of environmental stress that predicted changes in SLC6A4 proximal promoter methylation (Supplementary Table 4). Although SES significantly varied as a function of familial risk in the expected direction (i.e., lower in those with a positive family history), the effect of SES on SLC6A4 methylation was not moderated by family history for depression, Δχ2(1)<.001, p>.99. In other words, lower SES predicted an increase in SLC6A4 proximal methylation over time to the same degree in adolescents with or without a positive family history of mental illness.
Figure 1. Lower socioeconomic status at Wave 1 predicts greater increases in SLC6A4 methylation from Wave 1 to 2.
Standardized household SES was calculated by obtaining the mean of standardized scores of parents' education and income levels, as well as spouses' education and income levels if reporting parent was married. Lower SES at Wave 1 was associated with greater increases in methylation at Wave 2, B=−.33, Beta=−.24, SE=.1, p=.02, Δr2=.05. Shaded area represents 95% confidence intervals.
Increases in SLC6A4 proximal promoter methylation from Wave 1 to Wave 2 predict greater increases in threat-related amygdala reactivity over the same time period
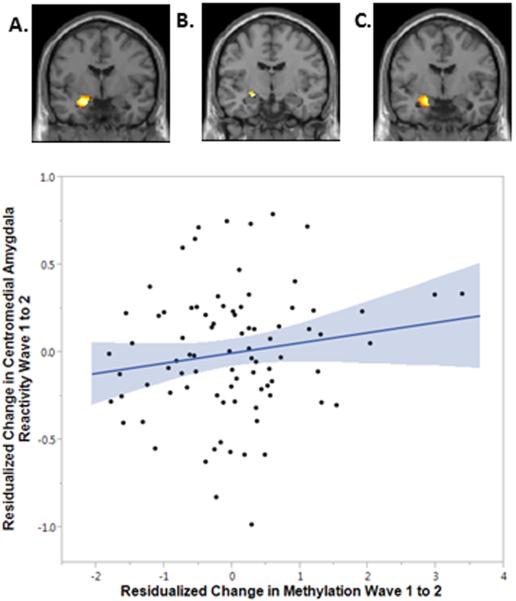
Having identified that lower SES is associated with an increase in SLC6A4 proximal promoter methylation over time, we next asked if this change in methylation was associated with the change in left amygdala reactivity to fearful facial expressions previously noted in this cohort.16 We found that greater increases in SLC6A4 proximal promoter methylation from Wave 1 to Wave 2 were associated with greater increases in reactivity during the same time window, B=.09, SE=.04, Beta=.21, p=.04, Δr2=.05. Subsequent analysis by amygdala sub-region revealed this effect was driven by increases in centromedial but not basolateral amygdala reactivity, B=.10, SE=.04, Beta=.26, p=.01, Δr2=.08 (Figure 2; Supplementary Table 5). As observed for SES effects on methylation, the association between SLC6A4 methylation and reactivity was not moderated by familial risk, Δχ2(1)=1.02, p=.31. Thus, increases in SLC6A4 proximal promoter methylation predict increases in threat-related centromedial amygdala reactivity independently of family history of depression.
Figure 2. Greater increases in SLC6A4 methylation are associated with greater increases in centromedial amygdala reactivity to fearful facial expressions from Wave 1 to Wave 2.
Mean parameter estimates were extracted from functional clusters activated at both Wave 1 and 2 within the whole left amygdala (A), the left centromedial amygdala (B), and the left basolateral amygdala (C) for the contrast of Fearful Faces > Shapes. Greater increases in SLC6A4 methylation were associated with greater increases in centromedial amygdala reactivity, B=.10, Beta=.26, SE=.04, p=.01, Δr2=.08.
Increases in threat-related amygdala reactivity from Wave 1 to Wave 2 predict future increases in depressive symptoms from Wave 2 to Wave 3 one year later
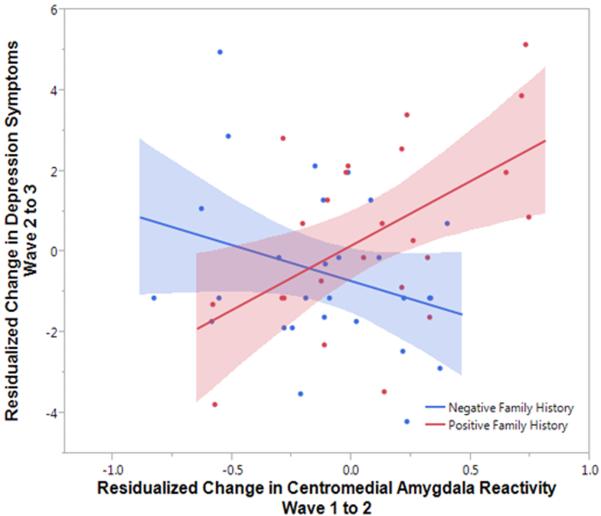
Analyses revealed a significant moderating effect of familial risk on the association between increases in amygdala reactivity and future depressive symptoms, Δχ2(1)=5.92, p=.01. In adolescents with a positive family history, greater increases in reactivity from Wave 1 to Wave 2 prospectively predicted greater increases in depressive symptoms from Wave 2 to Wave 3, B=2.71, SE=1.1, Beta=.46, p=.02, Δr2=.21 (Figure 3; Supplementary Table 6). There was no such relationship in adolescents without a family history of depression, B=−.95, SE=1.3, Beta=−.17, p=.47, Δr2=.02.
Figure 3. Greater increases in centromedial amygdala reactivity to fearful faces from Wave 1 to Wave 2 predict greater increases in depressive symptoms from Wave 2 to Wave 3 in adolescents with a positive family history of depression.
Residualized change scores were calculated with symptoms from the Youth Self Report (YSR) Affective Problems scale at Wave 2 and 3. Greater increases in centromedial amygdala reactivity predicted greater increases in symptoms one year later in the positive family history group, B=2.71, SE=1.1, Beta=.46, p=.02, Δr2=.21.
Lower socioeconomic status in adolescence has an indirect effect on future changes in depressive symptoms mediated by increased methylation and threat-related amygdala reactivity
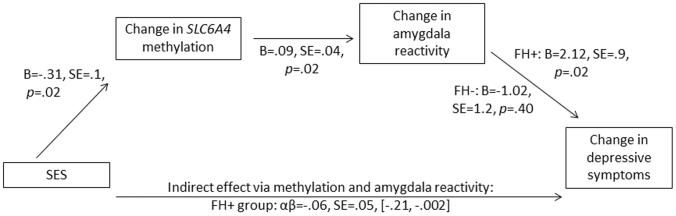
The above analyses separately established effects between lower SES and increases in SLC6A4 proximal promoter methylation, increases in methylation and increases in amygdala reactivity, and increases in reactivity and future depressive symptoms. Lastly, we constructed a moderated mediation model to simultaneously examine the indirect effect of lower SES measured at Wave 1 on changes in depressive symptoms from Wave 2 to Wave 3, mediated by changes in SLC6A4 methylation and threat-related amygdala reactivity. The path from amygdala reactivity to changes in symptoms was freed to vary between the family history groups given the finding of significant moderation. All other paths were constrained to be equal across groups. The indirect effect was estimated by generating bias-corrected 95% confidence intervals with 1,000 bootstrapped samples; confidence intervals that do not include 0 indicate a significant indirect effect. The final model had a good fit, Δχ2(38)=28.87, p=.86, RMSEA=.00, CFI=1.00, SRMR=.07. As expected, SES had an indirect effect on changes in depressive symptoms in the positive family history group only, αβ=−.06, SE=.05, 95% confidence intervals [−.21, −.002] (Figure 4). This negative indirect effect indicates that lower SES predicts greater than expected increases in SLC6A4 methylation and centromedial amygdala reactivity, which in turn predicts greater than expected increases in future depressive symptoms. In contrast, this indirect effect was not significant for the negative family history group, αβ=.03, SE=.05, 95% confidence intervals [−.02, .17].
Figure 4. Moderated mediation model examining indirect effect of SES on future changes in depressive symptoms, mediated by changes in SLC6A4 methylation and centromedial amygdala reactivity to fear.
Not shown in figure: covariates included on each path were age, gender, emotional neglect, stressful life events, SES, time between waves, and depressive symptoms at Wave 2 (except on the final path). 95% confidence intervals for the indirect effect were obtained by requesting 1,000 bootstrapped samples using Mplus v7. SES=socioeconomic status assessed at Wave 1; FH+=positive family history; FH−=negative family history.
Discussion
Our results provide initial evidence for a prospective biological pathway through which a common stressor, namely lower socioeconomic status, may increase risk for future depression in high-risk adolescents. Specifically, lower SES predicted residualized change in SLC6A4 proximal promoter methylation, which in turn predicted residualized change in threat-related reactivity of the centromedial amygdala, which drives a number of autonomic responses to stress including activation of the hypothalamic pituitary adrenal axis. These findings extend our earlier observation identifying a cross-sectional relationship between methylation and amygdala reactivity in two separate cohorts13 and suggest that relative methylation status of the SLC6A4 proximal promoter region is a reliable predictor of amygdala reactivity across time. Because socioeconomic status was on average lower amongst adolescents with a positive family history of depression, and this pathway is uniquely associated with the emergence of depressive symptoms in these adolescents, this may partially explain our prior finding that these same adolescents show greater increases in amygdala reactivity over time.16
Our results further demonstrate that an increase in risk-related brain function prospectively predicts an increase in depression symptoms approximately one year later particularly among high-risk adolescents. Notably, we found that sensitized amygdala reactivity only predicted increases in depressive symptoms amongst adolescents with a positive family history of depression. It is possible that these high-risk adolescents experience additional forms of chronic adversity such as parental neglect or familial discord uncommon amongst their low-risk counterparts, and that this additional exposure is necessary to trigger symptoms of depression. This is consistent with our finding in an independent sample of young adults that increased amygdala reactivity to threat only predicts future internalizing symptoms in response to higher levels of stress.14
As our follow-up measure of depressive symptoms was limited to self-report, however, it is unclear if this pathway predicts clinically significant levels of dysfunction. Of course, we did not have access to brain-derived DNA and were limited to assays of SLC6A4 methylation in DNA derived from peripheral tissues. But, there is evidence that methylation of SLC6A4 may be conserved across DNA derived from multiple tissue types including blood, saliva, and brain.13 These limitations notwithstanding, our results identify a specific biological pathway through which broader environmental adversity may act to drive individual vulnerability for depression amongst at-risk adolescents.
In addition to increased chronic stress experienced by parents and their offspring, lower SES is further associated with a range of environmental risk factors that may bias developmental changes in DNA methylation, including poor nutrition and smoking.30,31 Identifying specific environmental mechanisms contributing to the effects of SES on methylation observed here will help narrow targets for possible intervention. Moreover, preventive interventions such as training in mindfulness-based techniques may be effective in lowering threat-related amygdala reactivity in adolescents identified as high-risk due to increased SLC6A4 methylation.32 Isolation of family process factors (e.g., high family conflict) that help explain why higher amygdala reactivity predicts depressive symptoms only in FH+ adolescents will help further focus on targets for prevention within a family context. Thus, if replicated, the risk pathway identified here could represent a discrete biomarker that could be targeted by novel strategies for personalized treatment and prevention.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant R01AA016274 and the Dielmann Family (DEW), MH087493 (Consuelo Walss-Bass, Ph.D.), the National Institute on Drug Abuse grant R01DA033369 and R01DA031579 (ARH), the National Institute on Aging grant R01AG049789 (ARH), and the Center for the Study of Adolescent Risk and Resilience, NIH grant P30DA023026 (JRS). We thank Ryan Bogdan, Annchen Knodt, and Caitlin Carey for assistance with conducting analyses and Yuliya Nikolova for discussions of the manuscript.
Footnotes
Supplementary information is available at Molecular Psychiatry's website.
Author Contributions ARH and DEW designed the study; DEW oversaw analysis of DNA methylation; ARH and JRS oversaw analysis of functional imaging data; JRS conducted the statistical analyses and drafted the manuscript; all authors edited and approved the final manuscript.
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depresssion and obesity. Am J Public Health. 2003;93(11):1844–1850. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 4.Fryers T, Melzer D, Jenkins R. Social inequalities and the common mental disorders: A systematic review of the evidence. Soc Psychiatry Psychiatr Epidemiol. 2003;38(5):229–237. doi: 10.1007/s00127-003-0627-2. [DOI] [PubMed] [Google Scholar]
- 5.Barbeau EM, Krieger N, Soobader M. Working class matters: Socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2010;94(2):269–278. doi: 10.2105/ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagot RC, Meaney MJ. Epigenetics and the biological basis of gene x environment interactions. J Am Acad Child Adolesc Psychiatry. 2010;49(8):752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beach SR, Brody GH, Lei MK, Kim S, Cui J, Philibert RA. Is serotonin transpoter genotype associated with epigenetic susceptibility or vulnerability? Examination of the impact of socioeconomic status risk on African American youth. Dev Psychopathol. 2014;26:289–304. doi: 10.1017/S0954579413000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolova YS, Hariri AR. Can we observe epigenetic effects on human brain function? Trends Cogn Sci. 2015;19(7):366–373. doi: 10.1016/j.tics.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: An examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach SRH, Dogan MV, Brody GH, Philibert RA. Differential impact of cumulative SES risk on methylation of protein-protein interaction pathways as a function of SLC6A4 genetic variation in African American young adults. Biol Psychol. 2014;96:28–34. doi: 10.1016/j.biopsycho.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transpoter gene and depressive symptoms: A monozygotic twin study. Psychosom Med. 2013;75(6):523–529. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolova YS, Koenen KC, Galea S, Wang C, Seney ML, Sibille E, et al. Beyond genotype: Serotonin transporter epigenetic modification predicts human brain function. Nat Neurosci. 2014;17(9):1153–1155. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: Effects of family history for depression and stressful life events. Am J Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson DE, Birmaher B, Axelson D, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry. 2004;43(3):291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163:1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 19.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. PNAS. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, et al. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety. 2014;31(10):834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal component analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 22.Hanson JL, Hariri AR, Williamson DE. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry. 2015;78(9):598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 24.Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. SCAN. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, et al. The Stressful Life Events Schedule for children and adolescents: Development and validation. Psychiatry Res. 2003;119(3):225–241. doi: 10.1016/s0165-1781(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 26.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach TM. Manual for the Youth Self-Report and 1991 Profiles. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- 28.Achenbach TM, Dumenci L, Rescorla LA. DSM-oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adolesc Psychol. 2003;32(3):328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- 29.Ferdinand RF. Validity of the CBCL/YSR DSM-IV scales Anxiety Problems and Affective Problems. J Anxiety Disord. 2008;22:126–134. doi: 10.1016/j.janxdis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolsecent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153(7):1350–1354. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- 31.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr. 2009;139(6):1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- 32.Lutz J, Herwig U, Opialla S, HIttmeyer A, Jancke L, Rufer M, et al. Mindfulness and emotion regulation--an fMRI study. SCAN. 2013;9(6):776–785. doi: 10.1093/scan/nst043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.