Abstract
The oral microbiome is incredibly complex with the average adult harboring about 50 to 100 billion bacteria in the oral cavity, which represent about 200 predominant bacterial species. Collectively, there are approximately 700 predominant taxa of which less than 1/3 still have not yet been grown in vitro. Compared to other body sites, the oral microbiome is unique and readily accessible. There is extensive literature available describing the oral microbiome and discussing the roles that bacteria may play in oral health and disease. However, the purpose of this review is not to rehash these detailed studies but rather to educate the reader with understanding the essence of the oral microbiome, namely that there are abundant bacteria in numbers and types, that there are molecular methods to rapidly determine bacterial associations, that there is site-specificity for colonization of the host, that there are specific associations with oral health and disease, that oral bacteria may serve as biomarkers for non-oral diseases, and that oral microbial profiles may have potential use to assess disease risk.
Keywords: oral microbiome, 16S rDNA, bacterial associations, Next Generation Sequencing
Introduction
Although there have been wildly diverse estimates of the total number of oral bacterial taxa, it is generally accepted that, collectively speaking, there are 687 predominant species in the oral cavity (www.homd.org) (Dewhirst et al., 2010). These estimations are based on years of traditional identification of bacteria from cultural and phenotypic characterization studies, but mostly from identification of bacteria from culture-independent molecular studies using 16S rRNA gene comparative analyses (Paster et al., 2001, Aas et al., 2005, Dewhirst et al., 2010, Paster et al., 2006, Aas et al., 2008). As of May 2016, 31% of oral bacterial taxa have not been grown in vitro (www.homd.org). These not-yet-cultivated taxa are typically referred to as phylotypes, or colloquially as “uncultivables.” About 400 to 500 oral taxa have been detected in the subgingival crevice alone (Aas et al., 2005, Paster et al., 2001). The remaining taxa are distributed on the many oral habitats including different areas on the tongue, tooth surface, buccal mucosa, tonsils, soft and hard palate, and lip vestibule (Aas et al., 2005, Human Microbiome Project, 2012a, Human Microbiome Project, 2012c, Segata et al., 2012). The salivary microbiome would essentially be comprised of a mixture of microbes sloughed off from all sites. Although there is considerable overlap of species detected in all oral sites, such as certain species of Streptococcus, Gemella, Granulicatella, Neisseria, and Prevotella, there is often site-specificity. For example, species of Rothia typically colonize the tongue or tooth surfaces, Simonsiella colonizes only the hard palate, Streptococcus salivarius mainly colonizes the tongue and treponemes are typically restricted to the subgingival crevice (Aas et al., 2005, Kazor et al., 2003, Mager et al., 2003, Segata et al., 2012).
It is well known that specific bacterial taxa that colonize the oral cavity are associated with oral health and oral diseases or afflictions, such as dental caries, periodontal diseases, endodontic lesions, dry socket, halitosis, and odontogenic infections (Aas et al., 2008, Aas et al., 2005, Dewhirst et al., 2010, Johansson et al. 2016; Mager et al., 2003, Paster et al., 2001, Paster et al., 2006, Socransky et al., 1998, Socransky & Haffajee, 2005). Furthermore, oral bacteria may be linked or serve as biomarkers for certain systemic diseases, such as pancreatic cancer (Farrell et al., 2012), diabetes type II (Demmer et al, 2015), pediatric Crohn’s Disease (Docktor et al., 2012), heart disease (Leishman et al., 2010), and low weight, preterm birth (Shira Davenport, 2010). However, it is yet to be established if there is a causal relationship between the oral microbiome and these systemic disorders.
The Human Microbiome Project (HMP)
Our ability to study the human microbiome has been greatly improved by the advances made in sequencing technologies and recent developments in bioinformatics. These advances have led to a plethora of genomic and metagenomic studies investigating the role of microbes in several different ecosystems (Gilbert & Dupont, 2011). Established in 2008, the HMP aimed to determine the microbiomes from 242 healthy human subjects from sites including the oral cavity (7 sites), nasal cavity, skin, gastrointestinal tract and urogenital tract. The data obtained from sequencing was used for taxonomic assignment and is also available through the HMP Data Analysis and Coordination Center data browser. This enables the advance of research relating to the human microbiome by acting as a community resource that is widely accessible. The establishment of such an effort led to the development of a variety of new protocols including methods for laboratory and sequence processing, and analysis of 16S rDNA and whole genome shotgun sequences and profiles of the microbiome (Human Microbiome Project, 2012a). Results from the HMP analyses indicated that repertoire and abundance of microbiota found on individuals varies greatly depending on multiple factors, with ethnic/racial background having one of the strongest associations to microbes with clinical metadata (Human Microbiome Project, 2012c). Such studies provided insights into what constitutes the normal microbiota of each organ or mucosa in the body, enabling a better understanding of how they impact human health. As of April 2016, over 1,300 reference strains isolated from the human body were sequenced and the data publicly available for researchers.
The Human Oral Microbe Database (HOMD)
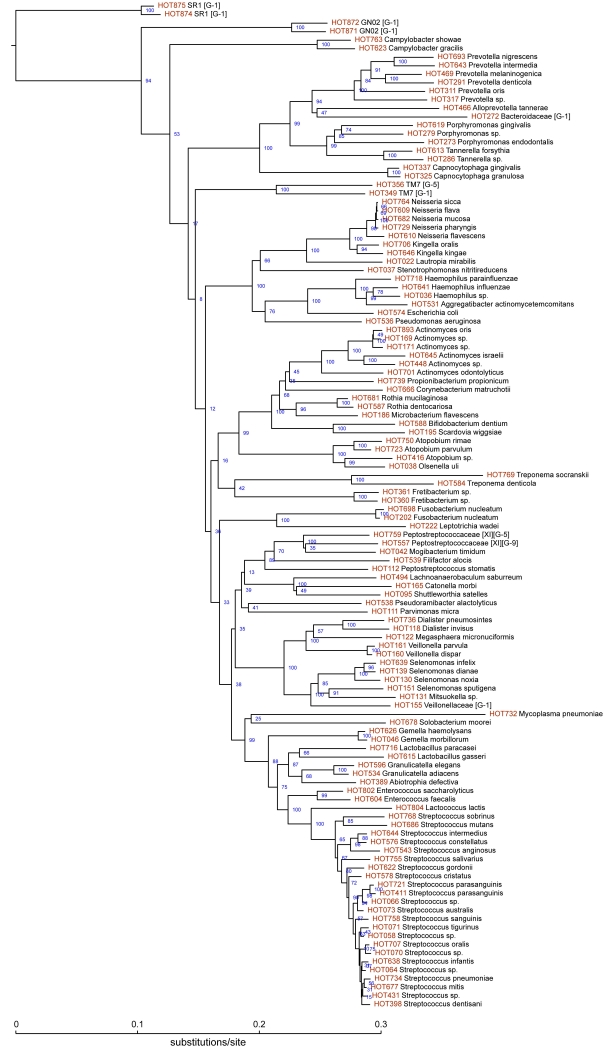
The purpose of HOMD (www.homd.org) is to provide the scientific community with comprehensive information on the approximately 700 predominant bacterial species that inhabit the human oral cavity (Dewhirst et al., 2010); www.homd.org. This curated 16S rDNA database provides a provisional naming scheme for currently unnamed species or phylotypes. The HOMD also links sequence data of 701 oral taxa with phenotypic, phylogenetic, clinical, and bibliographic information. A phylogenetic tree of 118 of the most predominant and other key taxa, i.e., identified in 16S rRNA cloning studies, is shown in Figure 1. Note that there are many well-known oral species, e.g., including species Prevotella, Porphyromonas, Treponema, Tannerella, Fusobacterium, and Streptococcus, as well as perhaps lessor known species, e.g., phylotypes of members of the phyla SR1, GN02 and TM7, Fretibacterium, Solobacterium, and Abiotrophia. Many are associated with oral health and disease and will be discussed in more detail below. Phylogenetic trees of members of each bacterial phylum or family can be downloaded from the HOMD website. As part of HOMD, HMP and other sequencing projects, genome sequences are available for approximately 400 oral bacterial taxa, which represent 58% of the known oral species. BLAST tools are available to rapidly determine oral bacterial identification from 16S rDNA sequences. Easy to use tools for viewing all publically available oral bacterial genomes are also offered on the HOMD site.
Figure 1.
CORE, another phylogenetically-curated 16S rDNA database of the oral microbiome, is also available and can be used identify bacterial taxa from large next generation sequence (NGS) 16S rDNA datasets (Griffen et al., 2011).
Bacterial-bacterial and host-bacterial interactions
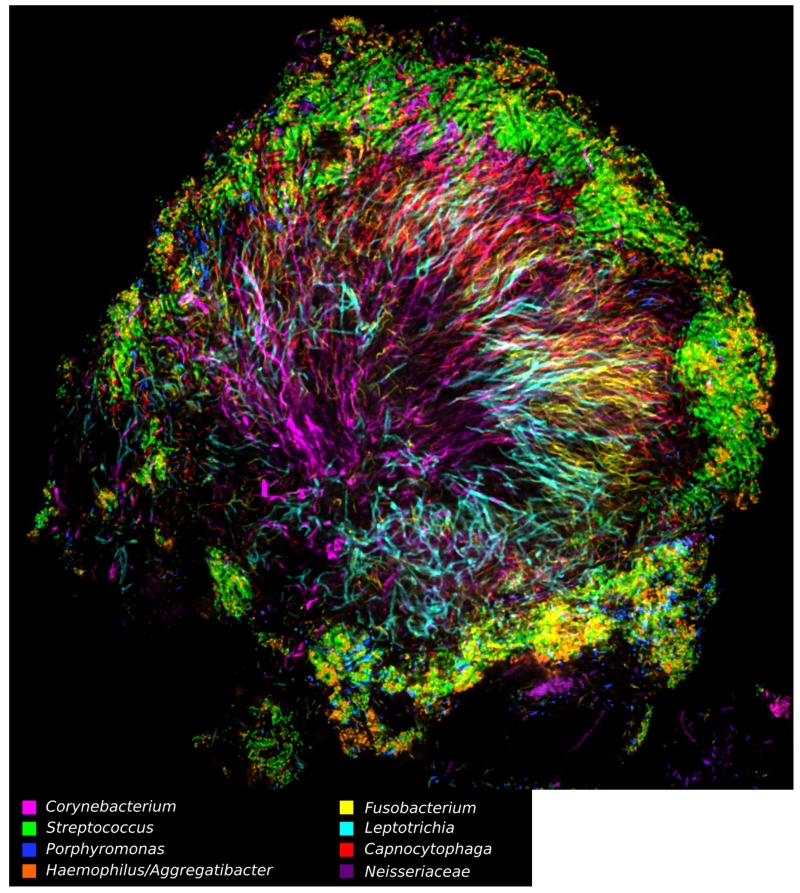
It has long been known that oral bacteria exhibit specificity for their respective colonization sites and to each other, directed by adhesin-receptor binding (Kolenbrander, 2000). Thus, adhesins on bacterial cells bind to receptors on epithelial cells or to other bacteria, including pili, auto-transporters, and extracellular matrix-binding proteins (Nobbs et al., 2011). Some receptors are derived from salivary components, such as proline- or serine-rich-proteins, that undergo conformational change when they are adsorbed onto surfaces such as the tooth surface. Consequently, bacteria do not simply bind or randomly pile on to oral surfaces or other bacteria—there is a specific interaction with a strong affinity. Such specificity can be readily seen in situ by using Combinatorial Labeling and Spectral Imaging FISH (CLASI-FISH) which utilizes multiple taxa-specific, fluorescently labeled probes. (Mark Welch et al., 2016, Valm et al., 2011). Figure 2 illustrates a specific spatial organization of bacterial taxa within dental plaque and that the bacteria do not randomly aggregate. Based on these CLASI-FISH data, the authors were able to propose a model for plaque microbiome development integrating known metabolic, adherence, and environmental information. Thus, we can deduce functional traits of the specific members of the consortium, e.g., anaerobic species are at the center with facultatively anaerobic or aerobic species are at the edge.
Figure 2.
Tools to define oral microbiome
Culture techniques
Historically, bacterial taxa were identified using culture-dependent methodologies such as microscopy, biochemical and other phenotypic tests, growth conditions, sugar utilization, and antibiotic sensitivity. Since 31% of the known oral taxa still cannot be grown in vitro, bacterial culture is still important in microbiology (Vartoukian et al., 2010). However for diagnostic purposes, except for antibiotic sensitivity, culture-dependent methods are labor-intensive, costly, and not as comprehensive as the molecular DNA-based technologies, which circumvent the need for culture.
Gel-based technologies
High-throughput analysis of microbial communities has been possible due to several culture-independent methodologies. Early on, community-fingerprinting techniques such as denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) separated DNA of the same size, which in turn could be sequenced for identification purposes (Anderson & Cairney, 2004, Deng et al., 2008, Nishigaki et al., 2000). Restriction fragment length polymorphism (RFLP) was used to digest homologous DNA sequences and variation in the resulting fragment length was then used as a tool for genome mapping (Deng et al., 2008). This enabled a macro-level analysis where large shifts or variations in the population of a particular microbial community could be identified. These community-profiling methods were based on PCR where specific primers were used to amplify regions of interest and then subjected to analysis.
DNA Microarrays
One of the first methods to rapidly assess specific bacterial associations in oral health and disease was DNA-DNA checkerboard hybridization on a solid membrane (Socransky et al., 1994). In the original method, 30 whole genomic probes were hybridized to 45 DNA samples bound to a membrane for up to 1,350 simultaneous hybridizations. Another version of checkerboard hybridization was a reverse-capture protocol that utilized labeled 16S rDNA PCR products that were hybridized to 16S rDNA taxa-specific oligonucleotide probes bound to the membrane (Paster et al., 1998, Socransky et al., 1994).
DNA microarrays used signals from hybridization of DNA fragments to hundreds or thousands of complementary probes arrayed on a glass slide for expression profiling (Schena et al., 1995). This was modified to identify microbial populations (Bodrossy & Sessitsch, 2004, Zhou, 2003), such as the PhyloChip to screen for 16S rDNA (Wilson et al., 2002) and GeoChip for functional analysis (He et al., 2007). The Human Oral Microbe Identification Microarray(HOMIM), another reverse-capture protocol, was developed using 379 species-level probes to identify ~290 oral bacterial species and has been used in several disease related and oral microbiome characterization studies (Colombo et al., 2009, Colombo et al., 2012, Olson et al., 2011, Lif Holgerson et al., 2011, Duran-Pinedo et al., 2011, Torlakovic et al., 2012, Belstrom et al., 2015). An array platform with broad range, higher taxa probes can help to identify/estimate a community population composition at a family or phylum level even if species level specificity is absent (Hamady & Knight, 2009).
16S rRNA gene sequencing
The 16S rRNA has been used as an evolutionary clock (Woese, 1987) for the identification and classification of pure cultures of bacteria as well as for estimation of bacterial diversity in environmental samples (Rajendhran & Gunasekaran, 2011). Comparative analyses of 16S rDNA sequences have been the primary basis of defining the microbiome from all environments, including the oral cavity. With pure bacterial cultures, PCR amplicons (approximately 1,500 bp) of 16S rRNA genes were simply sequenced using Sanger sequencing (Sanger et al., 1977). Phylogenetic identity of bacterial taxa, whether they are cultivable or not-yet-cultivated, in a mixed population, e.g., plaque, was determined using what has been referred to as the 16S rRNA approach (Paster et al., 2006). Briefly, DNA isolated from any given environment is amplified using universally conserved PCR primers for 16S rRNA genes. The resultant amplicons were cloned into Escherichia coli, and the 16S rDNA inserts were sequenced to determine species identity (Hugenholtz & Pace, 1996, Paster et al., 2001). Typically, a >98.5% identity defines a species/phylotype. Consequently, 16S rDNA sequences from an isolate or cloned insert with <98.5% similarity to previously defined phylotypes would be considered representatives of new species (Dewhirst et al., 2010).
Next Generation Sequencing (NGS) Platforms
In the last decade, next generation sequencing methods have revolutionized the study of microbial diversity, which allow for large-scale sequencing projects to be completed in a few days or sometimes hours. The main technologies for next-generation sequencing are as follows.
454 pyrosequencing. This method clonally amplified fragmented DNA on beads within an emulsion (Margulies et al., 2005). The sequencer was able to generate over 250 bp long reads and about 400,000 reads per run.
- Applied Biosystem (Life Technologies)
-
○Sequencing by Oligo Ligation Detection (SOLiD). This technique was similar to 454 pyrosequencing in that fragmented DNA was amplified on agarose beads. However, this technique utilized the incorporation of a ligase and universal oligonucleotides, which resulted in millions of reads.
-
○Personal Genome Machine, or Ion Torrent. Newer technology with a similar emulsion PCR for amplification technique but with an underlying semiconductor technology (Nakano et al., 2003).
-
○
- Illumina
-
○Early instruments utilized a sequencing-by-synthesis platform where DNA fragments were clonally amplified on a flow cell and binding of complementary fluorescently labeled dNTPs is detected. Millions of 35 bp reads could be produced in one run and, depending on the instrument, multiplexing of sample across lanes was possible.
-
○In the past few years, Illumina has emerged as the market leader with a suite of instruments like HiSeq, HiSeq X, NextSeq 500 and MiSeq with varying abilities for sequencing length and number of reads. The MiSeq can generate up to 2×300bps reads and HiseqX can produce ~600Gb of data. The MiSeq is generally used for 16S rDNA profiling.
-
○
Pacific Biosciences (PacBio), single molecule real-time (SMRT) technology. This instrument is sensitive enough to detect a single fluorescently labeled nucleotide (Korlach et al., 2008, Levene et al., 2003, Lundquist et al., 2008) and is purportedly able to generate ~10,000 bp reads. The PacBio platform is often used to determine whole genomic sequences, without the need for a reference genome.
Oxford Nanopore, MinION technology. One of the most recent technologies, released in May 2015, enables sequencing of single DNA molecules (Mikheyev & Tin, 2014, Quick et al., 2014). As with the PacBio, MinION would allow for de novo sequencing of whole genomes.
All NGS analysis requires extensive bioinformatic capabilities and involved data quality control, filtering for good quality reads, aligning and mapping to good reference genomes, removing chimeras, normalizations across samples and populations for meaningful interpretations.
16S rDNA profiling
The 16S rRNA approach was refined further using NGS methodologies to rapidly sequence hypervariable regions of the 16S rRNA genes (Caporaso et al., 2011). This involves amplification of DNA samples using universally conserved PCR primers of 16S rDNA and sequencing of the amplified regions to produce millions of reads enabling multiplexing of several samples in one run. The length of sequencing reads varies depending upon the primers used, but many studies utilize about 500 base-reads for a typical sequencing run, which allows for microbial community identification (Liu et al., 2007, Liu et al., 2008, Wang et al., 2007, Mougeot et al., 2016). At the present time, using an Illumina platform (described below), 500 to 600 base-reads is the size limit for sequencing. This technique does rely on PCR amplification and care should be taken on which region of the 16S rRNA gene is used in analysis for accurate classification of the population (Yang et al., 2016), but nevertheless is a valuable tool to identify species in a population.
A common bioinformatics tool for analysis has been Quantitative Insights Into Microbial Ecology (QIIME), which picks Operational Taxonomic Units (OTUs) and assigns taxonomic identities based on comparisons to sequences from a reference database (Caporaso et al., 2010). Typically, especially with only 500 or base pair reads, these analyses identify taxa at the genus level with some species level identification.
Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS), http://homings.forsyth.org, is the new HOMIM, which utilizes standard NGS methodologies (Caporaso et al., 2010), and is capable of species level identification for most of the prevalent oral bacterial taxa. This is achieved by an in silico search for specific ‘probe’ sequences, called ProbeSeq for HOMINGS, that targets approximately 600 species. ProbeSeq is an iterative process in which for those sequences that are not identified, the search is repeated with 129 genus-level probes that will identify those species at the genus level (Gomes et al., 2015). The advantages of HOMINGS are that it is computationally efficient, rapid, reproducible, and can identify the majority of the oral microbiome at the species level. There is good correlation between HOMINGS and HOMIM (Mougeot et al., 2016). HOMINGS has been used in several recent studies demonstrating bacterial associations with endodontic lesions (Gomes et al., 2015), salivary microbiomes in caries and periodontitis (Belstrom et al., 2016b), temporal differences in salivary microbiomes (Belstrom et al., 2016a), and in biofilm models in response to sucrose induced dysbiosis (Rudney et al., 2015).
Most recently, a multi-stage algorithm for 16S rDNA NGS reads was developed for species-level identification. Although this method requires more computing power, it is able to maximize the percentage of reads classified at the species level (Al-Hebshi et al., 2015). In that paper, this technique was used to determine the oral microbiome in subjects with oral cancer.
Whole-Genome Shotgun Metagenome Sequencing
The entire DNA (genome) of a single microbial culture or a complex microbial population can now be sequenced to great depth allowing us to generate reference genomes (de novo assembly) as a resource for future studies or identify the composition of microbial community respectively (mapping back to a reference genome). This culture and PCR independent technique allows parallel sequencing and identification of several organisms. Long read lengths enable a more accurate assembly of the genomes present in the population, however the huge volume of data generated still poses a bioinformatic challenge (Grice & Segre, 2012). Fortunately, at least for the oral microbiome, there are complete genomes for about 400 bacterial species that facilitate assembly. At great depth of short read sequencing, metagenomic analysis also allows quantification of copy number and allelic variants of genes within the microbial population (Vincent et al., 2016).
Microbial Metatranscriptomic Sequencing
Both 16S rDNA and metagenome sequencing allow us to determine ‘who is there’ however metatranscriptomic analysis would tell ‘what they are doing’. The metatranscriptome represents the RNA encoded by the microbial population; this functional analysis is performed by enriching for the mRNA, converting it into cDNA and sequencing the fragments. The reads are mapped back to reference genomes for gene expressions profiling within the microbial communities. Clinical samples can be sequenced to identify changes in gene expression between disease and normal states to identify key pathways upregulated in disease and expression patterns of potential pathogenic factors and microbial diversity (Yost et al., 2015, Duran-Pinedo et al., 2014, Benitez-Paez et al., 2014) (Wade, 2011).
Bacterial associations in health and disease
Unlike many human diseases, oral bacterial diseases, such as caries and periodontitis, are not caused by a single species, but by a consortium of species that are likely living harmlessly in very low numbers (often below the limit of detection) in the oral cavity. In essence, oral bacterial diseases are opportunistic infections and thus disease occurs under the proper circumstances and conditions, e.g., diet, host immune response, complicating systemic or genetic disorders, pH, poor oral hygiene, life style, and even bad luck.
In using the molecular techniques described above, bacterial associations have been determined in their relationships to health status. Such studies help to determine the role of specific species in oral health and disease, including extraoral sites in systemic diseases. However, note that these associations do not necessarily identify actual etiological agents, hence often the designation “putative” pathogens or biomarkers of disease. Also of note is that, in all cases, bacterial associations are usually more complex than previously believed.
Oral Health
The oral microbiome changes during the life of an individual from bacterial acquisition at birth (Berkowitz & Jones, 1985, Asikainen & Chen, 1999) to bacterial colonization of the elderly (Preza et al., 2008). Species of Streptococcus are usually the first pioneering microorganisms to colonize the oral cavity with Streptococcus salivarius found mostly on the tongue dorsum and in saliva, Streptococcus mitis on the buccal mucosa and Streptococcus sanguinis on the teeth (Gibbons & Houte, 1975, Socransky & Manganiello, 1971, Smith et al., 1993). The growth and metabolism of these pioneer species change local environmental conditions such as local redox potential, pH, coaggregation, and availability of nutrients, thereby enabling more fastidious organisms to colonize after them (Marsh, 2000). Over time, other microbial communities take over including Prevotella melaninogenica, Fusobacterium nucleatum, Veillonella, Neisseria and nonpigmented Prevotella (Kononen et al., 1992). With the development of teeth, an increase in the presence of genera such as Leptotrichia and Campylobacter is observed and along with colonization by additional species such as Prevotella denticola and members of the Fusobacterium and Selenomonas genera (Kononen et al., 1994). The eruption of teeth creates a new habitat, the gingival crevice, which is nourished by the gingival crevicular fluid (GCF). Along with saliva, GCF is critical for the maintenance of the integrity of the gingival crevices and contains antimicrobial peptides, immunoglobulins and a range of other active proteins that enable it to influence the ecology of the oral cavity. Moreover, it also contains nutrients that support the resident microflora (Marsh, 2000). This continual succession of microbes is eventually replaced with a stable homeostasis of microbial communities that is referred to as the climax community whereby different bacteria interact to establish an ecosystem where each community contributes in some form (Marsh, 2000). The “keystone pathogen” hypothesis suggests that specific low-abundance pathogens can influence periodontal disease by altering the “healthy” microflora to a disease state (Hajishengallis et al., 2012). However, you still have to know health before you know disease.
Depending upon the oral site and individuals, many health-associated species have been identified. By using the 16S rRNA approach, Aas et al (Aas et al., 2005) analyzed sites from five clinically healthy subjects. Sites included tongue dorsum, lateral sides of tongue, buccal mucosa, hard and soft palate, palate, supragingival and subgingival plaque, maxillary anterior vestibule, and tonsils. Species typically associated with periodontitis and caries were not detected. Using NGS, Zaura et al 2009 performed a similar analysis of multiple oral sites. In both of these studies, species and phylotypes of Streptococcus, Granulicatella, Neisseria, Haemophilus, Corynebacterium, Rothia, Actinomyces, Prevotella, Capnocytophaga, Porphyromonas and Fusobacterium were common. They concluded that most oral taxa found in unrelated healthy individuals was similar, and supported the concept of a healthy core microbiome. From the HMP 16S rRNA gene data of 200 subjects,(Segata et al., 2012) found similar taxa, but there was significant subject-to-subject variation.
Periodontitis
Putative pathogens have long been implicated in periodontal disease including Porphyromonas gingivalis, Tannerella forsythia, Aggregatibacter actinomycetemcomitans, and species of Treponema and Prevotella. However, using checkerboard hybridization, Socransky et al. (Socransky et al., 1998) determined that oral diseases were better defined as based on a combination of species, or complexes, rather than a single specific etiologic agent. The authors defined five complexes of which the “red complex” was the most pathogenic one. This complex contained P. gingivalis, T. forsythia, and Treponema denticola and depended on earlier colonization of the pocket by the orange complex (Socransky & Haffajee, 2005, Socransky et al., 1998).
The literature is quite extensive in regards to those species that are associated with periodontal disease (Socransky & Haffajee, 2005, Teles et al., 2013). Several studies suggested that there may be additional red complex species (Kumar et al., 2003, Paster et al., 2001) that are associated with chronic periodontitis. Recently, there was systematic review (Perez-Chaparro et al., 2014) of 1,450 bacterial association studies of subgingival plaque, of which 41 studies qualified for analysis. Consequently, based on these analyses, they concluded that there were 17 additional disease-associated species or phylotypes and these are listed in Table 1 along with their Human Oral Taxon (HOT) designations (www.homd.org).
Table 1.
Newly identified putative periodontal pathogens (from Perez-Chaparro et al 2014)
| Bacterial taxa | |
|---|---|
| Anaeroglobus geminatus HOT 121 | cultivable |
| Archaea spp. | cultivable |
| Bacteroidales [G-2] sp. oral taxon 274 | unnamed |
| Desulfobulbus sp. oral taxon 041 | phylotype |
| Eubacterium [XI] [G-5] saphenum HOT 759 | cultivable |
| Filifactor alocis HOT 539 | cultivable |
| Fretibacterium fastidiosum HOT 363 | cultivable |
| Fretibacterium sp. oral taxon 360 | phylotype |
| Fretibacterium sp. oral taxon 362 | phylotype |
| Mogibacterium timidum HOT 042 | cultivable |
| Peptostreptococcus stomatis HOT 112 | cultivable |
| Porphyromonas endodontalis HOT 273 | cultivable |
| Selenomonas sputigena HOT 151 | cultivable |
| TM7 [G-5] sp. oral taxon 356 | phylotype |
| Treponema lecithinolyticum HOT 653 | cultivable |
| Treponema medium HOT 667 | cultivable |
| Treponema vincentii HOT 029 | cultivable |
Refractory periodontal disease
Some subjects who have destructive periodontal disease do not respond to conventional therapy and continue to lose periodontal attachment. This has often been termed as refractory periodontal disease (Adams, 1992). It has been suggested that subjects with refractory disease may be mildly immunocompromised making them more susceptible to periodontal disease. Early studies of refractory periodontal disease demonstrated a lack of typical periodontal pathogens (Magnusson et al., 1991). In contrast, using newer molecular techniques such as HOMIM, Colombo et al. 2009 were able to demonstrate that refractory periodontitis differed from treatable periodontitis by having a higher frequency of putative periodontal pathogens as listed above and in Table 1. However, they also found additional species that are not commonly detected in treatable periodontal disease, including P. alactolyticus, Brevundimonas diminuta, Shuttleworthia satelles, D. invisus, Granulicatella adiacens, Veillonella atypica, and Mycoplasma salivarium. A more recent study implicated Actinetobacter baumannii, an important nosocomial pathogenthat is notoriously antibiotic resistant, as a risk factor for refractory periodontitis (Richards et al., 2015).
Aggressive periodontitis
This periodontal disease was previously referred to as localized juvenile periodontitis or generalized juvenile periodontitis. As the name implies, this is an aggressive form of periodontitis that typically affects only incisors and first molars in teenagers or young adults. There is usually a lack of gingival inflammation, even with deep probing depths (Albandar, 2014). Aggregatibacter (Actinobacillus) actinomycetemcomitans has long been considered the etiologic agent of aggressive periodontitis, however more recent evidence has shown that the subgingival plaque microbiome of aggressive periodontitis resembles that of chronic periodontitis (Kononen & Muller, 2014). Shaddox et al (2012) showed that A. actinomycetemcomitans, Filifactor alocis, Tannerella spp. Solobacterium moorei, Parvimonas micra, and Capnocytophaga spp were most abundant in aggressive periodontitis. A recent study showed that a consortium of A. actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis may serve as a biomarker of disease, i.e., predict bone loss before it occurs (Fine et al., 2013).
Caries
One of the most prevalent human bacterial infections is dental caries, which leads to tooth decay and potentially tooth loss. Streptococcus mutans has long been considered at the etiological agent of caries inasmuch as it not only produces lactic acid, but also thrives in the low pH environment. However, 10 to 20% of subjects with caries do not have detectable levels of S. mutans, so clearly other acid producing bacterial taxa must be involved. Molecular methods such as the 16S rRNA approach or microarrays (Aas et al., 2008) have demonstrated that in carious lesions with S. mutans, additional species belonging to the genera Atopobium, Propionibacterium, and Lactobacillus, were present at significantly higher levels. In those subjects with no detectable levels of S. mutans, Lactobacillus spp., Bifidobacterium dentium, and low-pH non-S. mutans streptococci were predominant. Based on these results, it was suggested that bacterial species other than S. mutans, e.g., species of the genera Veillonella, Lactobacillus, Scardovia, and Propionibacterium, low-pH non-S. mutans streptococci, Actinomyces spp., and Atopobium spp., may play an important role in caries progression. NGS analyses of the microbiome of populations with a low and high prevalence of caries found that adolescents in Romania, who had limited access to care, were colonized with S. mutans and S. sobrinus. In contrast, those adolescents in Sweden, who had very good care, were colonized only infrequently with S. mutans and S. sobrinus., but were colonized more with species of Actinomyces, Selenomonas, Prevotella, and Capnocytophaga (Johansson et al. 2016).
The oral microbiomes do differ between primary and secondary dentitions as well as in root surface caries. In the primary dentition (Becker et al., 2002), S. mutans was typically detected at high levels. Other disease associated species included Actinomyces gerencseriae, Scardovia wiggsiae, Veillonella, S. salivarius, S. constellatus, S. parasanguinis, and Lactobacillus fermentum. Root surface caries differ from primary and secondary caries in that root surfaces do not have enamel. Preza et al (Preza et al., 2008) demonstrated that S. mutans was also associated with root surface caries, but that the predominant taxa included Actinomyces spp., Lactobacillus, Enterococcus faecalis, Mitsuokella sp. HOT131, Atopobium and Olsenella spp., Prevotella multisaccharivorax, P. alactolyticus, and Propionibacterium acidifaciens. Of note, detectable levels of E. faecalis and P. alactolyticus are typically found only in endodontic lesions and not in dental plaque.
Odontogenic infections
These pus-laden infections typically originate within a tooth or surrounding structures resulting in swellings of the head, face and neck (Flynn et al., 2012). . Using the 16S rRNA approach, several predominant species that had been previously associated with odontogenic infections were detected (Flynn et al., 2012). These species included Fusobacterium spp, Parvimonas micra, Porphyromonas endodontalis, and Prevotella oris. However, they also detected newly-associated species including Dialister pneumosintes, D. invisus, and Eubacterium brachy, as well as several phylotypes. An interesting finding in this study was that species of Streptococcus were not detected.
Endodontic lesions
In primary infections, predominant taxa detected include species of Peptostreptococcus, P. micra, Filifactor alocis, P. alactolytcus, species of Dialister, Fusobacterium nucleatum, Treponema denticola, Porphyromonas endodontalis, P. gingivalis, T. forsythia, Prevotella baroniae, Prevotella intermedia, Prevotella nigrescens and Bacteroidaceae [G-1] HOT272 (Siqueira & Rocas, 2009). Enterococcus faecalis was detected, but as lower levels. However in retreatment cases, the predominant taxa include Enterococcus species such as E. faecalis, Parvimonas micra, Filifactor alocis, P. alactolytcus, Streptococcus constellatus and Streptococcus anginosus, and Propionibacterium propionicum. The microbiomes of endodontic-periodontal lesions had possessed similar profiles including E. faecalis, P. micra, Mogibacterium timidum, F. alocis, and Fretibacterium fastidiosum (Gomes et al., 2015).
Oral bacteria as biomarkers for non-oral diseases
Oral bacteria have been linked to a number of systemic diseases including bacterial endocarditis (Berbari et al., 1997), ischemic stroke (Joshipura et al., 2003), cardiovascular disease (Beck & Offenbacher, 2005; Teles & Wang, 2011); pancreatic cancer (Farrell et al 2012), pediatric Crohn’s Disease (Docktor et al., 2012), and pneumonia (Awano et al., 2008). Periodontal disease has been shown to predispose individuals to cardiovascular disease through its ability to induce chronic inflammation (Valtonen, 1991, Syrjanen, 1990). Similarly, the presence of several anaerobic oral bacterial species has been shown to predispose to bacterial pneumonia including Actinobacillus actinomycetemcomitans and Streptococcus constellatus (Venkataramani et al., 1994, Shinzato & Saito, 1994). In Alzheimer’s disease, inflammation, a key feature of the disease (Olsen & Singhrao, 2015), could be caused in part by peripheral infections, such as periodontal disease. Periodontal pathogens such as Aggregatibacter actinomycetemcomitans and Prevotella intermedia are capable of eliciting systemic inflammation, which results in the release of pro-inflammatory cytokines that traverse the blood-brain barrier.
An intriguing suggestion has been that oral bacteria may play a role in nitric oxide (NO) homeostasis, which is important in renal and cardiovascular health (Hezel & Weitzberg, 2015). Dietary nitrates can be reduced to nitrites by oral bacteria and nitrite, absorbed in the blood, is further reduced to NO by a variety of mechanisms. NO then acts on vascular smooth muscle to stimulate vasodilation.
Important Considerations and Conclusions
Researchers are now routinely identifying bacterial composition, and high-throughput sequencing of the microbiome will now progress into functional studies encompassing genomics, transcriptomics and metabolomics of both host and pathogens. Such analysis could provide insights into activity of the microbes, their relationship to hosts, and possible causative mechanisms. The second phase of the NIH Human Microbiome Project will study the host-microbiome relationship in longitudinal studies (Integrative, 2014). These data will guide researchers to develop new therapies that target key mechanisms. With such huge datasets, we will likely identify ‘community signatures’ of certain diseases. It can be envisioned that chairside or bedside, point-of-care diagnostics could be developed that target key bacterial taxa. Consequently, these potential biomarkers of disease, the proverbial “canary of the coal mine in human disease” could be used to warn dentists or physicians of disease yet to come or to assess risk of disease. Thus, for the dentist, oral medicine specialist, or periodontist, these warning “danger” microbial profiles would allow for early treatment to combat disease in the preclinical stages. The oral microbiome could be used further to monitor health status after treatment, i.e., is the treatment working to establish a more healthy microbial profile? Regardless of specific application, microbial analysis is in an exciting phase of research with huge prospects for the clinic.
Acknowledgements
Supported in part by National Institute of Dental and Craniofacial Research/National Institute of Health grant, R01 DE021565. We thank Rebecca Misra for her help in preparation of the manuscript.
Footnotes
Conflicts of interest: none to declare
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DF. Diagnosis and treatment of refractory periodontitis. Curr Opin Dent. 1992;2:33–8. [PubMed] [Google Scholar]
- Al-Hebshi NN, Nasher AT, Idris AM, Chen T. Robust species taxonomy assignment algorithm for 16S rRNA NGS reads: application to oral carcinoma samples. J Oral Microbiol. 2015;7:28934. doi: 10.3402/jom.v7.28934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar JM. Aggressive periodontitis: case definition and diagnostic criteria. Periodontol 2000. 2014;65:13–26. doi: 10.1111/prd.12014. [DOI] [PubMed] [Google Scholar]
- Anderson IC, Cairney JW. Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques. Environ Microbiol. 2004;6:769–79. doi: 10.1111/j.1462-2920.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- Asikainen S, Chen C. Oral ecology and person-to-person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol 2000. 1999;20:65–81. doi: 10.1111/j.1600-0757.1999.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, Yoshida A, Sonoki K, Fujisawa K, Takehara T. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87:334–9. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–9. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrom D, Fiehn NE, Nielsen CH, Klepac-Ceraj V, Paster BJ, Twetman S, Holmstrup P. Differentiation of salivary bacterial profiles of subjects with periodontitis and dental caries. J Oral Microbiol. 2015;7:27429. doi: 10.3402/jom.v7.27429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrom D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. Temporal Stability of the Salivary Microbiota in Oral Health. PLoS One. 2016a;11:e0147472. doi: 10.1371/journal.pone.0147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrom D, Paster BJ, Fiehn NE, Bardow A, Holmstrup P. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J Oral Microbiol. 2016b;8:30170. doi: 10.3402/jom.v8.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Paez A, Belda-Ferre P, Simon-Soro A, Mira A. Microbiota diversity and gene expression dynamics in human oral biofilms. BMC Genomics. 2014;15:311. doi: 10.1186/1471-2164-15-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari EF, Cockerill FR, 3rd, Steckelberg JM. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–42. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- Berkowitz RJ, Jones P. Mouth-to-mouth transmission of the bacterium Streptococcus mutans between mother and child. Arch Oral Biol. 1985;30:377–9. doi: 10.1016/0003-9969(85)90014-7. [DOI] [PubMed] [Google Scholar]
- Bodrossy L, Sessitsch A. Oligonucleotide microarrays in microbial diagnostics. Curr Opin Microbiol. 2004;7:245–54. doi: 10.1016/j.mib.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–22. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst FE, Paster BJ. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–87. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–32. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Jacobs DR, Jr, Singh R, Zuk A, Rosenbaum M, Papapanou PN, Desvarieux M. Periodontal bacteria and prediabetes prevalence in origins: the oral infections, glucose intolerance, and insulin resistance study. J Dent Res. 2015;94:201S–11S. doi: 10.1177/0022034515590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Xi D, Mao H, Wanapat M. The use of molecular techniques based on ribosomal RNA and DNA for rumen microbial ecosystem studies: a review. Mol Biol Rep. 2008;35:265–74. doi: 10.1007/s11033-007-9079-1. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docktor MJ, Paster BJ, Abramowicz S, Ingram J, Wang YE, Correll M, Jiang H, Cotton SL, Kokaras AS, Bousvaros A. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:935–42. doi: 10.1002/ibd.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659–72. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Paster B, Teles R, Frias-Lopez J. Correlation network analysis applied to complex biofilm communities. PLoS One. 2011;6:e28438. doi: 10.1371/journal.pone.0028438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DT. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582–8. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrendiz J, Furgang D, Paster BJ, Dewhirst FE. A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J Clin Microbiol. 2013;51:2850–61. doi: 10.1128/JCM.00729-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn TR, Paster BJ, Stokes LN, Susarla SM, Shanti RM. Molecular methods for diagnosis of odontogenic infections. J Oral Maxillofac Surg. 2012;70:1854–9. doi: 10.1016/j.joms.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Houte JV. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Dupont CL. Microbial metagenomics: beyond the genome. Ann Rev Mar Sci. 2011;3:347–71. doi: 10.1146/annurev-marine-120709-142811. [DOI] [PubMed] [Google Scholar]
- Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J Endod. 2015;41:1975–84. doi: 10.1016/j.joen.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–70. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Firestone ND, Gross EL, Difranco JM, Hardman JH, Vriesendorp B, Faust RA, Janies DA, Leys EJ. CORE: a phylogenetically-curated 16S rDNA database of the core oral microbiome. PLoS One. 2011;6:e19051. doi: 10.1371/journal.pone.0019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–25. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009;19:1141–52. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Gentry TJ, Schadt CW, Wu L, Liebich J, Chong SC, Huang Z, Wu W, Gu B, Jardine P, Criddle C, Zhou J. GeoChip: a comprehensive microarray for investigating biogeochemical, ecological and environmental processes. ISME J. 2007;1:67–77. doi: 10.1038/ismej.2007.2. [DOI] [PubMed] [Google Scholar]
- Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- Howe K, Bateman A, Durbin R. QuickTree: building huge Neighbour-Joining trees of protein sequences. Bioinformatics. 2002;18:1546–7. doi: 10.1093/bioinformatics/18.11.1546. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P, Pace NR. Identifying microbial diversity in the natural environment: a molecular phylogenetic approach. Trends Biotechnol. 1996;14:190–7. doi: 10.1016/0167-7799(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project C A framework for human microbiome research. Nature. 2012a;486:215–21. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012c;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrative HMPRNC The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;16:276–89. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Witkowska E, Kaveh B, Lif Holgerson P, Tanner AC. The microbiome in populations with a low and high prevalence of caries. J Dent Res. 2016;95:80–6. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- Kazor CE, Mitchell PM, Lee AM, Stokes LN, Loesche WJ, Dewhirst FE, Paster BJ. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J Clin Microbiol. 2003;41:558–63. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–37. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- Kononen E, Asikainen S, Jousimies-Somer H. The early colonization of gram-negative anaerobic bacteria in edentulous infants. Oral Microbiol Immunol. 1992;7:28–31. doi: 10.1111/j.1399-302x.1992.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Kononen E, Asikainen S, Saarela M, Karjalainen J, Jousimies-Somer H. The oral gram-negative anaerobic microflora in young children: longitudinal changes from edentulous to dentate mouth. Oral Microbiol Immunol. 1994;9:136–41. doi: 10.1111/j.1399-302x.1994.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Kononen E, Muller HP. Microbiology of aggressive periodontitis. Periodontol 2000. 2014;65:46–78. doi: 10.1111/prd.12016. [DOI] [PubMed] [Google Scholar]
- Korlach J, Marks PJ, Cicero RL, Gray JJ, Murphy DL, Roitman DB, Pham TT, Otto GA, Foquet M, Turner SW. Selective aluminum passivation for targeted immobilization of single DNA polymerase molecules in zero-mode waveguide nanostructures. Proc Natl Acad Sci U S A. 2008;105:1176–81. doi: 10.1073/pnas.0710982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–44. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- Leishman SJ, Do HL, Ford PJ. Cardiovascular disease and the role of oral bacteria. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v2i0.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–6. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- Lif Holgerson P, Harnevik L, Hernell O, Tanner AC, Johansson I. Mode of birth delivery affects oral microbiota in infants. J Dent Res. 2011;90:1183–8. doi: 10.1177/0022034511418973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, DeSantis TZ, Andersen GL, Knight R. Accurate taxonomy assignments from 16S rRNA sequences produced by highly parallel pyrosequencers. Nucleic Acids Res. 2008;36:e120. doi: 10.1093/nar/gkn491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lozupone C, Hamady M, Bushman FD, Knight R. Short pyrosequencing reads suffice for accurate microbial community analysis. Nucleic Acids Res. 2007;35:e120. doi: 10.1093/nar/gkm541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PM, Zhong CF, Zhao P, Tomaney AB, Peluso PS, Dixon J, Bettman B, Lacroix Y, Kwo DP, McCullough E, Maxham M, Hester K, McNitt P, Grey DM, Henriquez C, Foquet M, Turner SW, Zaccarin D. Parallel confocal detection of single molecules in real time. Opt Lett. 2008;33:1026–8. doi: 10.1364/ol.33.001026. [DOI] [PubMed] [Google Scholar]
- Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30:644–54. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Magnusson I, Marks RG, Clark WB, Walker CB, Low SB, McArthur WP. Clinical, microbiological and immunological characteristics of subjects with "refractory" periodontal disease. J Clin Periodontol. 1991;18:291–9. doi: 10.1111/j.1600-051x.1991.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–80. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. Oral ecology and its impact on oral microbial diversity. In: Kuramitsu HK, Ellen RP, editors. Oral Bacterial Ecology: The Molecular Basis. Horizon Scientific Press; Wymondham: 2000. [Google Scholar]
- Mikheyev AS, Tin MM. A first look at the Oxford Nanopore MinION sequencer. Mol Ecol Resour. 2014;14:1097–102. doi: 10.1111/1755-0998.12324. [DOI] [PubMed] [Google Scholar]
- Mougeot JL, Stevens CB, Cotton SL, Morton DS, Krishnan K, Brennan MT, Lockhart PB, Paster BJ, Bahrani Mougeot FK. Concordance of HOMIM and HOMINGS technologies in the microbiome analysis of clinical samples. J Oral Microbiol. 2016;8:30379. doi: 10.3402/jom.v8.30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Komatsu J, Matsuura S, Takashima K, Katsura S, Mizuno A. Single-molecule PCR using water-in-oil emulsion. J Biotechnol. 2003;102:117–24. doi: 10.1016/s0168-1656(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Nishigaki K, Naimuddin M, Hamano K. Genome profiling: a realistic solution for genotype-based identification of species. J Biochem. 2000;128:107–12. doi: 10.1093/oxfordjournals.jbchem.a022719. [DOI] [PubMed] [Google Scholar]
- Nobbs AH, Jenkinson HF, Jakubovics NS. Stick to your gums: mechanisms of oral microbial adherence. J Dent Res. 2011;90:1271–8. doi: 10.1177/0022034511399096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol. 2015;7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JC, Cuff CF, Lukomski S, Lukomska E, Canizales Y, Wu B, Crout RJ, Thomas JG, McNeil DW, Weyant RJ, Marazita ML, Paster BJ, Elliott T. Use of 16S ribosomal RNA gene analyses to characterize the bacterial signature associated with poor oral health in West Virginia. BMC Oral Health. 2011;11:7. doi: 10.1186/1472-6831-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ, Bartoszyk IM, Dewhirst FE. Identification of oral streptococci using PCR-based, reverse-capture, checkerboard hybridization. Methods in Cell Science. 1998;20:223–231. [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–7. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Perez-Chaparro PJ, Goncalves C, Figueiredo LC, Faveri M, Lobao E, Tamashiro N, Duarte P, Feres M. Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res. 2014;93:846–58. doi: 10.1177/0022034514542468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ. Bacterial profiles of root caries in elderly patients. J Clin Microbiol. 2008;46:2015–21. doi: 10.1128/JCM.02411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick J, Quinlan AR, Loman NJ. A reference bacterial genome dataset generated on the MinION portable single-molecule nanopore sequencer. Gigascience. 2014;3:22. doi: 10.1186/2047-217X-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendhran J, Gunasekaran P. Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiol Res. 2011;166:99–110. doi: 10.1016/j.micres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Richards AM, Abu Kwaik Y, Lamont RJ. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol Oral Microbiol. 2015;30:2–15. doi: 10.1111/omi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudney JD, Jagtap PD, Reilly CS, Chen R, Markowski TW, Higgins L, Johnson JE, Griffin TJ. Protein relative abundance patterns associated with sucrose-induced dysbiosis are conserved across taxonomically diverse oral microcosm biofilm models of dental caries. Microbiome. 2015;3:69. doi: 10.1186/s40168-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–70. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, Walker CB, Klepac-Ceraj V, Paster BJ. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012;91:927–33. doi: 10.1177/0022034512456039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato T, Saito A. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J Med Microbiol. 1994;40:118–23. doi: 10.1099/00222615-40-2-118. [DOI] [PubMed] [Google Scholar]
- Shira Davenport E. Preterm low birthweight and the role of oral bacteria. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v2i0.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira JF, Jr., Rocas IN. Diversity of endodontic microbiota revisited. J Dent Res. 2009;88:969–81. doi: 10.1177/0022034509346549. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Anderson JM, King WF, van Houte J, Taubman MA. Oral streptococcal colonization of infants. Oral Microbiol Immunol. 1993;8:1–4. doi: 10.1111/j.1399-302x.1993.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Manganiello SD. The oral microbiota of man from birth to senility. J Periodontol. 1971;42:485–96. doi: 10.1902/jop.1971.42.8.485. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–92. [PubMed] [Google Scholar]
- Syrjanen J. Vascular diseases and oral infections. J Clin Periodontol. 1990;17:497–500. doi: 10.1111/j.1365-2710.1992.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Teles R, Wang C-Y. Mechanisms involved in the association between peridontal diseases and cardiovascular disease. Oral Diseases. 2011;17:450–461. doi: 10.1111/j.1601-0825.2010.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 2013;62:95–162. doi: 10.1111/prd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ, Olsen I. Microbial community succession on developing lesions on human enamel. J Oral Microbiol. 2012;4 doi: 10.3402/jom.v4i0.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE, Borisy GG. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci U S A. 2011;108:4152–7. doi: 10.1073/pnas.1101134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen VV. Infection as a risk factor for infarction and atherosclerosis. Ann Med. 1991;23:539–43. doi: 10.3109/07853899109150515. [DOI] [PubMed] [Google Scholar]
- Vartoukian SR, Palmer RM, Wade WG. Cultivation of a Synergistetes strain representing a previously uncultivated lineage. Environ Microbiol. 2010;12:916–28. doi: 10.1111/j.1462-2920.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani A, Santo-Domingo NE, Main DM. Actinobacillus actinomycetemcomitans pneumonia with possible septic embolization. Chest. 1994;105:645–6. doi: 10.1378/chest.105.2.645. [DOI] [PubMed] [Google Scholar]
- Vincent AT, Derome N, Boyle B, Culley AI, Charette SJ. Next-generation sequencing (NGS) in the microbiological world: How to make the most of your money. J Microbiol Methods. 2016 doi: 10.1016/j.mimet.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38(Suppl 11):7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KH, Wilson WJ, Radosevich JL, DeSantis TZ, Viswanathan VS, Kuczmarski TA, Andersen GL. High-density microarray of small-subunit ribosomal DNA probes. Appl Environ Microbiol. 2002;68:2535–41. doi: 10.1128/AEM.68.5.2535-2541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–71. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wang Y, Qian PY. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinformatics. 2016;17:135. doi: 10.1186/s12859-016-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015;7:27. doi: 10.1186/s13073-015-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Microarrays for bacterial detection and microbial community analysis. Curr Opin Microbiol. 2003;6:288–94. doi: 10.1016/s1369-5274(03)00052-3. [DOI] [PubMed] [Google Scholar]