Abstract
Rationale: Recent advancements that have been made in magnetic resonance imaging (MRI) improve our ability to assess pulmonary structure and function in patients with cystic fibrosis (CF). A nonionizing imaging modality that can be used as a serial monitoring tool throughout life can positively affect patient care and outcomes.
Objectives: To compare an ultrashort echo-time MRI method with computed tomography (CT) as a biomarker of lung structure abnormalities in young children with early CF lung disease.
Methods: Eleven patients with CF (mean age, 31.8 ± 5.7 mo; median age, 33 mo; 7 male and 4 female) were imaged via CT and ultrashort echo-time MRI. Eleven healthy age-matched patients (mean age, 22.5 ± 10.2 mo; median age, 23 mo; 5 male and 6 female) were imaged via ultrashort echo-time MRI. CT scans of 13 additional patients obtained for clinical indications not affecting the heart or lungs and interpreted as normal provided a CT control group (mean age, 24.1 ± 11.7 mo; median age, 24 mo; 6 male and 7 female). Studies were scored by two experienced radiologists using a well-validated CF-specific scoring system for CF lung disease.
Measurements and Main Results: Correlations between CT and ultrashort echo-time MRI scores of patients with CF were very strong, with P values ≤0.001 for bronchiectasis (r = 0.96) and overall score (r = 0.90), and moderately strong for bronchial wall thickening (r = 0.62, P = 0.043). MRI easily differentiated CF and control groups via a reader CF-specific scoring system.
Conclusions: Ultrashort echo-time MRI detected structural lung disease in very young patients with CF and provided imaging data that correlated well with CT. By quantifying early CF lung disease without using ionizing radiation, ultrashort echo-time MRI appears well suited for pediatric patients requiring longitudinal imaging for clinical care or research studies.
Clinical Trial registered with www.clinicaltrials.gov (NCT01832519).
Keywords: ultrashort echo-time, magnetic resonance imaging, lung, cystic fibrosis
Magnetic resonance imaging (MRI) has become the modality of choice for the investigation of the central nervous system and for most indications in the abdomen and musculoskeletal system (1–3). MRI in the lung has lagged behind other areas because of the low proton density of the lungs, sensitivity to physiological motion (respiration, cardiac pulsation), and local magnetic-susceptibility gradients caused by the air–tissue interfaces of the alveoli, leading to an exceedingly short effective transverse relaxation time of the lung parenchyma. Together, these result in a loss of image quality and compromise the detection of pulmonary structural information (4, 5).
Despite these difficulties, advances have been made in obtaining structural and functional pulmonary information using techniques including ultrashort echo-time (UTE) imaging and new Fourier decomposition methods (5–11). These techniques can reduce the echo time to 100 microseconds or less, minimizing the parenchymal signal decay caused by short effective transverse relaxation time, and therefore, can detect very subtle lung physiology, including gravity-dependent lung-density gradients (11–15). We and others have recently developed a novel approach to lung MRI using a refinement of the UTE technique. This new technology allows improvement in lung image quality, which now closely approaches the image quality of computed tomography (CT) scanning (11–15). We performed this study using expert observer scoring to compare UTE MRI with CT in the evaluation of early cystic fibrosis (CF) lung disease in young patients.
CF is an autosomal recessive disorder caused by mutations encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein; it affects approximately 70,000 people worldwide (16). At birth, none of the features of CF lung disease are clinically evident, but airway abnormalities observed as air trapping and bronchiectasis develop within the first months of life (17–22). Despite improvements in treatment and a resulting increase in lifespan, with a median survival of patients with CF to >40 years, 95% of CF deaths are related to lung disease (23). Increased detection of CF-specific lung abnormalities can lead to personalized interventions and potentially faster assessment of treatment response or treatment impact in clinical trials (24).
Until recently, CF therapy has addressed the physiologic consequences of abnormal CFTR. Progress in disease management and therapeutic efficacy has improved our ability to deal with these consequences, and now therapy is directed toward improving the function of CFTR itself and thus preventing the development of CF lung disease (25–28). To provide maximum benefit, these therapies should begin before airway damage and the cycle of infection and inflammation have begun the progressive lung destruction that is the hallmark of CF (28). The development and most effective use of these therapies require outcome surrogates for drug development and methods to monitor the lungs closely for the first evidence of developing lung disease (29, 30).
Imaging has been suggested as a potentially valuable outcome surrogate for lung disease in young children with CF (29, 30). CT is the most well established modality because of its ability to localize and quantify the findings of CF lung disease (19, 31–34). However, CT exposes patients to ionizing radiation, which poses an undesirable risk for children in the lifelong longitudinal assessment of disease progression (35, 36). MRI does not involve ionizing radiation, which reduces the risk of future radiation-induced cancers (37), and can provide functional as well as anatomic information (5–15). Previous MRI studies using conventional sequences have found MRI to be inferior to CT in disease evaluation (38, 39). New MRI techniques can bridge this quality gap and open the way for a “one-stop shop” of anatomic and functional lung evaluation that cannot be performed in any other way (40). Furthermore, there is a great need for a common methodology that is able to detect and monitor early signs of morphological and functional lung changes in patients with CF from birth throughout their lifespan.
We hypothesized that UTE MRI may provide a sensitive tool for monitoring early CF lung disease without radiation exposure. In this study, we imaged patients with CF and age-matched healthy control subjects, 7 to 47 months of age, with CT and/or UTE MRI. The purpose of this study was to evaluate the ability of UTE MRI, compared with CT, to assess CF-specific structural lung abnormalities in young children with early lung disease. Our findings support the further development of UTE MRI to detect and quantify structural lung disease in young children with CF.
Methods
This study was approved by the institutional review board (IRB approval number 2012–2096) at Cincinnati Children’s Hospital Medical Center. The study was registered prospectively with a global trials registry site (NCT01832519). Informed consent was obtained from all guardians of the participants.
Patients
CT and UTE MRI were performed on the same day in 11 patients with CF (7 male and 4 female; mean ± SD age, 31.8 ± 5.7 mo; median age, 33 mo; age range, 23–47 mo). Diagnosis of CF was confirmed by sweat chloride >60 mM and/or two CF causing mutations in the CFTR gene. UTE MRI was performed in 11 age-matched control subjects (±12 mo; 5 male and 6 female; mean ± SD age, 22.5 ± 10.2 mo; median age, 23 mo; age range, 7–37 mo). These control subjects were selected from patients undergoing clinical MRI of the central nervous system. Subjects with pulmonary symptoms or a history of lung disease were excluded.
Because CT scans could not be performed without a clinical indication, CT scans in 13 additional control subjects (6 male and 7 female; mean age, 24. ± 11.7 mo; median age, 23 mo; age range, 8–46 mo) were evaluated in the same manner as that of the CT scans performed on the patients with CF. Clinical reading and review of these CT scans found no cardiopulmonary abnormalities. The clinical CT studies were obtained for multiple indications, primarily to evaluate for pulmonary metastases in the initial evaluation of children with abdominal tumors. Patients were intubated and ventilated as per routine clinical imaging of young patients at our institution. The total anesthesia time from induction to full recovery ranged from 100 to 140 minutes.
CT Examination
All patients with CF underwent clinically indicated CT imaging before MRI as part of their annual check-up. CT scanning was performed using our hospital’s Toshiba Aquilion One (Toshiba Medical Systems, Tustin, CA) 320-slice CT scanner. Volumetric CT scans (slice thickness acquired at 0.5 mm, and FC18 convolution kernel) were performed at inspiration and expiration using airway pressures of 30 and 0 cm water, respectively, after lung-recruitment maneuvers to reduce atelectasis (41). A weight-based CT technique was used to minimize radiation dose (42). CT parameters included axial orientation; voltage, 80–100 KVp; and current, 40–170 mA; and in-plane resolution varied from 0.37 × 0.37 mm2 to 0.51 × 0.51 mm2. Total time from induction to CT scan completion was 15 to 40 minutes.
UTE MRI Protocols
All MRI was performed in a 1.5T MR Philips Ingenia scanner (Philips Healthcare, Best, the Netherlands) with a 20-channel head and neck vascular phased-array coil that provided uniform radio frequency signal of the entire lungs. Lung images were acquired while the patients were ventilated using a radial three-dimensional UTE, stack-of-stars sequence, after lung-recruitment maneuvers to reduce atelectasis (41). Images were acquired with the following parameters: repetition time/echo time, 4.18/0.179 ms; flip angle, 5°; matrix size, 224 × 224; field of view, 200 × 200 mm; and voxel size, 0.89 × 0.89 × 2.0 mm. An echo navigator was positioned on the lung–liver interface, and image acquisition was gated during expiration to capture lung signal at approximately functional residual capacity (FRC). UTE image acquisition was approximately 5 to 10 minutes. Total time in the MRI scanner, which included patient positioning, localizer, UTE scan, and additional investigative imaging, was approximately 70–90 minutes.
Image Analysis
For the purposes of this study, image scoring was limited to previously validated and published scoring systems using CT and MRI (19, 31, 33, 34, 40, 43, 44). A recent CT scoring system that has been piloted in young patients with CF was not used because of the absence of a complementary scoring system for MRI (32). All identifying information was removed from the images, and the MRI scans were read in random order. The CT scans were then read, also in random order. Images were scored by two senior pediatric radiologists trained in pulmonary image analysis and CF-specific scoring. The overall score and subscores are reported as the mean value of the two readers’ scores.
CT Scoring
CT images were scored using a previously described scoring system modified from the Brody scoring system specifically for use in infants and young children (19, 31, 33, 34). Briefly, the lungs were divided into six lobar regions (five lobes plus lingula) and were assessed for bronchiectasis, bronchial wall thickening, mucus plugging, consolidation, ground-glass opacity (on inspiratory scans), and air trapping (on expiratory scans). The extent of each abnormality was scored on a 0–2 scale (0, not present; 1, <1/2 lobe; 2, >1/2 lobe) for each of these parameters in each of the lobes with a maximum score of 72. The slice thickness of the scored CT images was 1 mm obtained at intervals of 3–5 mm.
MRI Scoring
MRI scans were scored using a previously described MRI scoring system for lung morphology in CF (40, 43, 44). Here the lungs were divided into six lobar regions and were assessed for bronchial wall thickening, bronchiectasis, mucus plugging, ground glass opacities, consolidation, and air trapping. The extent of lobar morphology was scored on a 0 to 2 scale (0, not present; 1, <1/2 lobe; 2, >1/2 lobe) for each of these parameters in each of the lobes, with a maximum score of 72. The slice thickness of the scored MRI scans was 2 mm.
Statistical Analysis
The overall CF-specific scores for CT and MRI were calculated for each imaging study. Mean scores and SDs were calculated for CT and for UTE MRI scans. Pearson correlation coefficients were calculated to assess agreement between CF-specific scores determined from both CT and UTE MRI. P values <0.05 (two sided) were considered statistically significant. Interobserver agreement of CT and UTE MRI scores was examined using the intraclass correlation coefficient (ICC). ICC values between 0.40 and 0.60, between 0.60 and 0.80, and >0.80 were considered moderate, good, and very good agreement, respectively (45). Comparison of these CT and UTE MRI CF-specific scores between patients with CF and healthy control subjects was performed using an unpaired t test.
Results
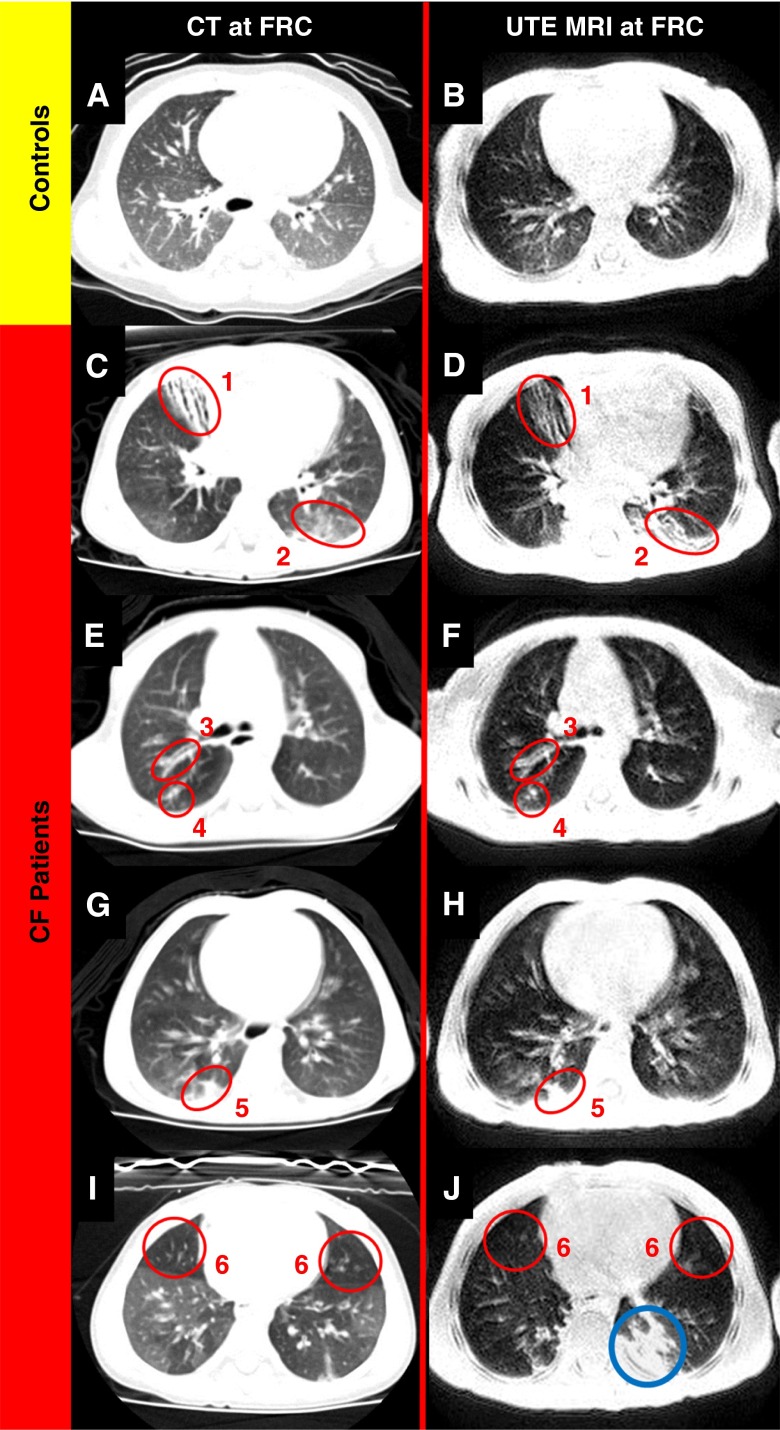
The demographic description of control subjects and patients with CF is listed in Table 1. The clinical history of the patients with CF is detailed in Table 2. CT and UTE MRI scans of four patients with CF (age range, 33–47 mo) with observed lung abnormalities compared with two control subjects are displayed in Figure 1. Visual assessment of CF-specific lung-structure abnormalities such as bronchiectasis, consolidation, ground-glass opacity, mucus plugging, bronchial wall thickening, and air trapping were detectable in both CT and UTE MRI scans of the patients with CF and are denoted in Figure 1.
Table 1.
Demographics of patients with CF and control subjects
| No. Patients/Subjects | Age (mo) | Sex (M/F) | |
|---|---|---|---|
| Patients with CF | 11 | 31.8 ± 5.7 | 7/4 |
| Control subjects: MRI | 11 | 22.5 ± 10.2 | 5/6 |
| Control subjects: CT | 13 | 24.1 ± 11.7 | 6/7 |
Definition of abbreviations: CF = cystic fibrosis; CT = computed tomography; MRI = magnetic resonance imaging.
Data are expressed as mean ± SD for age.
Table 2.
Clinical history of population of patients with CF
| Patient Number | Age (mo) | Sex | Genotype | Weight for Length Percentile | Dornase α | Hypertonic Saline | Prior CF Pathogens | Prior Antibiotic Courses | Prior Hospitalizations |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 23 | M | F508del/F508del | 83.8 | Yes | No | SA | Oral: 5 | 2 |
| PA | Inhaled: 1 | ||||||||
| IV: 8 | |||||||||
| 02 | 24 | F | F508del/F508del | 55.5 | Yes | No | SA | Oral: 6 | 2 |
| PA | Inhaled: 1 | ||||||||
| HI | IV: 8 | ||||||||
| 03 | 40 | M | F508del/F508del | 77.6 | Yes | No | SA | Oral: 5 | 2 |
| HI | Ear drops: 1 | ||||||||
| Eye drops: 1 | |||||||||
| 04 | 37 | M | F508del/F508del | 21.4 | Yes | No | SA | None | 0 |
| HI | |||||||||
| 05 | 27 | M | F508del/R117H | 96.1 | No | No | HI | Oral: 1 | 1 |
| SP | Eye drops: 2 | ||||||||
| SM | |||||||||
| 06 | 30 | M | F508del/Q493X | 45.1 | Yes | Yes | HI | Oral: 9 | 3 |
| ORSA | Inhaled: 1 | ||||||||
| IV: 2 | |||||||||
| Eye drops: 3 | |||||||||
| 07 | 36 | F | F508del/F508del | 72.5 | Yes | No | PA | Oral: 8 | 5 |
| HI | Inhaled: 4 | ||||||||
| ORSA | IV: 3 | ||||||||
| SM | |||||||||
| 08 | 37 | F | F508del/F508del | 90.3 | No | No | PA | Oral - 1 | 0 |
| HI | Inhaled: 1 | ||||||||
| SA | |||||||||
| 09 | 33 | F | F508del/F508del | 61.4 | Yes | Yes | PA | Oral: 17 | 4 |
| HI | Inhaled: 2 | ||||||||
| ORSA | IV: 3 | ||||||||
| SM | |||||||||
| 10 | 35 | M | F508del/F508del | 31.0 | Yes | Yes | ORSA | None | 0 |
| SM | |||||||||
| 11 | 28 | M | F508del/Unknown | 80.0 | Yes | Yes | ORSA | Oral: 15 | 0 |
| HI | Inhaled: 2 | ||||||||
| IV: 4 |
Definition of abbreviations: CF = cystic fibrosis; HI = Haemophilus influenzae; ORSA = oxacillin-resistant Staphylococcus aureus; PA = Pseudomonas aeruginosa; SA = Staphylococcus aureus; SM = Stenotrophomonas maltophilia; SP = Streptococcus pyogenes (strep A).
All patients were prescribed pancreatic enzyme replacement therapy, twice daily airway clearance, and fat-soluble vitamins.
Figure 1.
(A) Computed tomography (CT) image of an age-matched healthy patient with normal chest findings. (B) Ultrashort echo-time (UTE) magnetic resonance imaging (MRI) of an aged-matched healthy patient. (C–J) CT images (left) and matched UTE MRI scans (right) of four patients with cystic fibrosis aged 33 to 47 months old. Both CT and UTE MRI visualize bronchiectasis (1), ground-glass opacity (2), bronchial wall thickening (3), mucus plugging (4), consolidation (5), and air trapping (6). The blue circle captures the accumulated anesthesia induced-atelectasis.
Correlations between CT and UTE MRI in Young Patients with CF
The mean CF-specific score and subscores for patients with CF are listed in Table 3. Table 4 lists the ICCs of the two readers’ scores and subscores. For the CT images of the patients with CF, ICC was 0.84–0.98 for mucus plugging, consolidation, bronchiectasis, air trapping, and the overall score, indicating very good agreement. There was good agreement (ICC, 0.73–0.76) for bronchial wall thickening and ground-glass opacity for CT images of the patients with CF.
Table 3.
Comparison of mean CT and UTE MRI CF-specific scores in pediatric patients with CF and control subjects
| Subjects | Bronchiectasis | Mucus Plugging | Bronchial Wall Thickening | Consolidation | Ground-Glass Opacity | Air Trapping | Overall |
|---|---|---|---|---|---|---|---|
| Patients with CF – CT scores (Ttest of CF patients’ CT scores vs. UTE MRI scores) | 1.55 ± 2.78 (0.091) | 0.41 ± 0.89 (0.11) | 2.32 ± 1.81 (0.045) | 2.45 ± 1.44 (0.34) | 0.50 ± 0.71 (0.42) | 2.59 ± 3.12 (0.025) | 9.86 ± 7.04 (0.0053) |
| Patients with CF – UTE MRI Scores (Ttest of UTE MRI Scores of patients with CF vs. control subjects) | 0.64 ± 1.27 (0.32) | 0.091 ± 0.30(0.33) | 0.11 ± 1.32 (0.11) | 3.0 ± 1.92 (0.011) | 0.32 ± 0.34 (0.70) | 0.14 ± 0.32 (0.19) | 5.55 ± 3.70 (0.017) |
| Control subjects – CT scores (Ttest of CT Score of patients with CF vs. control subjects) | 0.19 ± 0.56 (0.14) | 0.039 ± 0.14 (0.20) | 0.039 ± 0.14 (0.0019) | 0.46 ± 0.95 (0.0011) | 0.039 ± 0.14 (0.057) | 0.0 ± 0.0 (0.020) | 0.77 ± 1.20 (0.0016) |
| Control subjects – UTE MRI scores | 0.23 ± 0.34 | 0.0 ± 0.0 | 0.50 ± 0.77 | 1.14 ± 0.84 | 0.41 ± 0.70 | 0.0 ± 0.0 | 2.27 ± 1.42 |
Definition of abbreviations: CF = cystic fibrosis; CT = computed tomography; MRI = magnetic resonance imaging; UTE = ultrashort echo-time.
Data are presented as mean ± SD (P value). Age-matched control subjects generally had lower scores compared with patients with CF.
Table 4.
Pearson correlations and ICC of CT and UTE MRI scores of patients with CF
| Patients with CF | Pearson Correlation Coefficient (r) (P Value) | Reader 1 vs. Reader 2, CT ICC (95% CI) | Reader 1 vs. Reader 2, MRI ICC (95% CI) |
|---|---|---|---|
| Overall | 0.90 (0.0002) | 0.98 (0.93-0.99) | 0.76 (0.31-0.93) |
| Bronchiectasis | 0.96 (<0.0001) | 0.94 (0.81-0.98) | 0.84 (0.54-0.95) |
| Bronchial wall thickening | 0.62 (0.043) | 0.73 (0.28-0.92) | 0.60 (0.06-0.87) |
| Air trapping | * | 0.98 (0.92-0.99) | * |
| Consolidation | 0.45 (0.16) | 0.84 (0.52-0.95) | 0.71 (0.23-0.91) |
| Mucus plugging | * | 0.84 (0.49-0.96) | * |
| Ground-glass opacity | 0.21 (0.54) | 0.76 (0.31-0.93) | No agreement |
Definition of abbreviations: CF = cystic fibrosis; CI = confidence interval; CT = computed tomography; ICC = intraclass correlation coefficient; MRI = magnetic resonance imaging; UTE = ultrashort echo-time.
If there were three or fewer observations, value was not calculated.
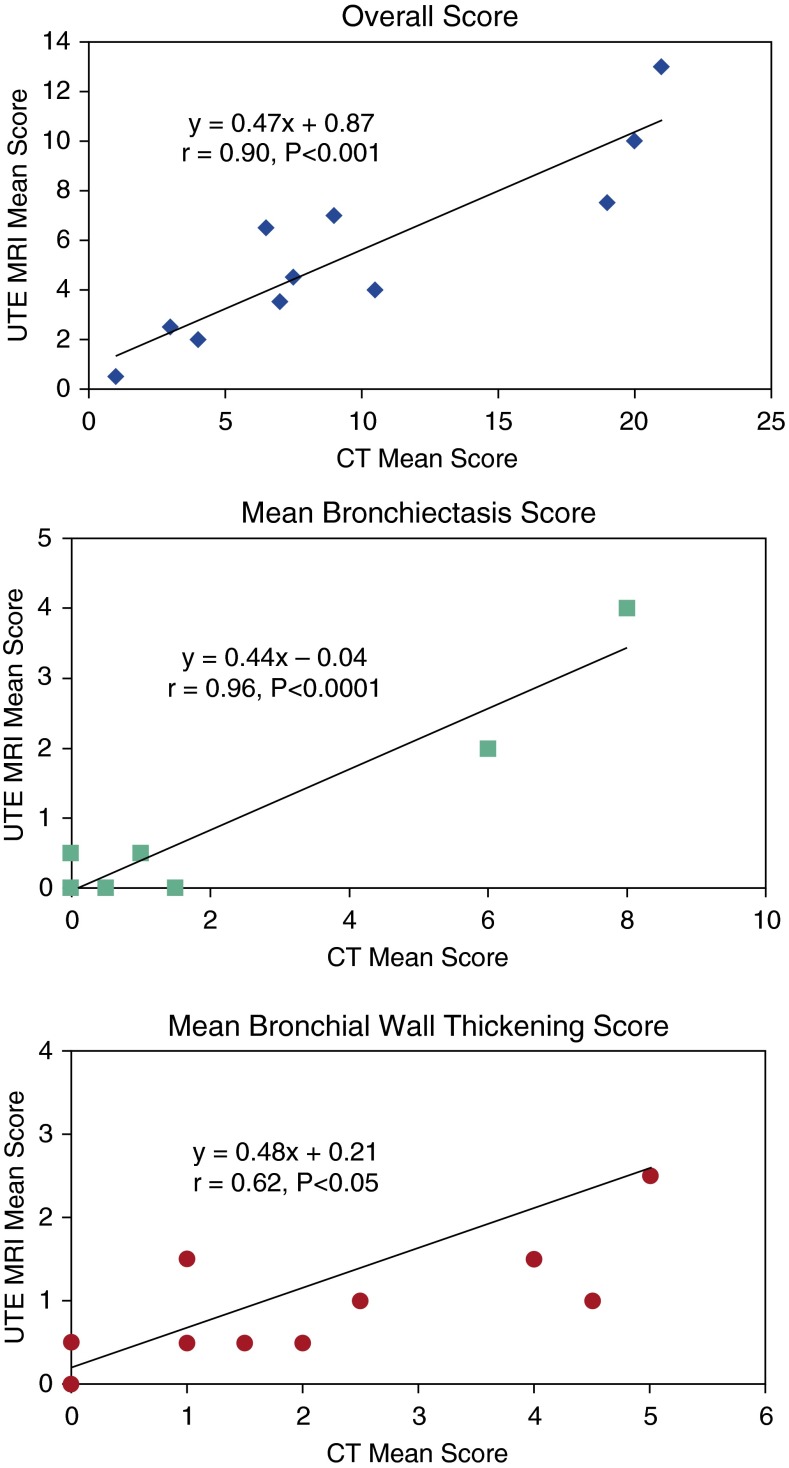
For the MR images of the patients with CF, there was good to very good agreement (ICC, 0.60–0.84) for the overall score, bronchiectasis, bronchial wall thickening, and consolidation, but no agreement for ground-glass opacity. Pearson correlation coefficients between the scores of CT and UTE MRI of the patients with CF are listed in Table 4 (mean scores for the two readers for each imaging modality). The correlation coefficient between the average overall score for CT and UTE MRI of the patients with CF was significant (r = 0.90, P < 0.001) (Figure 2). The correlations between the mean subscores for CT and UTE MRI were strong to very strong and significant for bronchial wall thickening (r = 0.62, P < 0.05) and bronchiectasis (r = 0.96, P < 0.0001). The impact of recent pulmonary exacerbations within 6 months of imaging was assessed in these same patients with CF.
Figure 2.
Correlation between the overall, bronchiectasis, and bronchial wall-thickening mean computed tomography (CT) and ultrashort echo-time (UTE) magnetic resonance imaging (MRI) scores of the two readers. The values for r are obtained from simple linear regression of UTE MRI (y axis) on CT (x axis) mean scores.
Ultimately, there was no difference (P > 0.13) in the overall score or in the majority of subscores between patients with recent exacerbations and those without exacerbations before imaging. However, there were two instances in which the differences in subscores of patients with recent exacerbation were almost greater than those of patients without recent exacerbations, specifically, CT bronchial wall thickening score (P = 0.054) and MRI consolidation score (P = 0.08).
Differentiation of Young Patients with CF and Control Subjects by CT and MRI
The CT scores of the patients with CF vs. control subjects (Table 3) were significantly higher for bronchial wall thickening (P = 0.0019), consolidation (P = 0.011), air trapping (P = 0.02), and the overall score (P = 0.0016). The UTE MRI scores of the patients with CF vs. the control subjects were significantly higher for consolidations (P = 0.011) and for the overall score (P = 0.017) in these young patients with early lung disease. Importantly, bronchiectasis scores were expectedly low for CT and UTE MRI in this study population relative to patients with moderate or severe disease (school-aged or older), reflecting the relatively early lung disease observed in this young population.
Discussion
To our knowledge, this is the first report of UTE MRI in the evaluation of early CF lung disease in young pediatric patients. The results demonstrated that features of lung disease detected by CT are also detected by UTE MRI. UTE MRI subscores for CF-specific abnormalities tended to be lower compared with those from CT but higher compared with non-CF control UTE MRI values. Moreover, when bronchial wall thickening, bronchiectasis, and overall scores were evaluated, correlations between CT and UTE MRI for patients with CF were high.
The results suggest that the use of UTE MRI to detect and quantify CF-specific abnormalities should include an evaluation of both individual pathologic subscores and comprehensive scoring. Our anecdotal impression that the images from the UTE MRI technique were similar in quality to the CT images is supported by the excellent agreement between the scores from CT and UTE MRI for many of the morphologic findings of CF lung disease. The robust nature of this UTE MRI technique is supported by our use of previously published scoring systems used in multiple investigations, rather than by developing a new scoring system optimized for our technique. The CT scoring system was developed specifically for CT scanning of young children with CF (19, 32, 33, 34). The CF MRI scoring system, developed by an independent research group, has also been used in multiple studies (40, 43, 44). If supported by future studies, UTE MRI may be used to provide the same information as CT for most findings of CF lung disease. In addition, UTE MRI may be integrated into routine clinical MRI sequences, such as contrast-enhanced T1-weighted imaging and perfusion imaging, in a more comprehensive evaluation of CF lung disease.
Limitations
The greatest limitation of CT scanning is that it requires the use of ionizing radiation. Although there is increasing scientific recognition that the perception of the risk of ionizing radiation from CT scanning may be greater than the true risk, this perception will likely continue to cause limited acceptance of serial CT scanning by both families and CF physicians. MRI does not use ionizing radiation, and there are no known health risks from MRI scanning. Unlike CT scanning, increasing the number of images obtained during an MRI examination does not add any risk. This allows the use of techniques that capture large numbers of images during free breathing that can be selected by phase of respiration and the combining of multiple images to provide high-quality images at any phase of the respiratory cycle (46).
MRI can also produce functional information such as regional lung ventilation with hyperpolarized gas and blood flow using time-of-flight techniques without intravenous contrast material. These modalities were not included in this report because of our current focus on developing UTE MRI to detect structural lung disease in CF, the limited experience of hyperpolarized gases in young children, and recent questions that have been raised regarding gadolinium deposition in tissues after repeated perfusion measurements (47–50).
Quantifying morphologic lung disease in CF from infancy to adulthood is increasingly important for clinical care and research and for the development of new therapies. Since the success of any individual lifelong monitoring tool will depend on its ability to detect early CF lung disease, we chose very young patients for this study. UTE MRI was able to visualize early structural abnormalities including bronchiectasis, bronchial wall thickening, consolidation, ground-glass opacity, mucus plugging, and air trapping (Figure 1). Our data indicate that it was less sensitive than CT in identifying bronchial wall thickening and air trapping in this young patient population (Table 3). However, these results do not account for potential perfusion deficits because perfusion was not measured in these patients. The difference in the scores was most likely a result of the higher image resolution achievable with CT and because one lung volume was included for UTE MRI, whereas two were included for CT.
Because UTE MRI was acquired at FRC, it is possible that it was less sensitive in scoring airway diseases such as bronchiectasis and bronchial wall thickening compared with inspiratory CT. A previous study has demonstrated that expiratory CT is less sensitive than inspiratory CT in assessing structural airway disease in young children with CF (51). Another limitation of our current UTE sequence is that it was less sensitive to air trapping compared with CT, as we were only able to acquire quality images at FRC; however, similar sensitivity issues in visualizing air trapping have been demonstrated in previous MRI studies (52, 53). Therefore, future endeavors should focus on accurately assessing trapped air via hyperpolarized gas and or proton ventilation maps (6, 54–56). Efforts should also be focused on imaging sedated or tranquil patients while free breathing, as opposed to the current standard of care for imaging these young patients, which involves ventilation and anesthesia. Furthermore, retrospective gating will allow continuous image acquisition during free breathing, with later separation of inspiration from expiration images (46).
A possible confounding factor contributing to the elevated UTE MRI scores of the control subjects was anesthesia-induced atelectasis. Because of the length of the scan (5–10 min), atelectasis may have accumulated during the scan despite the lung recruitment technique that was implemented prior to scanning (41). An advantage of MRI is that, unlike CT, scans can be repeated if atelectasis is detected without exposing patients to additional ionizing radiation. Overall, UTE MRI demonstrated the ability to quantify the extent and severity of lung abnormalities in very young patients with CF via a CF-specific scoring system, with overall results very similar to those of CT scanning. The overall scores for CT and UTE MRI demonstrated excellent agreement between the two modalities, highlighting the value of comprehensive scoring analysis to quantify lung disease in young patients with CF with generally subtle abnormalities. We attribute this excellent correlation to the superior image resolution and quality provided by UTE MRI, compared with more traditional MRI methods that have seen only modest correlations (38, 39).
Conclusions
In summary, using UTE MRI, we were able to identify CF-specific lung structural abnormalities caused by bronchiectasis, bronchial wall thickening, mucus plugging, ground-glass opacity, and consolidation. Pearson coefficients, determined from the CF scores of both CT and UTE MRI, displayed strong and significant correlations for the overall score, bronchiectasis, and bronchial wall thickening in this group of very young patients with early lung disease. UTE MRI involves no ionizing radiation and its continuing development places this technique as a potential longitudinal imaging surrogate for CT. Our results support the further development of UTE MRI techniques to longitudinally monitor pediatric patients with CF as a biomarker of both regional and total lung disease abnormalities. These imaging techniques may have direct application in detecting and quantifying the impact of new therapies in clinical trials and in monitoring interventions in clinical care.
Footnotes
Supported by the National Institutes of Health (R01 HL116226) and by Vertex Pharmaceuticals (J.C.W.).
The sponsors of this study had no role in the design of the study, the collection and analysis of data, or the preparation of the manuscript.
Author Contributions: J.C.W. is the guarantor of this paper and takes responsibility for the overall content and integrity of the work as a J.P.C. whole. J.C.W., J.P.C., D.J.R., Y.C., and S.D.S. contributed to the conception and design of the study; J.C.W., D.J.R., R.J.F., A.S.B., R.D.S., S.K., and J.P.C. contributed to the analysis and interpretation of the data; D.J.R. and J.C.W. contributed to the drafting of the manuscript; J.C.W., J.C.P., R.J.F., A.S.B., R.D.S., and Y.C. contributed to editing the manuscript for intellectual content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bradley WG., Jr Magnetic resonance imaging in the central nervous system: comparison with computed tomography. Magn Reson Annu. 1986:81–122. [PubMed] [Google Scholar]
- 2.Symms M, Jäger HR, Schmierer K, Yousry TA. A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry. 2004;75:1235–1244. doi: 10.1136/jnnp.2003.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt GP, Reiser MF, Baur-Melnyk A. Whole-body imaging of the musculoskeletal system: the value of MR imaging. Skeletal Radiol. 2007;36:1109–1119. doi: 10.1007/s00256-007-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergin CJ, Noll DC, Pauly JM, Glover GH, Macovski A. MR imaging of lung parenchyma: a solution to susceptibility. Radiology. 1992;183:673–676. doi: 10.1148/radiology.183.3.1584917. [DOI] [PubMed] [Google Scholar]
- 5.Kauczor HU, Kreitner KF. MRI of the pulmonary parenchyma. Eur Radiol. 1999;9:1755–1764. doi: 10.1007/s003300050919. [DOI] [PubMed] [Google Scholar]
- 6.Pennati F, Quirk JD, Yablonskiy DA, Castro M, Aliverti A, Woods JC. Assessment of regional lung function with multivolume (1)H MR imaging in health and obstructive lung disease: comparison with (3)He MR imaging. Radiology. 2014;273:580–590. doi: 10.1148/radiol.14132470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo JR, MacKay A, Müller NL. MR imaging of the lungs: value of short TE spin-echo pulse sequences. AJR Am J Roentgenol. 1992;159:951–956. doi: 10.2214/ajr.159.5.1414805. [DOI] [PubMed] [Google Scholar]
- 8.Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology. 1991;179:777–781. doi: 10.1148/radiology.179.3.2027991. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70:1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauman G, Puderbach M, Deimling M, Jellus V, Chefd’hotel C, Dinkel J, Hintze C, Kauczor HU, Schad LR. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn Reson Med. 2009;62:656–664. doi: 10.1002/mrm.22031. [DOI] [PubMed] [Google Scholar]
- 11.Bauman G, Lützen U, Ullrich M, Gaass T, Dinkel J, Elke G, Meybohm P, Frerichs I, Hoffmann B, Borggrefe J, et al. Pulmonary functional imaging: qualitative comparison of Fourier decomposition MR imaging with SPECT/CT in porcine lung. Radiology. 2011;260:551–559. doi: 10.1148/radiol.11102313. [DOI] [PubMed] [Google Scholar]
- 12.Togao O, Tsuji R, Ohno Y, Dimitrov I, Takahashi M. Ultrashort echo time (UTE) MRI of the lung: assessment of tissue density in the lung parenchyma. Magn Reson Med. 2010;64:1491–1498. doi: 10.1002/mrm.22521. [DOI] [PubMed] [Google Scholar]
- 13.Lederlin M, Crémillieux Y. Three-dimensional assessment of lung tissue density using a clinical ultrashort echo time at 3 tesla: a feasibility study in healthy subjects. J Magn Reson Imaging. 2014;40:839–847. doi: 10.1002/jmri.24429. [DOI] [PubMed] [Google Scholar]
- 14.Ma W, Sheikh K, Svenningsen S, Pike D, Guo F, Etemad-Rezai R, Leipsic J, Coxson HO, McCormack DG, Parraga G. Ultra-short echo-time pulmonary MRI: evaluation and reproducibility in COPD subjects with and without bronchiectasis. J Magn Reson Imaging. 2015;41:1465–1474. doi: 10.1002/jmri.24680. [DOI] [PubMed] [Google Scholar]
- 15.Dournes G, Menut F, Macey J, Fayon M, Chateil JF, Salel M, Corneloup O, Montaudon M, Berger P, Laurent F.Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution Eur Radiol(In press) [DOI] [PubMed] [Google Scholar]
- 16.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, Ramalho AS, Amaral MD, Dorfman R, Zielenski J, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 18.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 19.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623–628.e1. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175:943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 21.Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr. 2004;144:154–161. doi: 10.1016/j.jpeds.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Llapur CJ, Martínez TM, Coates C, Tiller C, Wiebke JL, Li X, Applegate K, Coxson HO, Tepper RS. Lung structure and function of infants with recurrent wheeze when asymptomatic. Eur Respir J. 2009;33:107–112. doi: 10.1183/09031936.00106607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuehn BM. Progress in treating cystic fibrosis means that many patients may now reach midlife and beyond. JAMA. 2014;312:1182–1183. doi: 10.1001/jama.2014.12285. [DOI] [PubMed] [Google Scholar]
- 24.Tiddens HA, Stick SM, Wild JM, Ciet P, Parker GJ, Koch A, Vogel-Claussen J. Respiratory tract exacerbations revisited: ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment. Pediatr Pulmonol. 2015;50:S57–S65. doi: 10.1002/ppul.23266. [DOI] [PubMed] [Google Scholar]
- 25.Stern M, Wiedemann B, Wenzlaff P German Cystic Fibrosis Quality Assessment Group. From registry to quality management: the German Cystic Fibrosis Quality Assessment project 1995 2006. Eur Respir J. 2008;31:29–35. doi: 10.1183/09031936.00056507. [DOI] [PubMed] [Google Scholar]
- 26.Simmonds NJ, D’Souza L, Roughton M, Alton EW, Davies JC, Hodson ME. Cystic fibrosis and survival to 40 years: a study of cystic fibrosis transmembrane conductance regulator function. Eur Respir J. 2011;37:1076–1082. doi: 10.1183/09031936.00079010. [DOI] [PubMed] [Google Scholar]
- 27.Cystic Fibrosis Foundation Patient Registry. 2014 Annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2014. [Google Scholar]
- 28.Bell SC, De Boeck K, Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther. 2015;145:19–34. doi: 10.1016/j.pharmthera.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc. 2007;4:418–430. doi: 10.1513/pats.200703-041BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiddens HA, Puderbach M, Venegas JG, Ratjen F, Donaldson SH, Davis SD, Rowe SM, Sagel SD, Higgins M, Waltz DA. Novel outcome measures for clinical trials in cystic fibrosis. Pediatr Pulmonol. 2014;50:302–315. doi: 10.1002/ppul.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr. 2004;145:32–38. doi: 10.1016/j.jpeds.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191:1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 33.Mott LS, Park J, Gangell CL, de Klerk NH, Sly PD, Murray CP, Stick SM Australian Respiratory Early Surveillance Team for Cystic Fibrosis Study Group. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr. 2013;163:243–248.e1–3. doi: 10.1016/j.jpeds.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 34.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 35.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, Khorasani R. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175–184. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 37.de Jong PA, Mayo JR, Golmohammadi K, Nakano Y, Lequin MH, Tiddens HA, Aldrich J, Coxson HO, Sin DD. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med. 2006;173:199–203. doi: 10.1164/rccm.200505-810OC. [DOI] [PubMed] [Google Scholar]
- 38.Tepper LA, Ciet P, Caudri D, Quittner AL, Utens EM, Tiddens HA. Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr Pulmonol. 2016;51:34–41. doi: 10.1002/ppul.23328. [DOI] [PubMed] [Google Scholar]
- 39.Ciet P, Serra G, Bertolo S, Spronk S, Ros M, Fraioli F, Quattrucci S, Assael MB, Catalano C, Pomerri F, et al. Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol. 2016;26:780–787. doi: 10.1007/s00330-015-3850-9. [DOI] [PubMed] [Google Scholar]
- 40.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, Ley S, Sumkauskaite M, Biederer J, Kauczor HU, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189:956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 41.Newman B, Krane EJ, Gawande R, Holmes TH, Robinson TE. Chest CT in children: anesthesia and atelectasis. Pediatr Radiol. 2014;44:164–172. doi: 10.1007/s00247-013-2800-4. [DOI] [PubMed] [Google Scholar]
- 42.Raman SP, Mahesh M, Blasko RV, Fishman EK. CT scan parameters and radiation dose: practical advice for radiologists. J Am Coll Radiol. 2013;10:840–846. doi: 10.1016/j.jacr.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 43.Eichinger M, Optazaite DE, Kopp-Schneider A, Hintze C, Biederer J, Niemann A, Mall MA, Wielpütz MO, Kauczor HU, Puderbach M. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol. 2012;81:1321–1329. doi: 10.1016/j.ejrad.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 44.Eichinger M, Puderbach M, Fink C, Gahr J, Ley S, Plathow C, Tuengerthal S, Zuna I, Müller FM, Kauczor HU. Contrast-enhanced 3D MRI of lung perfusion in children with cystic fibrosis--initial results. Eur Radiol. 2006;16:2147–2152. doi: 10.1007/s00330-006-0257-7. [DOI] [PubMed] [Google Scholar]
- 45.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 46.Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC.Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults Magn Reson Med(In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 48.Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37:1192–1198. doi: 10.3174/ajnr.A4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koumellis P, van Beek EJ, Woodhouse N, Fichele S, Swift AJ, Paley MN, Hill C, Taylor CJ, Wild JM. Quantitative analysis of regional airways obstruction using dynamic hyperpolarized 3He MRI-preliminary results in children with cystic fibrosis. J Magn Reson Imaging. 2005;22:420–426. doi: 10.1002/jmri.20402. [DOI] [PubMed] [Google Scholar]
- 50.van Beek EJ, Hill C, Woodhouse N, Fichele S, Fleming S, Howe B, Bott S, Wild JM, Taylor CJ. Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-Helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol. 2007;17:1018–1024. doi: 10.1007/s00330-006-0392-1. [DOI] [PubMed] [Google Scholar]
- 51.Mott LS, Graniel KG, Park J, de Klerk NH, Sly PD, Murray CP, Tiddens HA, Stephen MS AREST CF. Assessment of early bronchiectasis in young children with cystic fibrosis is dependent on lung volume. Chest. 2013;144:1193–1198. doi: 10.1378/chest.12-2589. [DOI] [PubMed] [Google Scholar]
- 52.Failo R, Wielopolski PA, Tiddens HA, Hop WC, Mucelli RP, Lequin MH. Lung morphology assessment using MRI: a robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients. Magn Reson Med. 2009;61:299–306. doi: 10.1002/mrm.21841. [DOI] [PubMed] [Google Scholar]
- 53.Ciet P, Tiddens HA, Wielopolski PA, Wild JM, Lee EY, Morana G, Lequin MH. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol. 2015;45:1901–1915. doi: 10.1007/s00247-015-3420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qing K, Ruppert K, Jiang Y, Mata JF, Miller GW, Shim YM, Wang C, Ruset IC, Hersman FW, Altes TA, et al. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging. 2014;39:346–359. doi: 10.1002/jmri.24181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, Szczesniak RD, Castro M, Woods JC. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274:250–259. doi: 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quirk JD, Lutey BA, Gierada DS, Woods JC, Senior RM, Lefrak SS, Sukstanskii AL, Conradi MS, Yablonskiy DA. In vivo detection of acinar microstructural changes in early emphysema with (3)He lung morphometry. Radiology. 2011;260:866–874. doi: 10.1148/radiol.11102226. [DOI] [PMC free article] [PubMed] [Google Scholar]