Abstract
Rationale: Radiographic lung cancer screening guidelines and coverage requirements warrant a shared decision-making process. Guidance is needed regarding how to conduct shared decision making effectively. A useful organizing theme should include consideration of a patient’s response to and tolerance of uncertainty associated with lung cancer screening.
Objectives: The objectives of this study are to: (1) describe how patients respond to specific categories of uncertainty in the context of lung cancer screening, and (2) inform strategies for addressing concerns about uncertainty as part of the shared decision making.
Methods: We performed two series of structured interviews on participants in a convenience sample of current or former cigarette smokers recruited from primary care and pulmonary practices in Philadelphia. An interview guide included prompts related to benefits, harms, and responses to general and specific types of uncertainty (stochastic, statistical, and evidentiary) associated with lung cancer screening. Interviews were audio-recorded, transcribed, and independently coded by two investigators. An inductive analysis was conducted, and major themes were identified.
Measurements and Main Results: Twenty-two adults participated in the study. Sixty-eight percent were men, 72% were black or African American, and 50% met U.S. Preventive Services Task Force criteria for lung cancer screening. The primary themes to emerge from our study were: (1) the desire to decrease uncertainty may motivate lung cancer screening decisions; (2) uncertainty is an attribute of health states that impacts how patients weigh benefits and harms of lung cancer screening; (3) patient understanding and tolerance of uncertainty varies across stochastic, statistical, and evidentiary uncertainty; and (4) provider–patient communication may mitigate intolerance of uncertainty in the context of lung cancer screening.
Conclusions: A systematic approach to understanding and addressing patients’ concerns about uncertainty in the context of lung cancer screening can guide a patient-centered approach to shared decision making. The results of this study can inform provider–patient communication strategies regarding the decision to perform radiographic lung cancer screening.
Keywords: lung cancer screening, communication, uncertainty, ambiguity, decision making
Lung cancer is the leading cause of cancer death among adults in the United States, with 221,200 new cases and 158,040 deaths forecasted for 2015 (1). In 2011, the results of the U.S. National Lung Screening Trial (NLST) of low-dose computed tomography scan compared with chest radiography demonstrated a 20% reduction in lung cancer–specific mortality after 6.5 years (2). The current paradigm of lung cancer prevention includes both lung cancer screening and smoking cessation.
On the basis of observations from the NLST and synthesis of evidence from smaller trials (3, 4), the U.S. Preventive Services Task Force, American Cancer Society, American College of Chest Physicians, American Thoracic Society, American College of Radiology, and other organizations issued guidelines recommending annual lung cancer screening using low-dose computed tomography imaging in older persons with a significant exposure to smoking (5–9). Medicare coverage for lung cancer screening was recently approved for beneficiaries between the ages of 55 and 78 years who: (1) are either current smokers or former smokers, (2) quit smoking within the last 15 years, and (3) have accumulated at least 30 pack-years of smoking. Medicare coverage requires, and other guidelines recommend, that a shared decision-making process occur before the initial lung cancer screening. However, little guidance is available for providers regarding how to conduct discussions that promote shared decision making in the context of lung cancer screening.
The elements of shared decision making set forth by the U.S. Centers for Medicare and Medicaid Services are consistent with generally accepted frameworks for shared decision making and include consideration of individual values and preferences. Communication regarding potential benefits and harms is complex, as it involves consideration of probabilistic outcomes. Potential benefits include a reduction in lung cancer and all-cause mortality. Potential harms have been categorized into four domains: physical, psychological, financial strains, and opportunity costs (10). Outcomes typically framed as harms include false-positive and incidental findings, health risks associated with radiation, overdiagnosis, and direct and indirect costs of screening (2, 3, 10, 11).
Patients considering lung cancer screening are faced with a number of uncertainties, including risk of cancer, implications of false-positive tests, overdiagnosis, and effects of possible treatment options (11–15). Efficacy estimates from scientific studies, precision of estimates, and evidence-based guidelines can provide data to inform patients considering lung cancer screening. A body of literature identifies effective graphic and numeric formats to communicate probabilistic information to patients in a way that supports informed decision making (16–19).
Individual characteristics that impact patient decision making about cancer screening and other medical interventions include one’s tolerance of uncertainty or ambiguity (20–22). These constructs are closely related but have been distinguished in one analysis by a focus on current (ambiguity) and future health states (uncertainty) (23). Previously developed taxonomies of uncertainty or ambiguity in the context of medical decision making include the domains of probabilistic outcomes, imprecision, conflicting expert opinions, complexity of medical outcomes, and individual response to uncertainty (16, 24).
In this study, we consider three types of uncertainty believed relevant to decisions regarding lung cancer screening: stochastic (or random), statistical (precision around estimates), and evidentiary (conflicting opinions and guidelines). The objectives of this study are to: (1) describe how patients respond to specific categories of uncertainty in the context of lung cancer screening decisions, and (2) inform strategies for addressing concerns about uncertainty as part of the shared decision-making process for lung cancer screening.
Methods
We performed semistructured interviews of participants in a purposive sample of adult current and former smokers. Participants were recruited from two affiliate hospitals and clinics associated with the University of Pennsylvania in Philadelphia. The initial round of interviews (n = 12) was conducted in a university-affiliated teaching hospital. To expand and broaden the study cohort and further explore understanding of uncertainty and overdiagnosis, we performed a second set of interviews on selected patients at the affiliated Veterans Affairs Medical Center (n = 10).
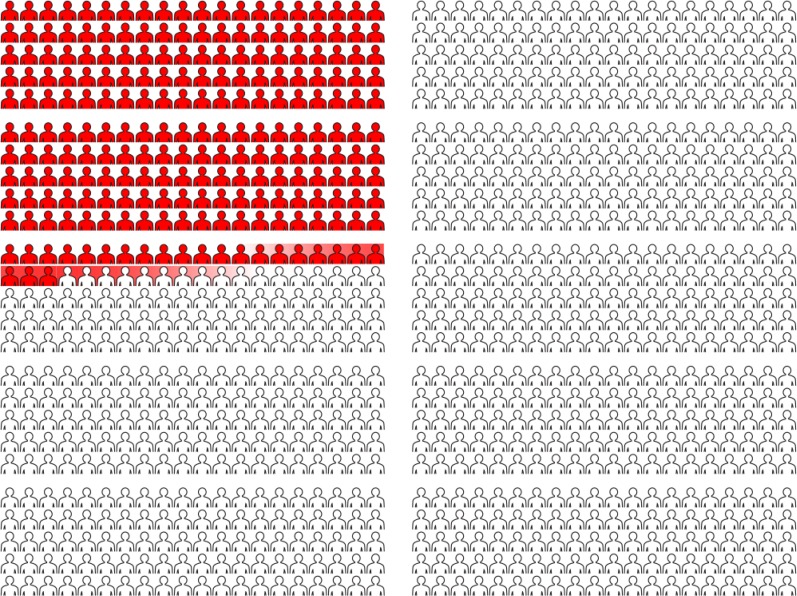
An interview guide was used for the interviews (online supplement). The research assistant (college degree but no medical training) reviewed educational materials about lung cancer screening with participants before conducting the interview (online supplement). Graphics were used to convey relevant outcomes reflecting data from the NLST (online supplement). The educational sessions took approximately 20 minutes, and the interview took up to 1 hour to complete. The first set of interviews used one graphic to indicate lung cancer mortality and the expected frequency of false-positive tests. Graphics were then expanded for the second set of interviews to include comparisons of lung cancer mortality, overall mortality, the incidence of false positives, the number of invasive tests, complications of invasive tests, and a 95% CI around an estimate of false-positive findings (Figure 1, and the online supplement).
Figure 1.
Graphic presentation of false-positive findings and statistical uncertainty. This graphic was used to represent an additional 223 false-positive findings when using low-dose computed tomographic imaging compared with chest radiography as a lung cancer screening test. Statistical uncertainty is indicated by shading at the transition from red to white, representing a CI of ±10.
Participants were recruited through study flyers placed in the clinic waiting areas and interviews were conducted in a private area after obtaining a signed informed consent. Participants received $25 to compensate them for their time. The protocol was approved by the Institutional Review Board of the Perelman School of Medicine of the University of Pennsylvania School of Medicine and the Corporal Michael J. Crescenz Veterans Affairs Medical Center of Philadelphia.
Analysis
The interviews were audio recorded, transcribed verbatim, and reviewed by two analysts. A coding scheme was developed, with input from a team of investigators including general internists, pulmonologists, radiation oncologists, and medical oncologists. The remaining transcripts were coded independently, and differences were reconciled by consensus. An inductive analysis approach was undertaken that used detailed reading of the data to derive concepts and themes from iterative review and interpretation of the data (25). Recruitment continued until the point of saturation, when the themes had depth and breadth and no new themes were emerging from the analysis (26).
Results
The study population (n = 22) was diverse in age, sex, and education (Table 1). The majority of participants were African American, and 50% met the U.S. Preventive Services Task Force criteria for lung cancer screening. Four themes emerged from our analysis of the recorded interviews: (1) the desire to decrease uncertainty may motivate lung cancer screening decisions; (2) uncertainty is an attribute of health that impacts how patients weigh benefits and harms of lung cancer screening; (3) patients’ understanding and tolerance of uncertainty varies across stochastic, statistical, and evidentiary uncertainty; and (4) provider–patient communication may mitigate intolerance of uncertainty in the context of lung cancer screening. We summarize our findings and support these themes with illustrative quotations in the text below and in Table 2.
Table 1.
Demographics of the study cohort
| Participant Characteristic | n (%) |
|---|---|
| Age, yr | |
| <52 | 5 (22.7) |
| 52–57 | 6 (27.3) |
| 58–64 | 5 (22.7) |
| ≥65 | 6 (27.3) |
| Sex | |
| Female | 7 (31.8) |
| Male | 15 (68.2) |
| Race | |
| White | 6 (27.3) |
| Black or African American | 16 (72.3) |
| Education | |
| Less than high school degree | 2 (9.1) |
| High school degree/GED | 9 (40.9) |
| Some college | 4 (18.2) |
| 4 yr college | 4 (18.2) |
| Graduate degree | 3 (13.6) |
| Medical care system | |
| Academic affiliate medical center | 10 (45.5) |
| Veterans Affairs medical center | 12 (54.6) |
| Smoking history, pack-years | |
| <30 | 9 (41) |
| 30–44 | 4 (18) |
| ≥45 | 9 (41) |
| Eligible for lung cancer screening by USPSTF Criteria | 11 (50%) |
Definition of abbreviations: GED = general education development; USPSTF = U.S. Preventive Services Task Force
Table 2.
Additional illustrative quotations to support findings
| Screening for lung cancer motivated by low tolerance of uncertainty |
| I would say peace of mind. When you have peace of mind you can really live your life. (Interview #11, male, HS/GED) |
| I tell you, I wouldn’t pay a dollar for this test if I didn’t think it was going to give me a real correct answer. (Interview #11, male, HS/GED) |
| I’m thinking I don’t want no test that isn’t going to tell me right away whether I have something as bad as cancer. (Interview #11, male, HS/GED) |
| Right, exactly and that solves the curiosity of the unknown. Because that unknown will stress you out and stress can kill you, you don’t only have that problem then you have a stress problem. You know, with the unknown. You want answers. And you want them now. And more and more the test shows, the more and more answers you can get. (Interview #5, male, HS/GED) |
| No, I think people would think like me and not want to be screened. Once you start taking tests you get tired of it and you get tired of the results because the results aren’t definite. (Interview # 3, male, some college) |
| Response to random (stochastic) uncertainty* |
| Uh, based on our conversation today, the benefit of screening would be certain to me. (Interview #2, male, 4 yr college) |
| Well it’s all uncertain. I mean. It’s basically a guess. It’s just a guess you know ain’t nothing wrong with guessing. You know you guess you gonna wake up tomorrow morning. (Interview #6, male, HS/GED) |
| Discomfort with statistical uncertainty |
| The imprecision. I am surprised that someone with a Ph.D. came up with these. I mean I can see right away that this doesn’t add up. So what the hell are we doing this for? I mean we are killing more people with our knife. (Interview #17, male, graduate school) |
| Comfort with statistical uncertainty |
| No, no, because you know what? Nothing is 100%. Doctors are still practicing medicine, even they might always have every operation a success, they’re still practicing. There’s the opportunity that the one surgery could go wrong. They’re practicing. Practicing law, practicing medicine, you know? You might ask another question that you don’t ask me, the next time you do a study. We’re always learning. Nothing is 100%. (Interview #1, male, HS/GED) |
| Uh, I, I, I mean, all things considered, uh, given the track record of, you know, research and study, uh, you know, I would have to continue to, uh given credence to and, uh, allow for, you know, the studies being accurate and, uh, a good tool. So you know, with the advance of, uh, technology and, uh, knowledge and all that, you know, science development stuff, I, I can’t help but continue to put my faith in it. You know what I’m saying? I mean, just look at us now, you know, considering the length of time that we’re living in these days and times, as comparison to. You know what I’m saying? (Interview #2, male 4 yr college) |
| Because that is how life is. You don’t know what’s going to happen from day to day. There’s always some wiggle room. (Interview #5, male, HS/GED) |
| No it doesn’t make me trust it less. Whatever you know whatever’s going to happen is going to happen and you’re going to fall in one of those places. (Interview #6, male, HS/GED) |
| Because um, like you said in real life that’s what happens, you know?” That’s just the way things happen, this is reality we’re looking at so you can’t—you can’t—I speak for myself. I can’t let trust a study less just because that’s the way—that’s the way reality is, you know there’s nothing I can do about it. So still the study is very—the study is still very important. And it’s still helping, right and it’s still helping no matter how—how you look at it, it’s still helping more than it’s hurting. (Interview #8, male, HS/GED) |
| No [would not trust study less], because, I mean, there’s, it’s going to be some glitches and, you know, uncertainties in anything that you do and as far as testing is concerned. (Interview #10, female, 4 yr college) |
| Response to evidentiary uncertainty: be proactive |
| I guess you can, you know, maybe, uh, take a little bit from both. If this is affecting you, you’re going to read up on it, research. So you’re going to be, like I said, proactive in your own health issues. So I’m going to read both studies or three studies and put my glasses on, get my highlighter and say okay, they’re pretty much saying the same thing. This guy says the same thing, but he has a, maybe a little bit something more, a little more to it. (Interview #1, male, HS/GED) |
| Response to evidentiary uncertainty: confusion |
| I don’t know. I mean, if they’re talking too much in medical terms instead of—I would want to hear somebody tell me, you know, what it is, you know, the medical terms might be a little confusing. But you know, just give it to me raw. I want to know. (Interview #1, male, HS/GED) |
| It would be confusing, because were getting back to overdiagnosing. (Interview #9, male, some college) |
| Response to evidentiary uncertainty: rely on doctor’s recommendation |
| As important as that may be, uh for me to, uh, be better armed with making a decision, uh, I, I would prefer to be in that camp where the doctors, uh, you know, feel strongly about, uh, you taking this test as opposed to uh, “well, we didn’t bring it to your attention because some people feel it’s important, some people don’t feel it’s important. It’s up to you to make the decision.” Well, I need the information to make the decision. So if I know that there’s these two different camps, you know what I’m saying. (Interview #2, male, 4 yr college) |
| There’s always going to be controversy, it’s always going to be difference of opinions (Interview #10, female, 4 yr college) |
| Importance of provider–patient communication in mitigating response to uncertainty |
| The positive aspect was that it [having a screening colonoscopy] allowed me not to be so afraid of dealing with something of the unknown with my health. And I could just be able to just get it over with. The doctors do what they do. They train for what they train for and—and if I have a medical problem that needs to be dealt with now, I’m like willing to speak about it. I put it in the doctor’s hand. (Interview #5, male, HS/GED) |
| If it’s explained to me correctly and you know, and in a manner, you know, where it’s like how can I say—concern or consideration you know of how, of my feelings and how I’m going to take it because this isn’t nothing to play with. (Interview #8, male, GED/HS) |
| What would really help me right is if it was explained to me that “Okay, now if we give you—if you get a positive don’t get excited right away because it could be a false positive.” If that’s explained to me in the beginning then I’d say “Oh God” no I’d say—hopefully I would be like “Okay no need to get”—as a matter of fact I don’t think I would be—I don’t think I would be really that you know, really go off the deep end that much because even—because all right, I feel this way. Even if it wasn’t a false positive and I was positive now we have to know how early it was caught. So maybe if I am positive that doesn’t mean it’s a death sentence because especially if you guys caught it early, right? (Interview #8, male, HS/GED) |
| I think that would be between me and the doctor. You know which one I’d rather be comfortable with. Yeah, you know because if they’re giving it to me straight. I’m certainly going to listen to them you know. (Interview #6, male, HS/GED) |
| Yes, I would probably follow any recommendations my doctor gives me. That’s why I pay her. (Interview #19, female, 4 yr of college) |
Definition of abbreviations: GED = General Education Development certificate; HS = high school graduate.
Responses to review of pictographs and uncertainty regarding whether a given individual will benefit from lung cancer screening (see the online supplement).
Desire to Decrease Uncertainty as a Factor in Decisions Regarding Lung Cancer Screening
The desire to decrease uncertainty emerged as a factor in decision making. For those with a strong desire to decrease uncertainty, this theme was expressed as seeking “peace of mind,” “wanting to know,” or “fear of the unknown.” The following statement illustrates the idea that lung cancer screening would provide a sense of control in the face of a threatening health condition.
I’ll put in this way: it’s the fear of the unknown. I’m really afraid of what I don’t know, okay? Some people don’t want to know because they’re afraid to find out the answer…I fear what I don’t know because what I know I feel like I can control it or I can get help—allow someone to control it, like a doctor or whatever. Interview #8, male, high school graduate or General Education Development certificate (HS/GED)
In contrast, some declined lung cancer screening, preferring uncertainty to the possibility of facing a cancer diagnosis, as expressed below.
Well, because I don’t want to know if I’ve got lung cancer. That’s it, because I don’t want to know. Interview #5, male, HS/GED
Uncertainty as an Attribute of Expected Health States in Lung Cancer Screening
Participants differed in the weight they placed on uncertainty as an attribute of a health state when balancing benefits and harms. For example, when asked to reflect on the balance of lung cancer mortality risk reduction and false-positive tests, some placed a high value on mortality benefits despite the low absolute number of lung cancer deaths averted (3 per 1,000 screened), as illustrated below.
It would be encouraging; if this could save three people’s lives, then there is a chance that early detection would be worth it. Interview #13, female, 4 years of college
Others expressed concern about the large number of individuals who would experience false-positive tests, noting the uncertainty that would be experienced in that health state.
Yeah, that is ridiculous. Honestly, I am not going to save lives to put 300 almost 400 people through all that trouble. Not only that but you have to think that it is a ripple effect. I mean people’s families as well have to go through this anxiety and pain. Interview #9, male, some college
A Taxonomy of Uncertainty Associated with Lung Cancer Screening
We explored responses to three specific categories of uncertainty relevant to cancer screening decisions: stochastic (random), statistical, and evidentiary. We defined stochastic uncertainty as the probabilistic estimate of possible outcomes, such as the expected decrease in lung cancer mortality of 3 per 1,000 persons who are screened. Statistical uncertainty, or imprecision, was defined as the range of probabilities that includes the true probability 95% of the time. Evidentiary uncertainty was defined as differences in the interpretation of evidence and professional guidelines offered by professional groups.
Response to Stochastic Uncertainty
We used graphics to display stochastic outcomes among of a cohort of 1,000 persons who underwent lung cancer screening and were expected to have diverse outcomes, including true positives, false positives, and lung cancer (see the online supplement). Participants conveyed understanding of the relative magnitude of benefits and harms depicted in the graphics. One participant noted that lung cancer screening would decrease uncertainty by determining what outcome category you will be in, as described below.
It seems certain to me [the benefit of screening] because you certainly know what’s going on after—after you had the screening you know whether you have it or you know what part of it—which one of those categories you fit in. Interview #8, male, HS/GED
However, the concept that not all who undergo lung cancer screening will incur benefit was difficult for some to grasp. When considering the possibility that some individuals’ screen-detected cancers will not lead to improvements in survival, the reason was attributed to a delay in testing, rather than a stochastic, or random, outcome that all had a chance to incur.
Let me ask you a question about what you just said. Now, at what point is it because it was diagnosed too late or it was so bad that there was nothing that they could do, or should I have went earlier? Interview #8, male, HS/GED
Response to the Uncertainty of Overdiagnosis
Overdiagnosis was framed as a potential harm of lung cancer screening with the following definition: “It is possible that the test will detect cancers that never would have become active or caused symptoms in a patient’s lifetime.” The stochastic uncertainty presented about overdiagnosis was as follows: “overdiagnosis may occur in as many as one out of every four lung cancers that are detected by this screening.” The interviewer encouraged a dialogue, asking participants to convey their understanding of overdiagnosis in their own words. Some conveyed they would respond to this uncertainty by seeking further testing and second opinions as indicated below.
Well, it wouldn’t [impact my decision]. And if possible, there’s only one thing that that tells me personally, is that I need a second opinion. I would go someplace else and even if I had to pay for it, as long as it wasn’t too expensive or if my insurance covered it, I would get a second opinion. Interview #1, male, HS/GED
The perception that overdiagnosis is a conduit for additional surveillance and testing led one participant to reject the label of harm, considering overdiagnosis a benefit.
I’m like, overdiagnosis to me would be beneficial. Interview #10, female, 4 years of college
An alternative strategy for reducing the uncomfortable state of uncertainty associated with overdiagnosis was to avoid knowledge of a diagnosis that required no treatment.
Well, in that case, you know, I mean, if the doctor is on point and he feels confident, you know, that this is nothing we should be concerned about, don’t tell me. Okay? Interview #2, male, 4 years of college
Response to Statistical Uncertainty
We explored responses to statistical uncertainty through graphics depicting a confidence interval around a point estimate of false-positive tests, illustrated as shadowing of figures (indicating a confidence interval of ±10 persons) to either side of the estimate (Figure 1). Participants understood that a confidence interval represented uncertainty around the estimate but found the degree of uncertainty to be relatively small and not salient to their decision, as illustrated below.
Hmm. Uh, I mean, wiggle room almost in any situation is good, because you don’t feel like you’re, and you’re boxed in. You know, I’ve got a little wiggle room and wiggle room would be in my mind, you know, okay. Interview # 1, male, HS/GED
Nothing is certain. With so many people you are going to have plus or minus, up or down on both sides so it really wouldn’t make a difference to me…Yeah. It’s a small percentage up or down and I think it wouldn’t make a difference. Interview # 3, male, some college
Response to Evidentiary Uncertainty
Participants were asked how they would respond to conflicts in guidelines regarding lung cancer screening. Some expressed the expectation that there would be disagreement among experts.
It wouldn’t be confusing because I know everybody…people have different opinions, different organizations have different procedures of how they would handle problems. To me it wouldn’t be confusing. I would just listen to all of the objective views or ideas and making an informed decision with me and my physician. Interview #3, male, some college
One strategy in response to the scenario of disagreement among experts was to take it upon themselves to evaluate the evidence.
If this is affecting you, you’re going to read up on it, research. So you’re going to be, like I said, proactive in your own health issues. So I’m going to read both studies or three studies and put my glasses on, get my highlighter and say okay, they’re pretty much saying the same thing. Interview #1, male, HS/GED
In contrast, others desired definitive strong statements and guidance from providers.
How they get there, that’s what they do. But just come tell me what’s what and then let me deal with it my way. Interview #5. male, HS/GED
Provider–Patient Communication and Patient Tolerance of Uncertainty
A final theme to emerge was that effective provider–patient communication can mitigate intolerance of uncertainty in the context of lung cancer screening. Participants recognized that reassurance and the ability to place information in context could mitigate concern about uncertainty, as indicated in the statements below and in Table 2.
I think that the way that the doctor would present it to me is that, hey, we found these and they are abnormal but we are going to watch them for a year then I would trust the doctor because I know the people here. Interview #13, female, 4 years of college
So, I would feel like if it was me and I was diagnosed with one of those slow-growing tumors I’d just listen to what the doctor said and I would go along with what he thinks. But if he explained it to me that it’s not—it’s not really life threatening right now, I’d say “oh okay whatever you think doc.” Interview #8, male, HS/GED
However, some participants stated that they did not want the clinician to discuss uncertainty, as illustrated by the following statement.
I don’t even know if I would want to hear that. It’s not up to you to be uncertain. We want to be certain, so we want to have these tests done. You know, just look at it as we’re going to do it and see what we see, whatever we see. And then you’re the doctor, you’re the professional. You tell me how to proceed or how I should proceed. And then if I’m not satisfied with what he tells me, than I’m going for that second opinion. Interview #1, male, HS/GED
Discussion
In this study we explore variation in how patients view and weigh the benefit and harms of lung cancer screening and the role of uncertainty in the context of lung cancer screening decision making. Our findings suggest that tolerance for uncertainty impacts how people balance the potential benefits and harms associated with lung cancer screening. Furthermore, we found that a taxonomy of uncertainty, including stochastic, statistical, and evidentiary uncertainty, can help to clarify the aspects of uncertainty most concerning to patients in the context of this decision.
We explored the general construct of uncertainty and its impact on decision making for lung cancer screening. Our findings suggest a complex relationship between tolerance for uncertainty and cancer screening. Some sought to decrease uncertainty through lung cancer screening and, if needed, reduce the ambiguity of false positive findings or overdiagnosis with additional testing. Others stated they would decline lung cancer screening to avoid the uncertainty associated with indefinite screening results. Previous studies indicate that greater uncertainty about the benefits of cancer screening may decrease perceived benefits, increase perceived harms, and reduce certainty about desire to screen (27). Our study supports the finding that individual tolerance of uncertainty about future events will impact choices regarding lung cancer screening.
We propose a taxonomy of uncertainty that is salient to lung cancer screening decisions. The taxonomy includes stochastic (random) uncertainty, statistical (imprecision) uncertainty, and evidentiary uncertainty. Our study used pictographs to convey probabilistic outcomes of lung cancer screening (stochastic uncertainty). Stochastic uncertainty has also been referred to as “aleatory uncertainty,” arising from the unpredictability of future events (28). Participants conveyed that graphics were effective in showing the relative magnitude of benefits and harms, consistent with previous literature in risk communication (23, 24). However, some persisted in the belief that all would benefit from screening with respect to health outcomes. The reasons for this finding require further study but could include misunderstanding of the natural history of lung cancer or selective attention to stochastic outcomes conveyed.
We used elaboration of graphics to indicate statistical uncertainty around probabilistic estimates. In a previous study that sought to communicate colorectal cancer risk, imprecision was conveyed with both text and visual formats. Although presenting a numeric range around a point estimate increased cancer worry, the effect was mitigated when a visual representation was used to indicate uncertainty (29). In a study of the communication of patient-reported outcomes, six graphic formats were used to present data, including one format of line graphs with CIs; although clinicians valued the CIs, patients found them to be confusing (19). Our study presented statistical uncertainty through shading of pictograph symbols. Participants stated that this degree of uncertainty was expected and did not pose a barrier to decision making. These findings may differ with estimates that have greater degrees of imprecision. We found that people expected to hear controversy regarding guidelines. A range of approaches to evidentiary uncertainty were expressed, from taking it upon themselves to evaluate the evidence to seeking guidance from providers.
Our study suggests that misconceptions regarding the natural history of cancer confound the relationship between tolerance of uncertainty and lung cancer screening decisions. Observations from the NLST database indicate that 18% of screen-detected lung cancers in the intervention arm are potentially attributable to overdiagnosis (30). We explored conceptual understanding of overdiagnosis after the provision of a definition and opportunity for clarification about the construct. Some of the participants conveyed an understanding that a proportion of lung cancers diagnosed will never cause harm in one’s lifetime. However, confusion persisted regarding whether this uncertainty could be resolved with further testing or second opinions.
Previous qualitative and quantitative studies found overdiagnosis to be poorly understood among the Australian public (31, 32). Misconceptions about overdiagnosis persist in our population as well. In particular, patients do not understand the inability to identify, at diagnosis, whether a specific cancer is clinically significant. Some participants in our study perceived overdiagnosis as a benefit, rather than a harm, a concept that was also reported in a previously published study of patients participating in the US Veterans Health Administration Lung Cancer Screening Clinical Demonstration Project (33).
Limitations
This was a qualitative study, and the findings are exploratory in nature. Participants were recruited from one city and academic medical center. Compared with the participants in the NLST, our study subjects were disproportionately African American. Prior lung cancer screening was not systematically assessed. However, we did include two hospital systems, one representing the traditional fee-for-service system and the other the publically financed Veterans Affairs system. Future studies with more diverse population and prospective designs are needed to validate these findings and assess their impact on lung cancer screening decisions.
Conclusions
Our findings indicate that a discussion of a patient’s tolerance for uncertainty can enhance a shared process of decision making regarding lung cancer screening. Discussions with patients should identify and address the types of uncertainty most concerning to them and incorporate that assessment in the decision-making process. In some cases, this may mitigate concerns about uncertainty. A frank discussion by clinicians regarding the limits of medical testing to resolve all uncertainty will help patients to make decisions that align with their individual values, including their tolerance for uncertainty. Education about the natural history of lung cancer will help patients to understand the benefits and limitations of cancer screening.
Living with uncertainty is a challenge for many people, but a systematic approach to understanding and addressing individuals’ concerns in the context of lung cancer screening can guide a patient-centered approach to shared decision making.
Footnotes
Supported by National Institutes of Health grant P30 CA016520 and the Department of Veterans Affairs Health Services Research and Development Service Program grant HX001334-01A1.
Author Contributions: M.M.S. contributed to conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, supervision, original draft, and review and editing. C.A., S.A., J.A., A.V., C.L., C.B.S., E.S., and L.F. contributed to conceptualization and review and editing. D.I. contributed to data curation and review and editing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 6.Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest Chest. 2015;147:295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg. 2012;144:25–32. doi: 10.1016/j.jtcvs.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 10.Harris RP Sheridan SL, Lewis CL, Barclay C, Vu MB, Kistler CE, Golin CE, DeFrank JT, Brewer NT. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174:281–285. doi: 10.1001/jamainternmed.2013.12745. [DOI] [PubMed] [Google Scholar]
- 11.Patz EF Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemagi MC, Chiles C, Black WC, Aberle DR; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355–365. doi: 10.1111/hex.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan DR, Golden SE, Ganzini L, Hansen L, Slatore CG. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028. doi: 10.1038/npjpcrm.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharf BF, Stelljes LA, Gordon HS. ‘A little bitty spot and I’m a big man’: patients’ perspectives on refusing diagnosis or treatment for lung cancer. Psychooncology. 2005;14:636–646. doi: 10.1002/pon.885. [DOI] [PubMed] [Google Scholar]
- 15.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making. 2008;28:917–925. doi: 10.1177/0272989X08322013. [DOI] [PubMed] [Google Scholar]
- 16.Politi MC, Han PK, Col NF. Communicating the uncertainty of harms and benefits of medical interventions. Med Decis Making. 2007;27:681–695. doi: 10.1177/0272989X07307270. [DOI] [PubMed] [Google Scholar]
- 17.Zipkin DA, Umscheid CA, Keating NL, Allen E, Aung K, Beyth R, Kaatz S, Mann DM, Sussman JB, Korenstein D, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270–280. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 18.Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Med Decis Making. 2001;21:459–467. doi: 10.1177/0272989X0102100604. [DOI] [PubMed] [Google Scholar]
- 19.Brundage MD, Smith KC, Little EA, Bantug ET, Snyder CF PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24:2457–2472. doi: 10.1007/s11136-015-0974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taber JM, Klein WM, Ferrer RA, Han PK, Lewis KL, Biesecker LG, Biesecker BB. Perceived ambiguity as a barrier to intentions to learn genome sequencing results. J Behav Med. 2015;38:715–726. doi: 10.1007/s10865-015-9642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein WM, Ferrer RA, Graff KA, Kaufman AR, Han PK. Perceived ambiguity, fatalism, and believing cancer is more prevalent than heart disease. Am J Prev Med. 2014;46:e45–e47. doi: 10.1016/j.amepre.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han PK, Reeve BB, Moser RP, Klein WM. Aversion to ambiguity regarding medical tests and treatments: measurement, prevalence, and relationship to sociodemographic factors. J Health Commun. 2009;14:556–572. doi: 10.1080/10810730903089630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grenier S, Barrette AM, Ladouceur R. Intolerance of uncertainty and intolerance of ambiguity: similarities and differences. Pers Individ Dif. 2005;39:593–600. [Google Scholar]
- 24.Han PKJ, Klein WMP, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31:828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27:237–246. [Google Scholar]
- 26.Corbin JSA. Basics of qualitative research. 3rd ed. Thousand Oaks, California: Sage Publications; 2008. [Google Scholar]
- 27.Han PK, Williams AE, Haskins A, Gutheil C, Lucas FL, Klein WM, Mazor KM. Individual differences in aversion to ambiguity regarding medical tests and treatments: association with cancer screening cognitions. Cancer Epidemiol Biomarkers Prev. 2014;23:2916–2923. doi: 10.1158/1055-9965.EPI-14-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pate-Cornell E. On “black swans” and “perfect storms”: risk analysis and management when statistics are not enough. Risk Anal. 2012;32:1823–1833. doi: 10.1111/j.1539-6924.2011.01787.x. [DOI] [PubMed] [Google Scholar]
- 29.Han PK, Klein WM, Lehman T, Killam B, Massett H, Freedman AN. Communication of uncertainty regarding individualized cancer risk estimates: effects and influential factors. Med Decis Making. 2011;31:354–366. doi: 10.1177/0272989X10371830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, Chiles C, Black WC, Aberle DR NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moynihan R, Nickel B, Hersch J, Doust J, Barratt A, Beller E, McCaffery K. What do you think overdiagnosis means? A qualitative analysis of responses from a national community survey of Australians. BMJ Open. 2015;5:e007436. doi: 10.1136/bmjopen-2014-007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moynihan R, Nickel B, Hersch J, Beller E, Doust J, Compton S, Barratt A, Bero L, McCaffery K. Public Opinions about overdiagnosis: a national community survey. Plos One. 2015;10:e0125165. doi: 10.1371/journal.pone.0125165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeliadt SB, Sekaran NK, Hu EY, Slatore CC, Au DH, Backhus L, Wu DY, Crawford J, Lyman GH, Dale DC. Comparison of demographic characteristics, surgical resection patterns, and survival outcomes for veterans and nonveterans with non-small cell lung cancer in the Pacific Northwest. J Thorac Oncol. 2011;6:1726–1732. doi: 10.1097/JTO.0b013e31822ada77. [DOI] [PubMed] [Google Scholar]