Abstract
Rationale: Unlike tuberculosis, nontuberculous mycobacterial disease is not reportable to public health authorities in the United States, and the total burden of disease is uncertain.
Objectives: To estimate the mortality of nontuberculous mycobacterial disease in the United States over a 15-year period and to identify temporal trends.
Methods: The U.S. Multiple Cause of Death Files from 1999 through 2014 were searched for a listing of nontuberculous mycobacterial disease by International Classification of Diseases, Tenth Revision code as either the underlying or a contributing cause of death. Characteristics of individuals with nontuberculous mycobacteria–related deaths in the United States were summarized according to demographic characteristics. Age-adjusted mortality rates and rate ratios were calculated using bridged-race population estimates of U.S. census population data. Time trends were evaluated with negative binomial regression.
Measurements and Main Results: There was a significant increase in nontuberculous mycobacteria–related deaths among individuals without a diagnosis of HIV infection (P = 0.004). Mortality rates increased with advancing age. Age-adjusted mortality rate ratios were lower for men (risk ratio [RR], 0.84; 95% confidence interval [CI], 0.80–0.87) compared with women, and were lower for Hispanic individuals (RR, 0.53; 95% CI, 0.49–0.56) and black, non-Hispanic persons (RR, 0.83; 95% CI, 0.77–0.88) compared with white, non-Hispanic individuals.
Conclusions: The mortality rate of nontuberculous mycobacterial disease among HIV-uninfected individuals has increased in the United States between 1999 and 2014. These deaths occurred disproportionately in older white women. Considering the concurrent decline in tuberculosis-related deaths, these findings demonstrate a shift in the epidemiology of fatal mycobacterial infections in the United States.
Keywords: nontuberculous mycobacteria, epidemiology, mortality
Nontuberculous mycobacteria are a diverse group of organisms related to Mycobacterium tuberculosis and they cause a wide range of human disease (1). Infection is likely acquired from environmental exposures, particularly from water sources, although the mechanism of transmission to humans is poorly defined (2). Pulmonary disease is the most common clinical presentation; nontuberculous mycobacteria pulmonary infection must be distinguished from colonization of the respiratory tract in patients with chronic lung disease (3). Mycobacterium avium complex opportunistic infections were once a significant cause of morbidity and mortality among HIV-infected patients; however, since the advent of highly effective antiretroviral therapy, the prevalence of Mycobacterium avium complex disease among HIV-infected patients has declined (4).
Unlike tuberculosis, nontuberculous mycobacterial disease is not reportable to public health authorities in the United States, so the total burden of disease is uncertain. Analysis of U.S. Medicare Part B beneficiaries demonstrated a rising prevalence of nontuberculous mycobacterial disease between 1997 and 2007, greater among women compared with men (5), with a substantial financial burden for treatment (6). Similarly, analysis of data from integrated health care delivery systems revealed an increasing prevalence of nontuberculous mycobacterial disease from 1994–1996 to 2004–2006 (7). Interestingly, prior analysis of the publicly available multiple cause of death data from the National Center for Health Statistics, which aggregates U.S. death certificate data, did not identify significant temporal trends in the incidence rate of fatal nontuberculous mycobacterial disease between 1999 and 2010 (8). This analysis excluded deaths in which nontuberculous mycobacterial disease was a contributing cause of death and did not distinguish between nontuberculous mycobacteria–related deaths occurring in the presence and those occurring in the absence of HIV coinfection.
Our objective was to estimate the mortality burden of nontuberculous mycobacterial disease in the United States over a 15-year period and to identify temporal trends. We hypothesized that a rising incidence of nontuberculous mycobacteria–related deaths during this period would be limited to individuals without a comorbid diagnosis of HIV infection. In secondary analyses, we sought to characterize the demographic factors and comorbid conditions associated with nontuberculous mycobacterial deaths during this period.
Methods
Setting
Data were obtained from the National Center for Health Statistics, which receives information, including demographic information and cause of death, from death certificates from all 50 states. Data from 1999 through 2014 were included; deaths that occurred before 1999 were not included because of the change in cause of death codes from the International Classification of Diseases, Ninth Revision (ICD-9) to the Tenth Revision (ICD-10) that occurred in 1999 (9). The multiple cause of death data file includes both underlying and contributing causes that ultimately led to the individual’s death.
Case Definition
The U.S. multiple cause of death files from 1999 through 2014 were searched for a listing of nontuberculous mycobacterial disease (ICD-10 codes A31.0, A31.1, A31.8, A31.9) as either the underlying or a contributing cause of death. In the instructions for completion of the death certificate, the underlying cause of death is defined as “the disease or injury which initiated the train of morbid events leading directly or indirectly to death or the circumstances of the accident or violence which produced the fatal injury” (10). Thus, nontuberculous mycobacterial disease associated with a comorbid condition, such as chronic lung disease, may be recorded as a contributing cause of death, rather than as the underlying cause of death. For comparison, we also examined tuberculosis-related deaths during the same time period, as either the underlying or a contributing cause of death (ICD-10 codes A15–A19).
Nontuberculous Mycobacteria–related Mortality Rates Adjusted for Age, Race/Ethnicity, and Sex
The characteristics of individuals with nontuberculous mycobacteria–related deaths in the United States were summarized according to demographics. Age at death was defined on the basis of the following age groups: <1, 1–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years. Age-adjusted mortality rates and rate ratios were calculated with 95% confidence intervals (CIs) using bridged-race population estimates of U.S. census population data, which allowed for comparison of different race categories across various collection systems (11).
Time Trend Analysis
Count data were modeled using maximal likelihood analysis in a negative binomial regression model (12). To test for a temporal trend in the incidence rate of nontuberculous mycobacterial–related deaths in the population, year was included as a dummy variable in the negative binomial regression model, with the population as the offset, grouped by age category, race/ethnicity, and sex.
Matched Case-Control Analysis for Associated Diagnoses
To identify comorbid conditions associated with nontuberculous mycobacteria–related deaths, a previously validated approach developed by Redelings and colleagues (13) was used. For each record of nontuberculous mycobacteria–related death during the study period, a random sample of deaths during the same year from the multiple cause of death dataset was selected and was matched at a 10:1 ratio (control subjects:case) by age category, sex, and race/ethnicity. Groups of comorbidities were defined on the basis of the leading ICD-10 code diagnoses among all nontuberculous mycobacteria–related deaths. We selected diagnoses for evaluation on the basis of previous work with administrative data (5) and on conditions known to be related to nontuberculous mycobacterial disease (14). For each comorbidity, we calculated a matched odds ratio and the associated 95% CI. All analyses were performed in Stata v13.0 (StataCorp, College Station, TX).
Results
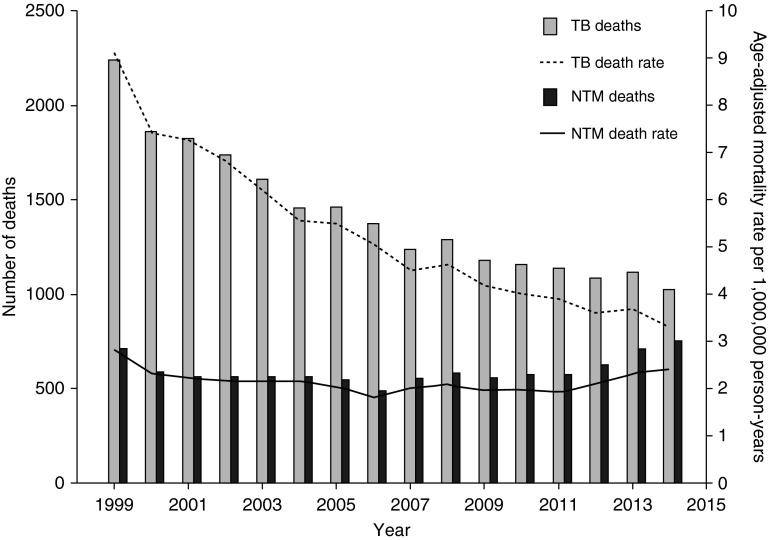
Between 1999 and 2014, a total of 9,490 deaths in the United States were attributed to nontuberculous mycobacteria infection, corresponding to an overall mortality rate of 2.3 deaths per 1,000,000 person-years, without a significant time trend (Figure 1). During this time period, nontuberculous mycobacterial disease was reported as the underlying cause of death in 6,483 individuals (69%) and as a contributing cause of death in 2,876 individuals (31%). By comparison, tuberculosis-related deaths decreased from 2,241 in 1999 (corresponding to a mortality rate of 9.1 deaths per 1,000,000 person-years) to 1,021 in 2014 (corresponding to a mortality rate of 3.3 deaths per 1,000,000 person-years).
Figure 1.
Comparison of the annual frequency and rate of deaths related to NTM and TB in the United States, 1999–2014. TB = tuberculosis; NTM = nontuberculous mycobacterial disease.
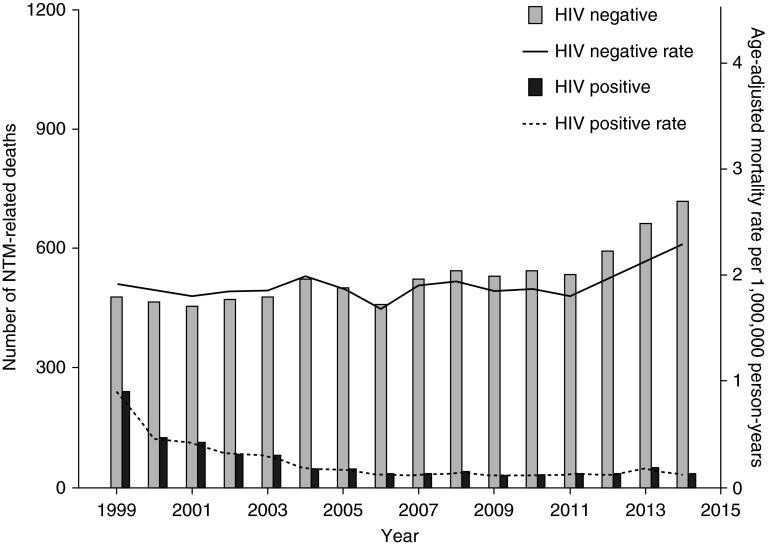
We identified HIV-associated nontuberculous mycobacteria–related deaths by the presence of an ICD-10 diagnosis code for HIV infection (B20-B24). Overall, HIV coinfection was present in 1,014 nontuberculous mycobacterial deaths during the study period (11%). The proportion of HIV-associated nontuberculous mycobacteria–related deaths decreased from 33% in 1999 to 4% in 2014 (Figure 2). In contrast, there was a significant increase in nontuberculous mycobacteria–related deaths among individuals without a diagnosis of HIV infection during this time period (P = 0.004).
Figure 2.
Deaths related to NTM in the United States, 1999–2014, according to the presence or absence of HIV infection on the death certificate. NTM = nontuberculous mycobacterial disease.
Age-adjusted mortality rates among 9, 490 nontuberculous mycobacteria–related deaths are shown in Table 1. Among individuals without a diagnosis of HIV infection, age-specific mortality rate ratios increased with increasing age category. Age-adjusted mortality rate ratios were lower in men (risk ratio [RR], 0.84; 95% CI, 0.80–0.87) compared with women and were lower in Hispanic individuals (RR, 0.53; 95% CI, 0.49–0.56), Asian individuals (RR, 0.78; 95% CI, 0.71–0.85), and black individuals (RR, 0.83; 95% CI, 0.77–0.88) compared with white, non-Hispanic individuals. In contrast, the peak age-specific mortality rate ratio among HIV-infected individuals was observed in the 35- to 44-year-old age group.
Table 1.
Nontuberculous mycobacteria–related mortality rates per 1,000,000 person-years
| Characteristic | Deaths Among Individuals without a Diagnosis of HIV Infection (n = 8,476) |
Deaths Among Individuals with a Diagnosis of HIV Infection (n = 1,014) |
||||
|---|---|---|---|---|---|---|
| No. Deaths | Age-adjusted Mortality Rate per 1,000,000 Person-Years (95% CI) | Age-adjusted Mortality Rate Ratios (95% CI) | No. Deaths | Age-adjusted Mortality Rate per 1,000,000 Person-Years (95% CI) | Age-adjusted Mortality Rate Ratios (95% CI) | |
| Age categories,* yr | ||||||
| <1 | 6 | 0.12 (0.02–0.22) | 0.35 (0.07–0.63) | 0 | † | † |
| 1–4 | 12 | 0.06 (0.031–0.10) | 0.17 (0.07–0.27) | 1 | 0.01 (0–0.02) | 0.01 (0–0.02) |
| 5–14 | 23 | 0.04 (0.03–0.06) | 0.12 (0.07–0.18) | 8 | 0.02 (0–0.03) | 0.02 (0.01–0.04) |
| 15–24 | 50 | 0.09 (0.076–0.12) | 0.26 (0.22–0.29) | 27 | 0.05 (0.03–0.07) | 0.07 (0.04–0.09) |
| 25–34 | 91 | 0.17 (0.14–0.21) | 0.49 (0.37–0.61) | 251 | 0.48 (0.42–0.54) | 0.65 (0.55–0.75) |
| 35–44 | 206 | 0.36 (0.31–0.40) | Reference | 425 | 0.73 (0.66–0.80) | Reference |
| 45–54 | 563 | 0.93 (0.86–1.01) | 2.63 (2.21–3.05) | 240 | 0.40 (0.35–0.45) | 0.54 (0.46–0.63) |
| 55–64 | 1,225 | 2.59 (2.45–2.74) | 7.29 (6.22–8.37) | 49 | 0.10 (0.07–0.13) | 0.14 (0.10–0.18) |
| 65–74 | 1,926 | 6.28 (6.00–6.56) | 17.65 (15.122–20.19) | 13 | 0.04 (0.02–0.07) | 0.06 (0.03–0.09) |
| 75–84 | 2,697 | 13.77 (13.25–14.29) | 38.70 (33.22–44.19) | 0 | † | † |
| >84 | 1,677 | 21.82 (20.78–22.87) | 61.34 (52.46–70.22) | 0 | † | † |
| Sex | ||||||
| Female | 5,237 | 1.90 (1.85–1.96) | Reference | 690 | 0.28 (0.26–0.30) | Reference |
| Male | 3,239 | 1.59 (1.55–1.64) | 0.84 (0.80–0.87) | 324 | 0.14 (0.12–0.15) | 0.48 (0.42–0.54) |
| Race/ethnicity | ||||||
| White, non-Hispanic | 7,058 | 2.65 (2.57–2.72) | Reference | 308 | 0.14 (0.12–0.16) | Reference |
| Black, non-Hispanic | 731 | 2.19 (2.06–2.31) | 0.83 (0.77–0.88) | 534 | 1.27 (1.17–1.38) | 9.06 (7.80–10.32) |
| Hispanic | 360 | 1.39 (1.32–1.46) | 0.53 (0.49–0.56) | 143 | 0.28 (0.23–0.32) | 1.98 (1.59–2.37) |
| Asian, non-Hispanic | 235 | 2.06 (1.89–2.24) | 0.78 (0.71–0.85) | 10 | 0.05 (0.02–0.09) | 0.39 (0.12–0.65) |
| American Indian or Alaskan Native, non-Hispanic | 47 | 2.63 (2.14–3.11) | 0.99 (0.81–1.18) | 14 | 0.41 (0.24–0.77) | 3.60 (1.67–5.52) |
Definition of abbreviation: CI = confidence interval.
Age-specific mortality rates are reported.
No deaths in this category.
We performed a matched case-control study to identify comorbid diagnoses associated with nontuberculous mycobacteria–related deaths (Table 2). As expected, comorbid diagnoses related to pulmonary disease demonstrated the greatest association with nontuberculous mycobacteria–related deaths, compared with population control subjects. We observed an inverse association between nontuberculous mycobacterial–related deaths and heart, kidney, and liver disease. Bronchiectasis showed the strongest association overall (matched odds ratio, 104.80; 95% CI, 83.38–131.73), followed by HIV infection (matched odds ratio, 21.46; 95% CI, 19.05–24.19).
Table 2.
Medical conditions associated with nontuberculous mycobacteria–related deaths in the United States, 1999–2014
| Condition | ICD-10 Codes | Nontuberculous Mycobacteria–related Deaths (%) (n = 9,490) | Matched Control Subjects* (%) (n = 94,900) | Matched Odds Ratio (95% CI) |
|---|---|---|---|---|
| Pulmonary conditions | ||||
| Lung cancer | C34–C34.9 | 451 (4.75) | 6954 (7.33) | 0.63 (0.57–0.69) |
| Cystic fibrosis | E84.0–E84.9 | 27 (0.3) | 17 (0.02) | 16.46 (8.91–30.39) |
| Emphysema or chronic obstructive pulmonary disease | J43–J44.9 | 3,092 (32.58) | 10,116 (10.66) | 4.05 (3.86–4.25) |
| Bronchiectasis | J47–J47.9 | 768 (8.09) | 86 (0.001) | 104.80 (83.38–131.73) |
| Pneumoconiosis | J60–J65.9 | 53 (0.56) | 103 (0.11) | 5.20 (3.73–7.25) |
| Asthma | J45–J45.9 | 49 (0.5) | 423 (0.4) | 1.16 (0.84–1.56) |
| Alveolar proteinosis | J84–J84.9 | 622 (6.55) | 837 (0.88) | 7.91 (7.10–8.80) |
| Nonpulmonary conditions | ||||
| HIV infection | B20–B24.9 | 1,014 (10.68) | 1,186 (1.25) | 21.46 (19.05–24.19) |
| Diabetes mellitus | E10–E14.9 | 377 (3.97) | 8,936 (9.42) | 0.39 (0.35–0.44) |
| Heart disease | I00–I99.9 | 3,298 (34.75) | 50,670 (53.39) | 0.45 (0.43–0.47) |
| Liver failure | K70–K77.9 | 197 (2.08) | 3,631 (3.83) | 0.52 (0.45–0.61) |
| Kidney disease | N17–N19.9 | 596 (6.28) | 7,937 (8.36) | 0.73 (0.67–0.80) |
Definition of abbreviations: CI = confidence interval; ICD-10 = International Classification of Diseases, Tenth Revision.
Each case of nontuberculous mycobacteria–related death was matched to 10 control subjects by year of death, age category, sex, and race/ethnicity.
Discussion
Our analysis of U.S. death certificate data revealed a shift in the epidemiology of fatal mycobacterial infections between 1999 and 2014. Concurrent with a decline in tuberculosis-related deaths during this period, nontuberculous mycobacteria–related deaths increased among individuals without an associated diagnosis of HIV infection. If these trends continue, nontuberculous mycobacterial disease among HIV-uninfected individuals will soon surpass tuberculosis as the leading cause of fatal mycobacterial disease in the United States.
In an adjusted analysis based on population census data, we observed that the greatest mortality burden of fatal nontuberculous mycobacterial disease occurred among older white women. These findings are in agreement with the findings of previous studies of nontuberculous mycobacterial morbidity using data from Medicare beneficiaries (5) and geographically diverse integrated health care delivery systems (7). Also in agreement with these studies, comorbid pulmonary disease was identified as a risk factor for nontuberculous mycobacteria–related deaths among HIV-uninfected patients.
Nontuberculous mycobacterial transmission is thought to occur via inhalation of aerosolized water or dust particles containing these organisms, swallowing with subsequent aspiration of nontuberculous mycobacteria, and less commonly via direct inoculation (15). Because of impairments in mucociliary clearance and airway secretion drainage, as well as local immune dysfunction, patients with structural lung disease are more susceptible to the development of pulmonary infection after a respiratory exposure (8). Patients with immune dysfunction, both intrinsic and iatrogenic, are also at risk of progressive disease.
A subset of patients with pulmonary nontuberculous mycobacterial disease lack the classic risk factors and exhibit a specific phenotype, known as “Lady Windermere syndrome,” which disproportionately affects postmenopausal, thin, nonsmoking females (16). The pathophysiology underlying the increased burden of nontuberculous mycobacterial disease among this population is uncertain. An increased prevalence of heterozygous mutations in the cystic fibrosis gene has been observed, suggesting a genetic predisposition to infection (17). Whether the higher mortality burden of fatal nontuberculous mycobacterial disease in this population subset reflects an increased susceptibility to the development of nontuberculous mycobacterial infection or a greater risk of treatment failure remains unknown.
Limitations
This study has several important limitations. Errors in the population estimates from the census data will be reflected in the calculated mortality rates. The completeness of death certificate data depends on the information available to the clinician at the time of death and the appropriate recognition of all relevant contributing causes of death (18). In addition, relevant factors such as income level and health insurance status are not included on the death certificate, and there is limited information regarding the severity of comorbid factors. Furthermore, the risk factors associated with nontuberculous mycobacterial disease acquisition may be different from those associated with death attributed to this disease. For example, other investigators have reported an association between Asian race and the development of nontuberculous mycobacterial disease (5), which was not observed in our analysis of mortality data. A major strength of this study is the completeness of death certificate reporting in the United States, supporting the epidemiologic study of a wide range of fatal conditions.
Conclusions
We estimate the U.S. mortality burden of nontuberculous mycobacterial disease to have been 2.3 deaths per 1,000,000 person-years over the past 15 years, with an increasing annual incidence rate among HIV-uninfected individuals, and the greatest mortality burden among older white women. Prospective observational studies are needed to identify the patient factors associated with the development and progression of nontuberculous mycobacterial disease.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases grant K23AI102639 (C.V.).
Author Contributions: All authors contributed to the design of the study; C.V. performed the data analysis; C.V., S.L., and K.H. wrote the manuscript; all authors participated in the revision of the manuscript and approved the final version.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148:1517–1527. doi: 10.1378/chest.15-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falkinham JO., III Current epidemiologic trends of the nontuberculous mycobacteria (NTM) Curr Environ Health Rep. 2016;3:161–167. doi: 10.1007/s40572-016-0086-z. [DOI] [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416.[Published erratum appears in Am J Respir Crit Care Med 175:744–745.] [DOI] [PubMed] [Google Scholar]
- 4.Karakousis PC, Moore RD, Chaisson RE. Mycobacterium avium complex in patients with HIV infection in the era of highly active antiretroviral therapy. Lancet Infect Dis. 2004;4:557–565. doi: 10.1016/S1473-3099(04)01130-2. [DOI] [PubMed] [Google Scholar]
- 5.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc. 2015;12:1458–1464. doi: 10.1513/AnnalsATS.201503-173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirsaeidi M, Machado RF, Garcia JGN, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One. 2014;9:e91879. doi: 10.1371/journal.pone.0091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health OrganizationInternational classification of diseases, 10th revision. Available from: http://www.who.int/classifications/icd/en/
- 10.National Center for Health Statistics. Instructions for classifying the underlying cause of death, 2013. Hyattsville, MD: National Center for Health Statistics; 2013. Available from: www.cdc.gov/nchs/data/dvs/2a_2013.pdf
- 11.Centers for Disease Control and Prevention National Center for Health StatisticsU.S. census populations with bridged race categories. Available from: http://www.cdc.gov/nchs/nvss/bridged_race.htm
- 12.Lloyd-Smith JO. Maximum likelihood estimation of the negative binomial dispersion parameter for highly overdispersed data, with applications to infectious diseases. PLoS One. 2007;2:e180. doi: 10.1371/journal.pone.0000180. 10.1371/journal.pone.0000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. Am J Epidemiol. 2007;166:104–108. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]
- 14.Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med. 2013;34:110–123. doi: 10.1055/s-0033-1333573. [DOI] [PubMed] [Google Scholar]
- 15.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101:1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- 17.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 18.Messite J, Stellman SD. Accuracy of death certificate completion: the need for formalized physician training. JAMA. 1996;275:794–796. [PubMed] [Google Scholar]