Abstract
Rationale: Exacerbations are key events in chronic obstructive pulmonary disease (COPD), affecting lung function decline and quality of life. The effect of exposure to different air pollutants on COPD exacerbations is not clear.
Objectives: To carry out a systematic review, examining associations between air pollutants and hospital admissions for COPD exacerbations.
Methods: MEDLINE, Embase, BIOSIS, Science Citation Index, and the Air Pollution Epidemiology Database were searched for publications published between 1980 and September 2015. Inclusion criteria were focused on studies presenting solely a COPD outcome defined by hospital admissions and a measure of gaseous air pollutants and particle fractions. The association between each pollutant and COPD admissions was investigated in metaanalyses using random effects models. Analyses were stratified by geographical clusters for investigation of the consistency of the evidence worldwide.
Measurements and Main Results: Forty-six studies were included, and results for all the pollutants under investigation showed marginal positive associations; however, the number of included studies was small, the studies had high heterogeneity, and there was evidence of small-study bias. Geographical clustering of the effects of pollution on COPD hospital admissions was evident and reduced heterogeneity significantly.
Conclusions: The most consistent association was between a 1-mg/m3 increase in carbon monoxide level and COPD-related admissions (odds ratio, 1.02; 95% confidence interval, 1.01–1.03). The heterogeneity was moderate, and there was a consistent positive association in both Europe and North America, although levels were clearly below World Health Organization guideline values. There is mixed evidence on the effects of environmental pollution on COPD exacerbations. Limitations of previous studies included the low spatiotemporal resolution of pollutants, inadequate control for confounding factors, and the use of aggregated health data that ignored personal characteristics. The need for more targeted exposure estimates in a large number of geographical locations is evident.
Keywords: patient admission, particulate matter, gases, environmental monitoring
Intense energy consumption, together with industrial and transportation emissions, has led to population exposure to a diverse variety of unhealthy concentrations of air pollution, leading to increased morbidity and mortality primarily due to cardiovascular and respiratory causes (1). Vulnerable groups include patients with chronic obstructive pulmonary disease (COPD), which is currently the fourth leading cause of death worldwide (2). Total deaths due to COPD are predicted to increase by more than 30% in the next 10 years, and economic costs for the management of COPD are estimated at $36 billion annually in the United States (3). Although smoking is the most important cause of COPD, a substantial proportion of cases cannot be explained by this lifestyle factor alone (4).
Exacerbations of COPD are a common cause of adult emergency hospital admissions and are associated with increased mortality and decreased quality of life. Patients may experience at least one exacerbation per year (5), and, as the disease worsens, exacerbations become more frequent and more severe (6). The effect of environmental exposure on COPD exacerbations is not clear. A number of variables may trigger COPD exacerbations (7), and understanding and addressing the effects of air quality may be key in managing COPD exacerbations. From a policy perspective, detecting air pollution–induced health effects early can lead to more effective control of exposures and more appropriate interventions.
In this systematic review, we evaluated the strength and consistency of current literature documenting the effect of different air pollutants on hospital admissions for COPD exacerbations. Previous metaanalytic studies on the effects of air pollution on COPD-related hospital admissions and mortality were focused on the effects of particles (8–11) or the effects of gases such as ozone and nitrogen dioxide (12, 13). This review is unique in that we simultaneously assessed the effects of key atmospheric pollutants, including gases and particulate matter, on hospital admissions of patients with an established diagnosis of COPD in a large number of studies globally.
Methods
Search Strategy
We endeavored to assess the effects of air pollutants on COPD hospital admissions by reviewing the literature from time-series and case-crossover studies. Two conceptual terms were developed for the search strategy relating to COPD: “environmental factors” and “health outcomes.” Search terms were developed using combinations of controlled vocabulary and free-text terms. Only papers with title, keywords, or abstracts including records from the search categories were included. Search terms from these categories were combined using the AND Boolean logic operator. “Environmental factors” refers to air pollution, including gases and particles suspected of affecting human health, such as carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), and particulates with diameters less than 10 μm and less than 2.5 μm (PM10 and PM2.5, respectively). The primary health outcome of interest in this review was COPD exacerbation qualified by hospital admissions.
The MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, Embase, BIOSIS, and Science Citation Index were searched for publications published between 1980 and September 2015 using Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology guidelines (14). We accessed the gray literature to address potential publication bias and searched additional sources, including reports by the World Health Organization (WHO) and the Committee on the Medical Effects of Air Pollution, as well as the Air Pollution Epidemiology Database hosted by St. George’s University. The search strategy is described in the online supplement.
Inclusion and Exclusion Criteria
While using the search strategy described above, we applied inclusion and exclusion criteria (Table 1) to titles, keywords, and abstracts before obtaining full reports on the studies that appeared to meet the criteria.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Reports a specific outcome of COPD exacerbation defined by hospital or emergency department admissions | Studies by the same author that repeat results (The most recent were selected.) |
| Recorded by clinician or in hospital records using the codes for the International Classification of Diseases, ninth and tenth revisions (ICD-9 codes 490–496, excluding code 493 for asthma; and ICD-10 codes J44.1–J44.9) | Studies that included asthma (ICD-9 code 493) were excluded because of clinical and pathological differences between COPD and asthma (19) |
| Reports a measure of air quality from a fixed monitoring station, indoor environment, or personal exposure (indoor to be analyzed separately from outdoor) | Incorrect outcome: included other respiratory diseases combined with COPD in the statistical analysis |
| Reports the findings of a primary research study or secondary analysis | Uncertain diagnosis of COPD |
| Published in English | Did not report or provide calculable odds ratio, relative risk, or percent change and 95% confidence intervals |
| Reported results derived from single-pollutant models | Poor quality: lacked adjustment for potential confounders, missing data, inadequate statistical analysis |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Classification and Quality Assurance
Two authors independently reviewed titles and abstracts for relevance and assessed whether they were related to the scope of this study. Relevant papers were included for full-text review and tested against the inclusion and exclusion criteria. The methodological quality of the studies was assessed on the basis of population size, study duration and design, air pollutant exposure measurement, diagnosis of COPD, potential confounding factors, controls used, statistical methods, and length of follow-up. A descriptive summary of the studies is provided in Table E1 in the online supplement.
Risk-of-Bias Assessment
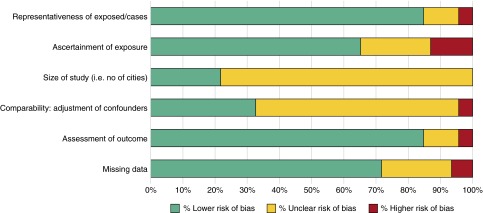
A risk-of-bias assessment based upon the Newcastle-Ottawa scale was devised (15), and assessment domains included the representativeness of exposure, ascertainment of exposure, comparability (i.e., controlling for confounders), and reporting of missing data (Figure 8). Small-study bias was assessed using the “trim-and-fill” method (16) (Figure E1). The percentage of variation between studies due to heterogeneity was assessed with Galbraith (radial) plots (Figure E2) and quantified with Cochran’s Q measure in random effects models. The I2 statistic was calculated as the weighted sum of squared differences between individual study effects and the pooled effects across studies as follows: I2 = 100% × (Q − df)/Q.
Figure 8.
Risk of bias assessment for studies included in the meta-analysis.
Data Extraction and Synthesis of Evidence
Relevant full-text studies were coded to address the topic focus of the review: study type (e.g., primary research, metaanalysis), focus of the study (e.g., health outcomes), country in which the research was conducted, duration of the study, and methodology employed (e.g., epidemiological study). Estimates of effects extracted from included studies were presented as odds ratios (OR), relative risks, or percentage changes in COPD hospital admissions (see online supplement for details).
Results
Methodological Classification of Studies
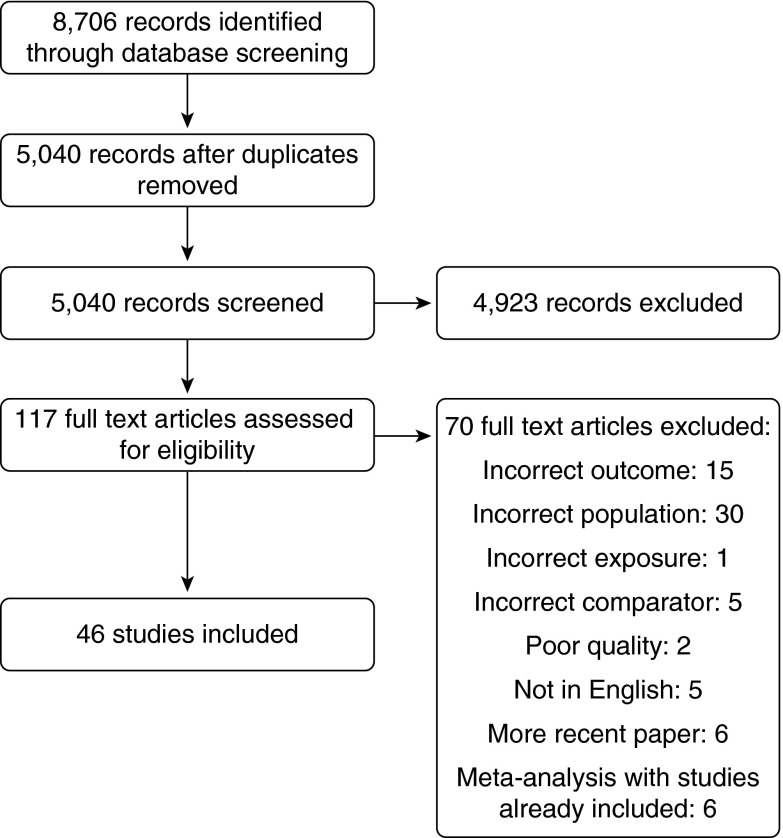
Figure 1 describes the literature search and screening process. Among the 46 studies included in the metaanalysis, 15 were performed in North America and 19 in Europe. Eight studies were conducted in Asia, while limited information from South America and Australia was available. Therefore, three geographical clusters were defined on the basis of available evidence in the literature.
Figure 1.
Flowchart of the literature search and screening process.
Two methodological approaches were identified: time-series and case-crossover studies. The most common approach was ecological time-series investigations, where aggregated health outcomes of the total population were associated with daily variations in air pollutants after controlling for confounding factors, such as temperature or influenza epidemics. That approach has the potential for including a large number of days over several years for a large population, with advantages of improving the precision of estimate of effect of exposure–response relationships. The researchers in the vast majority of these studies analyzed exposure–response relationships at a single-city level, and we found only six time-series multicity investigations (17–22).
Case-crossover studies measured COPD exacerbations in cohorts of patients with COPD. Conceptually, a case-crossover design is different from a time-series study, as the unit of assessment is at the individual level, where each patient acts as his or her own control, accounting for variation at the individual level. A total of 11 studies with a case-crossover design were included in the metaanalysis. The study population in those studies was relatively small compared with the time-series investigations, which would result in a smaller precision of the estimate. Only one case-crossover study (23) was organized as a multicity study, in 36 cities.
Air pollution exposure in all time-series and case-crossover studies was measured at the nearest fixed air quality monitoring station. The number of fixed monitoring stations employed in each study was not always reported, but it ranged from a single monitoring station to 31 (24). The time resolution of the measurements was most often the 24-hour average value for meteorological parameters and particles and 1-hour to 8-hour maximum levels for gaseous pollutants.
Metaanalysis of Studies Using Single-Pollutant Models
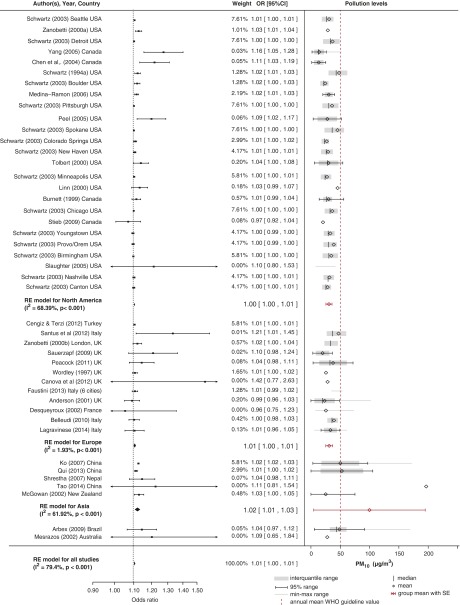
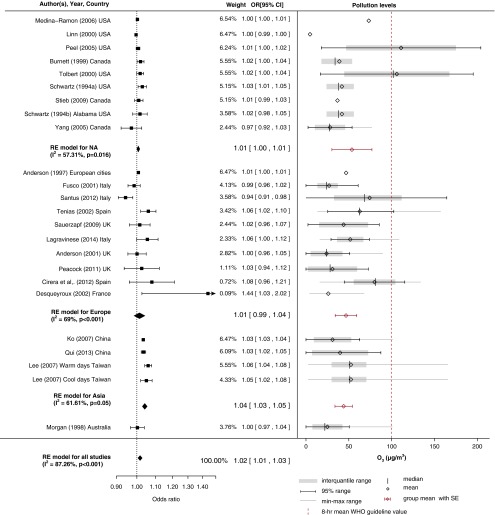
Figures 2–7 show forest plots for the converted odds ratios of COPD hospital admissions for PM10, PM2.5, CO, SO2, NO2, and O3 from single-pollutant models.
Figure 2.
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 10-μg/m3 increase in particulates with a diameter less than 10 μm in diameter (PM10). OR = odds ratio; RE = random effects; WHO = World Health Organization.
Figure 7.
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 10-μg/m3 increase in O3 levels. NA = North America; OR = odds ratio; RE = random effects; WHO = World Health Organization.
Figure 5.
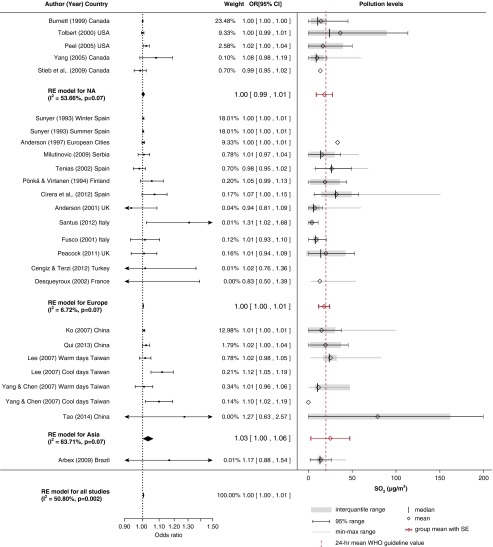
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 10-μg/m3 increase in SO2 levels. NA = North America; OR = odds ratio; RE = random effects; WHO = World Health Organization.
Effect of PM10
The pooled estimates of a total of 31 studies included in the metaanalysis for PM10 showed a marginal effect of a 10-μg/m3 increase of PM10 on COPD hospital admissions (Figure 2) with very high heterogeneity (I2 = 79.4%) between studies. Of these, 23 were ecological time-series studies, while the rest were organized as case-crossover investigations. While in 28 studies researchers estimated a positive association between COPD-related hospital admissions and PM10 exposure, the association was significant in only 15.
Most of the studies were conducted in Europe and North America, where a marginal effect was estimated (OR, 1.01; 95% confidence interval [CI], 1.00–1.01 for Europe; OR, 1.00; 95% CI, 1.00–1.01 for America), while a stronger effect was reported for studies conducted in Asia (OR, 1.02; 95% CI, 1.01–1.03). The stronger effect reported in Asian studies might be explained by the approximately threefold higher mean pollution levels of 99.8 ± 48.4 μg/m3 compared with 30.7 ± 2.6 μg/m3 and 31.1 ± 3.0 μg/m3 for North America and Europe, respectively, which were lower than the annual mean WHO guideline values. The metaregression model also indicates a nonlinear relationship, with stronger effects reported at higher ambient concentrations (Figure E3).
The heterogeneity among European studies (I2 = 1.93%) was significantly less than in the other two subgroups. With use of the trim-and-fill method, we identified evidence of small-study bias on the effect estimates of PM10. Contrary to single-city studies, in three multicity studies—one in Europe (25) and two in North America (20, 21)—a significant association was not found, while in a case-crossover study (23) and a time-series study (22) study in 10 U.S. cities a marginal association between PM10 and COPD hospital admissions was found.
There is insufficient evidence to assess the lagged effects of particle exposure on COPD morbidity, as most studies did not specify the temporal lags of the dependent variables in the regression. A further limitation includes the low temporal resolution of collected PM10 data, which in most studies was the daily average.
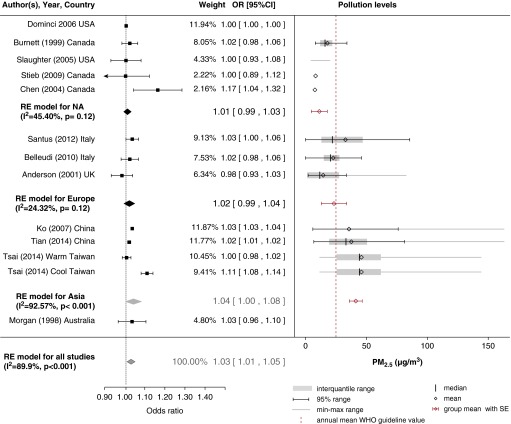
Effect of PM2.5
Due to the lack of available outdoor measurements of PM2.5 or smaller particles, evidence available on their potential association with COPD morbidity is limited, and the heterogeneity of the pooled metaanalysis was high (I2 = 89.9%). Of the 12 studies included in the metaanalysis (Figure 3), a positive association was found in 10; however, the association was significant in only 4. Studies in which researchers collected measurements of both PM2.5 and PM10 fractions, similar associations between COPD hospital admissions and these fractions were found (21, 22, 26–31). However, overall, a stronger association was found with PM2.5 (OR, 1.03; 95% CI, 1.01–1.05) than with PM10, which might be explained by the fact that smaller particles may penetrate deeper into the lungs.
Figure 3.
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 10-μg/m3 increase in particulates with a diameter less than 2.5 μm in diameter (PM2.5). NA = North America; OR = odds ratio; RE = random effects; WHO = World Health Organization.
Similarly, with PM10, the majority of studies were performed as time-series investigations, and most of the evidence comes from North America. When the effect estimates in the European and North American results were pooled, the heterogeneity was significantly reduced (I2 < 50%). The effect of PM2.5 was stronger in Asia (OR, 1.04; 95% CI, 1.00–1.08), but there was large heterogeneity. The highest concentration levels of PM2.5 (41.2 ± 2.7 μg/m3) were reported in Asia (where the effect was stronger and significant), and they were twice as high as in Europe (23.4 ± 5.3 μg/m3) and four times higher than in North America (11.3 ± 3.3 μg/m3), where the effects were lower and nonsignificant and levels were below annual mean WHO guideline values. The metaregression model also pointed toward a nonlinear relationship between COPD hospital admissions and ambient pollution levels, as there was a higher effect at higher concentrations (Figure E4).
Although there appears to be a relationship between PM2.5 and COPD hospital admissions, the results should be interpreted with caution because of the limited number of included studies. We did not detect any small-city bias with the trim-and-fill method. Two studies were organized as multicity investigations—one in 202 U.S. cities (18) and one in 7 Canadian cities (21)—and the researchers reported a nonsignificant association between PM2.5 exposure and COPD exacerbations.
As in the case of PM10, limited information exists on seasonal effects of fine particles on health outcomes, with researchers in only one study in a tropical climate estimating larger effects in the cool season (32). Limited evidence is available on the lagged effects of PM2.5 exposure on COPD morbidity and points toward a shorter temporal lag than PM10 of up to 2 days (30).
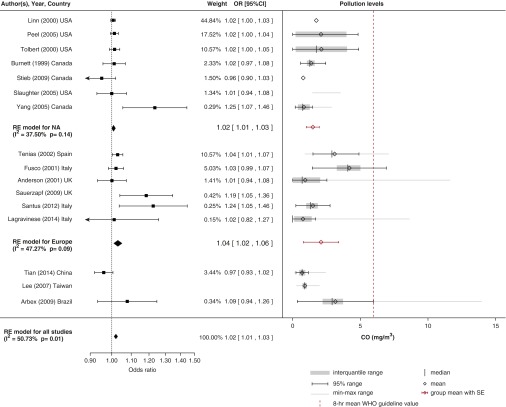
Effect of CO
Pooled results of 15 studies for CO (Figure 4) showed a small but significant effect of a 1-mg/m3 increase in CO on COPD admissions (OR, 1.02; 95% CI, 1.01–1.03) with moderate heterogeneity (I2 = 50.73%) between studies. We excluded one study done in Asia from the pooled estimate (33) that increased the heterogeneity significantly (I2 = 83.6%). The rescaled ORs for this study were 1.67 (95% CI, 1.37–2.04) in the warm season and 2.70 (95% CI, 2.04–3.58) in the cool season. Apart from two European case-crossover studies (21, 30) in which researchers found a strong positive association, all included studies were time-series investigations. There was some evidence of small-study bias in the trim-and-fill funnel plot, supported by the nonsignificant negative association estimated by the only multicity investigation, which was done in seven Canadian cities (21).
Figure 4.
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 1-mg/m3 increase in CO levels. NA = North America; OR = odds ratio; RE = random effects; WHO = World Health Organization.
There is insufficient evidence of an association between COPD hospital admissions and CO exposure in geographical locations other than Europe (six studies) and North America (seven studies). The heterogeneity between studies was significantly reduced in both these geographical subgroups. Researchers in studies done in Europe estimated an overall stronger association (OR, 1.04; 95% CI, 1.02–1.06; I2 = 47.3%) than in North America (OR, 1.02; 95% CI, 1.01–1.03; I2 = 37.5%), possibly because CO concentrations in Europe were higher (2.1 ± 0.7 mg/m3) than in North America (1.5 ± 0.2 mg/m3), and the metaregression indicated that there is a nonlinear association between effect and ambient concentrations (Figure E5). Researchers in most studies found significant associations with acute (25, 34) or lagged effects of up to 3 days (21, 30, 31, 35).
Effect of SO2
The overall pooled estimate of SO2 exposure indicated a borderline effect with COPD admissions, with moderate heterogeneity between studies (I2 = 50.8%). Of the 23 studies included in the metaanalysis, a time-series methodology was employed in 18. There was evidence of small-study bias (Figure E1).
Most of the studies were performed in Europe, with small heterogeneity (I2 = 6.72%) between studies. The effects were clustered in geographical locations, with a stronger positive effect estimate in Asia (OR, 1.03; 95% CI, 1.00–1.06) than in North America, where researchers did not detect a significant association, and there was only a borderline effect in Europe. SO2 levels in North America and Europe were similar, with a small SE (18.1 ± 4.7 μg/m3 and 18.0 ± 3.2 μg/m3, respectively), while levels in Asia were higher, with a large SE (25.1 ± 11.30 μg/m3). The metaregression model approximated a linear relationship between effect size and pollution levels (Figure E6).
Apart from the spatial variation of the effect of SO2, a seasonal effect might also underpin the estimated association. In two studies in a tropical climate in Taiwan (33, 36), researchers found a significant association between SO2 and COPD hospital admissions only in the cool season (temperatures <25°C). A possible explanation might be increased coal burning for heating during the cool season in developing countries where levels were higher. However, seasonal differences were estimated in a 5-year European study (37), with a very small but insignificant association observed in winter and no relationship seen in the summer. Most studies estimated acute effects for SO2 (21, 34) or 2-day lagged effects (21, 30, 38). In only one study (35) did researchers estimate longer lagged effects of up to 13 days.
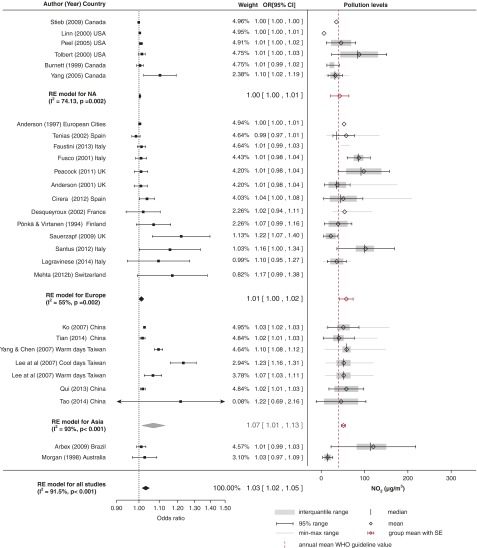
Effect of NO2
Results for NO2 (Figure 6) showed an association (OR, 1.03; 95% CI, 1.02–1.05) between a 10-µg/m3 increase in NO2 and COPD admissions, with high heterogeneity (I2 = 91.5%). We found evidence of small-study bias in single-city studies (Figure E1). A positive association was reported in 25 of 27 studies, and a significant association was reported in 11 studies. Only one multicity study in North America (21) and one in Europe (39) reported a negative nonsignificant effect.
Figure 6.
Pollution levels and summary estimates (95% confidence intervals [CIs]) for chronic obstructive pulmonary disease–related hospital admissions per 10-μg/m3 increase in NO2 levels. NA = North America; OR = odds ratio; RE = random effects; WHO = World Health Organization.
The majority of the evidence comes from Europe, where the heterogeneity between studies was moderate (I2 = 55%). The estimated effects in Europe and North America were similar (OR, 1.01; 95% CI, 1.00–1.02), but they were lower than the effects in Asia, where the CIs were wider (OR, 1.07; 95% CI, 1.01–1.13). The highest NO2 levels were measured in Europe (57.9 ± 8 μg/m3) and Asia (51.2 ± 2.4 μg/m3), and the lowest levels were measured in North America (42.7 ± 10.8 μg/m3), but in all geographical clusters the measurements were above mean annual WHO guideline values. A nonlinear relationship between mean levels and effect estimates was estimated in the metaregression, with stronger effects found at higher concentrations (Figure E7).
The findings on lagged effects of NO2 exposure are inconsistent. In three studies, researchers found significant acute effects of same-day NO2 exposure with COPD exacerbations (25, 40, 41) or 1- to 2-day lagged effects (33, 42). Longer 3-day lag effects were reported in three large studies (21, 27, 35), while researchers in four smaller studies reported longer lagged effects lasting up to 8 days (30, 39, 43, 44).
Effect of O3
In total, there were 23 studies in which researchers investigated the effect of O3 on COPD hospital admissions. Nine were performed in North America, nine in Europe, three in Asia, and one in Australia (Figure 7). As in the case of NO2, the heterogeneity between studies was large (I2 = 87.23%). Researchers in 18 of the 22 studies reported a positive effect; however, it was significant in only 10 studies. Overall, the pooled estimates showed that there was a small positive effect of O3 on COPD hospital admissions (OR, 1.02; 95% CI, 1.01–1.03). Mean levels of O3 were similar in all geographical locations, ranging from 43.9 μg/m3 in Asia to 53.6 μg/m3 in North America, and, using the metaregression model, we estimated a linear relationship with the effects (Figure E8).
The heterogeneity between studies in geographical subgroups remained high. The pooled models showed that the strongest effect (OR, 1.04; 95% CI, 1.03–1.05) was estimated for Asian countries, while the effects were marginally significant for North America (OR, 1.01; 95% CI, 1.00–1.02) and insignificant for Europe (OR, 1.01; 95% CI, 0.99–1.04). Contrary to the pooled models, researchers in the multicity studies found an insignificant effect in North America (21, 23) and a significant positive effect in Europe (17). There was no evidence of small-study bias.
The effect of seasonality on the association between O3 and COPD-related hospital admissions is unclear. Researchers in one study in Canada (21) estimated that the effect was nearly twice as large during the warm season as over the whole year. To the contrary, researchers in a study in a tropical climate (33) estimated that the effect was twice as large in the cool season.
Risk-of-Bias Assessment
The risk of bias for the studies included in this review (and the proportion of studies that had low, unclear, or high risk) is shown in Figure 8. Detailed descriptions for each individual study are included in the online supplement.
Discussion
To our knowledge, this is the first study in which metaanalytic techniques have been used to pool the effect estimates of the associations between COPD admissions with gaseous pollutants (NO2, O3, CO, and SO2) and particulate matter simultaneously. The models revealed suggestive evidence that all investigated pollutants may have a small but significant effect on COPD hospital admissions. These findings come from a relative small number of studies with high heterogeneity between them, however. Geographical clustering of the effects of pollution on COPD hospital admissions was evident and reduced heterogeneity significantly.
Particulate Matter
Previous systematic metaanalyses have been focused on the association between COPD exacerbations (8, 9) and exposure to particulate matter. Zhu and colleagues (11) estimated a 2.7% increase for COPD hospital admissions (95% CI, 1.9–3.6%) for every 10-μg/m3 increase in PM10, and they reported large heterogeneity in effect estimates from I2 values of 83.9% (11) to 79.4% (9). We found a marginally significant effect estimate for PM10 (OR, 1.01; 95% CI, 1.0–1.02), similar to that reported by Song and colleagues (9) but smaller than that reported by Zhu and colleagues (11). Song and colleagues (9) found that the strength of the association of COPD hospital admissions with PM10 varied among geographical locations, with an effect of 1% in China and Europe but a larger effect of 2% in the United States. We estimated a similar effect of 1% in Europe, with very little heterogeneity (I2 = 1.93%); however, we observed a smaller effect of 1% in North America and a larger effect in Asia of 3% (95% CI, 2–5%). A possible explanation might be that, unlike the investigators in both previous metaanalyses (9, 11), we did not include studies in which asthma was not separated in the diagnosis. Moreover, we found evidence of a nonlinear relationship where higher effects were reported at higher concentrations.
In only one metaanalysis (45) have researchers estimated the association of COPD admissions (excluding asthma) with PM2.5 exposure, and they found an association 1.02 (95% CI, 1.01–3.71) similar to the one in the present study (OR, 1.03; 95% CI, 1.01–1.05). In line with the findings of Atkinson and colleagues (45), we found large heterogeneity between studies but no evidence of small-study bias in the effect estimates of PM2.5 for COPD hospital admissions.
It is possible that the marginal effect of particulate matter estimated in this and previous metaanalytic studies might be influenced by small-study bias. In large multicity studies in North America and Europe (19–21), researchers failed to detect a significant association between outdoor PM10 levels and COPD hospital admissions. Similarly, researchers in the two multicity studies in North America (18, 21) did not find a significant association between PM2.5 exposure and COPD hospital admissions.
The effect of seasonal variation on the association between PM10 exposure and COPD exacerbations is not clear. Researchers in one multicity study in Italy (19) reported that the association is 7.5 times stronger in the summer season. In a study in Taiwan, however (33), researchers found a stronger effect in the cool season. A potential explanation for the observed differences might be related to behavioral patterns of the populations regarding time spent outdoors, which may vary in different climates. Another possible explanation in the Asian study may be related to the extensive use of mechanical cooling and air filtration in the tropical climate during the warm season, which may reduce exposure to PM. Similar results have been reported by Janssen and colleagues (46) in the reanalysis of the National Morbidity Mortality Air Pollution study in 14 U.S. states, where the percentage of households with air-handling units had a significant modification effect on COPD hospital admissions.
Gaseous Pollutants
The systematic evaluation of the association between COPD exacerbations with gaseous pollutants indicates a potential link between CO and SO2 levels, with moderate heterogeneity and strong geographical clustering. Both pollutants appeared in most studies to have acute effects or short lagged effects of up to 3 and 2 days, respectively, on COPD admissions, and a stronger effect in the winter season. Marginally stronger effects of CO were estimated in Europe than in North America. A potential explanation for the difference in effects estimated between Europe and North America may be related to different levels of ambient CO concentrations or to the methodological design of studies, such as the absence of multicity investigations in Europe.
The effect estimates of SO2 in each geographical subgroup indicated that the association was significant only in Asian countries, with stronger effects in the winter season, marginally significant in Europe, and insignificant in North America, where the majority of the evidence comes from, possibly because SO2 remains a predominant pollutant in developing countries. The researchers in the only two available multicity studies on the effects of SO2 found contradicting results, with one study in Europe showing a marginal positive association (17) and one study in North America (21) demonstrating a negative nonsignificant association. A possible explanation is that the chronological difference between these two studies reflects differences in outdoor SO2 concentrations.
The associations between NO2 and O3 exposure with COPD hospital admissions is less well understood, as the heterogeneity between studies in this review was large. Both pollutants showed marginal associations in Europe and North America, and stronger effects in Asia. Researchers in only three studies in Asia found an association with both O3 and NO2 (29, 33, 40). Studies in Europe and North America showed an association either with NO2 (24, 30, 35, 43) or O3 alone (17, 27, 39, 47, 48).
Limitations
A number of limitations in the methodological design of the studies included in this review do not allow us to establish a clear link between the effects of environmental pollution on COPD exacerbations. Using hospital admissions as an indication of exacerbation is a potential source of ecological fallacy, as it ignores individual-level characteristics and assesses health outcomes at a group level. Relationships at an individual level might not reflect group-level relationships and vice versa. Health-care use in COPD can vary depending on access, and it was not always possible to separate emergency from scheduled admissions, adding further uncertainty to the estimation of exacerbations.
Although both single-pollutant and multipollutant models were employed in many studies, results were included only from single-pollutant models, and the findings do not account for any covariance between air pollutants (such as NO2 and O3 or NO2 and PM). Other unmeasured pollutants in the mixture might also be important in the observed health outcomes (such as ultrafine particles). The confounding effects of temperature and humidity add further challenges. Although we know that there are seasonal effects on COPD exacerbations in northern and southern regions (49), the relationships between temperature and humidity and COPD admissions are not clear.
The studies were grouped on the basis of geographical location, which had the potential to reduce the heterogeneity of the subgroups; however, the small sample size limited the interpretation of the results. We used random effects models, which can account for the heterogeneity between studies better than fixed models. While this standardized method may reduce small-study bias, it cannot differentiate multicity from single-city studies if the SE is similar. Moreover, while the random effects pooled models assumed a linear relationship between air pollutants and effect estimates, we found evidence of a nonlinear relationship with higher effects reported at higher concentrations for all pollutants apart from O3 an SO2 that exhibited a linear relationship.
A significant limitation of the studies included the low spatiotemporal resolution of air pollution measurements from fixed monitoring stations as a surrogate for personal exposure. However, in practice, air quality is highly granular, and people, particularly those with chronic respiratory diseases, may spend a large fraction of their time indoors, where they might be exposed to a mixture of emissions from indoor sources. Missing daily monitoring data add further uncertainty in the analysis of time-series studies with daily lags in the exposure variable. Rather than using fixed site monitors as a proxy for “true” exposures, the development of hybrid models that combine pollutant dispersion models with space–time–activity models may prove to be a more effective way of examining the effects of personal environmental exposure on health (50).
Conclusions
A key finding of this review is that the effects of separate pollutants on COPD admissions appear to vary across geographical regions. Effects were evident even at concentrations below current guideline values, indicating a need to lower thresholds to protect such vulnerable groups.
Footnotes
This research was funded by the Medical Research Council (MR/L019744/1 [B.B.]). It was also supported by the Medical Research Council-Public Health England (MRC-PHE) Centre for Environment and Health and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author Contributions: E.M. and L.C.: performed the background research, literature search, screening of articles, data extraction, and data interpretation; produced the figures; and wrote the first draft of the manuscript; M.-O.K.: assisted with screening and data extraction; J.K.Q.: conceived of the study, made critical revisions to the manuscript for intellectual content, and supported completion of the first draft of the manuscript; B.B.: provided oversight for the study, commented on subsequent drafts of the manuscript, and approved the final version of the manuscript; and R.L.J. and F.J.K.: commented on the first draft of the manuscript and gave advice on data interpretation and analysis. All other authors commented on subsequent drafts and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; 2008. World health statistics 2008. [accessed 2015 Jul 8]. Available from: http://www.who.int/whosis/whostat/EN_WHS08_TOCintro.pdf. [Google Scholar]
- 3.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147:31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 4.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 7.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson RW, Mills IC, Walton HA, Anderson HR. Fine particle components and health—a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J Expo Sci Environ Epidemiol. 2015;25:208–214. doi: 10.1038/jes.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Q, Christiani DC, Wang X, Ren J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:11822–11832. doi: 10.3390/ijerph111111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunyer J, Schwartz J, Tobías A, Macfarlane D, Garcia J, Antó JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 11.Zhu R, Chen Y, Wu S, Deng F, Liu Y, Yao W. The relationship between particulate matter (PM10) and hospitalizations and mortality of chronic obstructive pulmonary disease: a meta-analysis. COPD. 2013;10:307–315. doi: 10.3109/15412555.2012.744962. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson RW, Mills IC, Walton HA, Kang S, Anderson HR.Systematic review and quantitative meta-analysis of the evidence for associations between chronic and short-term exposure to outdoor air pollutants and healthDepartment of Health Policy Research Programme. Project 002/0037. London: Department of Health; 2014[accessed 2016 Jul 13]. http://www.prp-ccf.org.uk/PRPFiles/SFR_April_2011/0020037%20SFR_Atkinson.pdf [Google Scholar]
- 13.Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 2015;5:e006946. doi: 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Ottawa, ON, Canada: Ottawa Hospital; 2015. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [accessed 2015 Dec 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 17.Anderson HR, Spix C, Medina S, Schouten JP, Castellague J, Rossi G, Zmirou D. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997;10:1064–1071. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]
- 18.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faustini A, Stafoggia M, Colais P, Berti G, Bisanti L, Cadum E, Cernigliaro A, Mallone S, Scarnato C, Forastiere F EpiAir Collaborative Group. Air pollution and multiple acute respiratory outcomes. Eur Respir J. 2013;42:304–313. doi: 10.1183/09031936.00128712. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz J, Zanobetti A, Bateson T. Boston: Health Effects Institute; 2003. Special report: revised analyses of time-series studies of air pollution and health. [Google Scholar]
- 21.Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health. 2009;8:25. doi: 10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanobetti A, Schwartz J, Dockery DW. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ Health Perspect. 2000;108:1071–1077. doi: 10.1289/ehp.001081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163:579–588. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Chen Y, Krewski D, Burnett RT, Shi Y, McGrail KM. Effect of short-term exposure to low levels of gaseous pollutants on chronic obstructive pulmonary disease hospitalizations. Environ Res. 2005;99:99–105. doi: 10.1016/j.envres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Lagravinese R, Moscone F, Tosetti E, Lee H. The impact of air pollution on hospital admissions: evidence from Italy. Reg Sci Urban Econ. 2014;49:278–285. [Google Scholar]
- 26.Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, Forastiere F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010;21:414–423. doi: 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- 27.Burnett RT, Smith-Doiron M, Stieb D, Cakmak S, Brook JR. Effects of particulate and gaseous air pollution on cardiorespiratory hospitalizations. Arch Environ Health. 1999;54:130–139. doi: 10.1080/00039899909602248. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Yang Q, Krewski D, Shi Y, Burnett RT, McGrail K. Influence of relatively low level of particulate air pollution on hospitalization for COPD in elderly people. Inhal Toxicol. 2004;16:21–25. doi: 10.1080/08958370490258129. [DOI] [PubMed] [Google Scholar]
- 29.Ko FW, Tam W, Wong TW, Chan DPS, Tung AH, Lai CKW, Hui DS. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62:780–785. doi: 10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santus P, Russo A, Madonini E, Allegra L, Blasi F, Centanni S, Miadonna A, Schiraldi G, Amaducci S. How air pollution influences clinical management of respiratory diseases: a case-crossover study in Milan. Respir Res. 2012;13:95. doi: 10.1186/1465-9921-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol. 2005;15:153–159. doi: 10.1038/sj.jea.7500382. [DOI] [PubMed] [Google Scholar]
- 32.Tsai SS, Chiu HF, Liou SH, Yang CY. Short-term effects of fine particulate air pollution on hospital admissions for respiratory diseases: a case-crossover study in a tropical city. J Toxicol Environ Health A. 2014;77:1091–1101. doi: 10.1080/15287394.2014.922388. [DOI] [PubMed] [Google Scholar]
- 33.Lee IM, Tsai SS, Chang CC, Ho CK, Yang CY. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2007;19:393–398. doi: 10.1080/08958370601174818. [DOI] [PubMed] [Google Scholar]
- 34.Fusco D, Forastiere F, Michelozzi P, Spadea T, Ostro B, Arcà M, Perucci CA. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J. 2001;17:1143–1150. doi: 10.1183/09031936.01.00005501. [DOI] [PubMed] [Google Scholar]
- 35.Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16:164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- 36.Yang CY, Chen CJ. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2007;70:1214–1219. doi: 10.1080/15287390701380880. [DOI] [PubMed] [Google Scholar]
- 37.Sunyer J, Sáez M, Murillo C, Castellsague J, Martínez F, Antó JM. Air pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year study. Am J Epidemiol. 1993;137:701–705. doi: 10.1093/oxfordjournals.aje.a116730. [DOI] [PubMed] [Google Scholar]
- 38.Arbex MA, de Souza Conceição GM, Cendon SP, Arbex FF, Lopes AC, Moysés EP, Santiago SL, Saldiva PH, Pereira LA, Braga AL. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health. 2009;63:777–783. doi: 10.1136/jech.2008.078360. [DOI] [PubMed] [Google Scholar]
- 39.Tenías JM, Ballester F, Pérez-Hoyos S, Rivera ML. Air pollution and hospital emergency room admissions for chronic obstructive pulmonary disease in Valencia, Spain. Arch Environ Health. 2002;57:41–47. doi: 10.1080/00039890209602915. [DOI] [PubMed] [Google Scholar]
- 40.Qiu H, Yu ITS, Wang X, Tian L, Tse LA, Wong TW. Season and humidity dependence of the effects of air pollution on COPD hospitalizations in Hong Kong. Atmos Environ. 2013;76:74–80. [Google Scholar]
- 41.Morgan G, Corbett S, Wlodarczyk J. Air pollution and hospital admissions in Sydney, Australia, 1990 to 1994. Am J Public Health. 1998;88:1761–1766. doi: 10.2105/ajph.88.12.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirera L, García-Marcos L, Giménez J, Moreno-Grau S, Tobías A, Pérez-Fernández V, Elvira-Rendeles B, Guillén JJ, Navarro C. Daily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of Cartagena. Allergol Immunopathol (Madr) 2012;40:231–237. doi: 10.1016/j.aller.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Sauerzapf V, Jones AP, Cross J. Environmental factors and hospitalisation for chronic obstructive pulmonary disease in a rural county of England. J Epidemiol Community Health. 2009;63:324–328. doi: 10.1136/jech.2008.077024. [DOI] [PubMed] [Google Scholar]
- 44.Peacock JL, Anderson HR, Bremner SA, Marston L, Seemungal TA, Strachan DP, Wedzicha JA. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011;66:591–596. doi: 10.1136/thx.2010.155358. [DOI] [PubMed] [Google Scholar]
- 45.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69:660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen NAH, Schwartz J, Zanobetti A, Suh HH. Air conditioning and source-specific particles as modifiers of the effect of PM10 on hospital admissions for heart and lung disease. Environ Health Perspect. 2002;110:43–49. doi: 10.1289/ehp.0211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desqueyroux H, Pujet JC, Prosper M, Le Moullec Y, Momas I. Effects of air pollution on adults with chronic obstructive pulmonary disease. Arch Environ Health. 2002;57:554–560. doi: 10.1080/00039890209602088. [DOI] [PubMed] [Google Scholar]
- 48.Tolbert PE, Klein M, Metzger KB, Peel J, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Interim results of the Study of Particulates and Health in Atlanta (SOPHIA) J Expo Anal Environ Epidemiol. 2000;10:446–460. doi: 10.1038/sj.jea.7500106. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, Yates JC, Calverley PM. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39:38–45. doi: 10.1183/09031936.00194610. [DOI] [PubMed] [Google Scholar]
- 50.Beevers SD, Kitwiroon N, Williams ML, Kelly FJ, Ross Anderson H, Carslaw DC. Air pollution dispersion models for human exposure predictions in London. J Expo Sci Environ Epidemiol. 2013;23:647–653. doi: 10.1038/jes.2013.6. [DOI] [PubMed] [Google Scholar]
- 51.Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canova C, Dunster C, Kelly FJ, Minelli C, Shah PL, Caneja C, Tumilty MK, Burney P. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology. 2012;23:607–615. doi: 10.1097/EDE.0b013e3182572563. [DOI] [PubMed] [Google Scholar]
- 53.Cengiz MA, Terzi Y. Comparing models of the effect of air pollutants on hospital admissions and symptoms for chronic obstructive pulmonary disease. Cent Eur J Public Health. 2012;20:282–286. doi: 10.21101/cejph.a3757. [DOI] [PubMed] [Google Scholar]
- 54.Linn WS, Szlachcic Y, Gong H, Jr, Kinney PL, Berhane KT. Air pollution and daily hospital admissions in metropolitan Los Angeles. Environ Health Perspect. 2000;108:427–434. doi: 10.1289/ehp.00108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGowan JA, Hider RN, Chacko E, Town GI. Particulate air pollution and hospital admissions in Christchurch, New Zealand. Aust N Z J Public Health. 2002;26:23–29. doi: 10.1111/j.1467-842x.2002.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 56.Mehta AJ, Schindler C, Perez L, Probst-Hensch N, Schwartz J, Brändl O, Karrer W, Tschopp JM, Rochat T, Künzli N SAPALDIA Team. Acute respiratory health effects of urban air pollutants in adults with different patterns of underlying respiratory disease. Swiss Med Wkly. 2012;142:w13681. doi: 10.4414/smw.2012.13681. [DOI] [PubMed] [Google Scholar]
- 57.Mészáros D, Markos J, FitzGerald DG, Walters EH, Wood-Baker R. An observational study of PM10 and hospital admissions for acute exacerbations of chronic respiratory disease in Tasmania, Australia 1992–2002. BMJ Open Respir Res. 2015;2:e000063. doi: 10.1136/bmjresp-2014-000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milutinović S, Nikić D, Stosić L, Stanković A, Bogdanović D. Short-term association between air pollution and emergency room admissions for chronic obstructive pulmonary disease in Nis, Serbia. Cent Eur J Public Health. 2009;17:8–13. doi: 10.21101/cejph.a3508. [DOI] [PubMed] [Google Scholar]
- 59.Pönkä A, Virtanen M. Chronic bronchitis, emphysema, and low-level air pollution in Helsinki, 1987–1989. Environ Res. 1994;65:207–217. doi: 10.1006/enrs.1994.1032. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz J. Air pollution and hospital admissions for the elderly in Detroit, Michigan. Am J Respir Crit Care Med. 1994;150:648–655. doi: 10.1164/ajrccm.150.3.8087333. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz J. Air pollution and hospital admissions for the elderly in Birmingham, Alabama. Am J Epidemiol. 1994;139:589–598. doi: 10.1093/oxfordjournals.aje.a117048. [DOI] [PubMed] [Google Scholar]
- 62.Shrestha SL. Time series modelling of respiratory hospital admissions and geometrically weighted distributed lag effects from ambient particulate air pollution within Kathmandu Valley, Nepal. Environ Model Assess. 2007;12:239–251. [Google Scholar]
- 63.Tao Y, Mi S, Zhou S, Wang S, Xie X. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ Pollut. 2014;185:196–201. doi: 10.1016/j.envpol.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Tian L, Ho KF, Wang T, Qiu H, Pun VC, Chan CS, Louie PK, Yu IT. Ambient carbon monoxide and the risk of hospitalization due to chronic obstructive pulmonary disease. Am J Epidemiol. 2014;180:1159–1167. doi: 10.1093/aje/kwu248. [DOI] [PubMed] [Google Scholar]
- 65.Wordley J, Walters S, Ayres JG. Short term variations in hospital admissions and mortality and particulate air pollution. Occup Environ Med. 1997;54:108–116. doi: 10.1136/oem.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect. 2000;108:841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 68.Higgins JPT, Green S.Cochrane handbook for systematic reviews of interventionsVersion 5.1.0. London: The Cochrane Collaboration; 2011[accessed 2015 Sep 2]. Available from: http://handbook.cochrane.org/ [Google Scholar]
- 69.Gleser LJ, Olkin I. Stochastically dependent effect sizes. In: Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009. pp. 358–376. [Google Scholar]
- 70.R Core TeamR: A language and environment for statistical computing. 2014[cited 2015 Dec 11]. Available from: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf
- 71.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 72.World Health Organization. Ambient (outdoor) air quality and health: fact sheet 313. 2014 [cited 2016 May 9]. Available from: http://www.who.int/mediacentre/factsheets/fs313/en/