Abstract
Rationale: Patients with idiopathic bronchiectasis are predominantly female and have an asthenic body morphotype and frequent nontuberculous mycobacterial respiratory infections. They also demonstrate phenotypic features (scoliosis, pectus deformity, mitral valve prolapse) that are commonly seen in individuals with heritable connective tissue disorders.
Objectives: To determine whether lumbar dural sac size is increased in patients with idiopathic bronchiectasis as compared with control subjects, and to assess whether dural sac size is correlated with phenotypic characteristics seen in individuals with heritable connective tissue disorders.
Methods: Two readers blinded to diagnosis measured anterior–posterior and transverse dural sac diameter using L1–L5 magnetic resonance images of 71 patients with idiopathic bronchiectasis, 72 control subjects without lung disease, 29 patients with cystic fibrosis, and 24 patients with Marfan syndrome. We compared groups by pairwise analysis of means, using Tukey’s method to adjust for multiple comparisons. Dural sac diameter association with phenotypic and clinical features was also tested.
Measurements and Main Results: The L1–L5 (average) anterior–posterior dural sac diameter of the idiopathic bronchiectasis group was larger than those of the control group (P < 0.001) and the cystic fibrosis group (P = 0.002). There was a strong correlation between increased dural sac size and the presence of pulmonary nontuberculous mycobacterial infection (P = 0.007) and long fingers (P = 0.003). A trend toward larger dural sac diameter was seen in those with scoliosis (P = 0.130) and those with a family history of idiopathic bronchiectasis (P = 0.149).
Conclusions: Individuals with idiopathic bronchiectasis have an enlarged dural sac diameter, which is associated with pulmonary nontuberculous mycobacterial infection, long fingers, and family history of idiopathic bronchiectasis. These findings support our hypothesis that “idiopathic” bronchiectasis development reflects complex genetic variation in heritable connective tissue and associated transforming growth factor-β–related pathway genes.
Keywords: bronchiectasis, nontuberculous mycobacteria, heritable connective tissue disorders, dural sac diameter
Idiopathic bronchiectasis, or bronchiectasis without a known cause, affects more than 100,000 Americans and carries substantial morbidity and mortality. Impaired mucus clearance results in recurrent infections, persistent inflammation, and dilation of the airways, reflecting loss of integrity of the supportive connective tissue network of the airways.
The authors of prior publications have reported that idiopathic bronchiectasis has a female predilection and a high prevalence of an asthenic body morphotype, as well as an association with pulmonary nontuberculous mycobacterial infection. In addition, other phenotypic abnormalities occur at a higher frequency in these individuals than in the general population, including pectus deformity, scoliosis, mitral valve prolapse, high-arched palate, arachnodactyly, and hypermobility (1–5). These skeletal and cardiovascular manifestations are similar to phenotypic features noted in individuals with heritable connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, and Ehlers-Danlos syndrome.
Patients with these disorders demonstrate diverse manifestations of disorganized connective tissue matrices, particularly in the cardiovascular and skeletal systems, but also in the lung (6–9). Autosomal dominant mutations result in excessive transforming growth factor (TGF)-β activation and signaling, which are important factors in the connective tissue abnormalities seen in individuals with these syndromes.
Phenotypic manifestations in Marfan syndrome include 25–60% with scoliosis or kyphoscoliosis (10); more than 65% with pectus abnormalities, including excavatum and carinatum (10, 11); 85% with joint hypermobility (11); 91% with mitral valve prolapse (12); and 60% with aortic root dilation (12). High-arched palate and long fingers (arachnodactyly) are also frequent findings in Marfan syndrome (11). Manifestations in Loeys-Dietz syndrome are similar: 70% with scoliosis (13), 51% with pectus deformity (13), more than 20% with mitral valve prolapse (14), 87% with aortic root dilation (13), and at least half with joint hypermobility and long fingers (13). High-arched palate is also seen (15). In individuals with Ehlers-Danlos syndrome, the prevalence of these clinical features is more varied because of subclassifications within this diagnosis. Joint hypermobility is the hallmark characteristic of all Ehlers-Danlos syndrome subtypes (16). Aortic root dilation is seen in 10–28% (17, 18), and mitral valve prolapse is seen in 6–66% (17, 18). High-arched palate, scoliosis, and pectus abnormalities may also be seen (19).
Individuals with Marfan syndrome and other patients with heritable connective tissue disorders have lung disease, with an increased frequency of pneumonia and bronchiectasis (19–23) as well as apical blebs and spontaneous pneumothorax; the latter is included in the systemic score of the modified Ghent criteria for Marfan syndrome (24). Dural ectasia, or dilation of the dural sac surrounding the spinal cord, is present in Marfan syndrome (25), Loeys-Dietz syndrome (26–28), and variant Ehlers-Danlos syndromes (29–31). It does not occur in the general population in the absence of neurofibromatosis type 1, severe scoliosis, trauma, or tumors (32). The prevalence of dural ectasia in the Marfan syndrome population is estimated to be between 63 and 90% (10, 25), and its prevalence in patients with Loeys-Dietz syndrome is estimated to be between 50 and 73% (26–28). Only case reports of dural ectasia occurring in Ehlers-Danlos syndrome and other heritable connective tissue disorders have been published.
Dural sac size can easily be measured using sagittal images of the lumbar spine obtained by magnetic resonance (MR) imaging or computed tomography (CT) (32–34). Measurements obtained in this way can be reproduced by readers with different experience using a standardized and validated method, and they are not influenced by age, sex, height, or race (34).
Following the observation of an apparent increase in dural sac size in a few individuals with idiopathic bronchiectasis, and in light of the overlap of heritable connective tissue disorder features in individuals with idiopathic bronchiectasis and the increasingly recognized presence of bronchiectasis in heritable connective tissue disorders, we sought to examine lumbar MR images of individuals with idiopathic bronchiectasis to determine if there was also a significant increase in dural sac size as compared with control individuals. If true, this would be congruent with the concept that genetic variation in heritable connective tissue disorder genes and TGF-β–related pathways contributes to the risk of bronchiectasis development.
Our secondary aim was to determine if increased dural sac size in subjects with bronchiectasis was associated with physical characteristics that overlap with heritable connective tissue disorders, which would further support our hypothesis that bronchiectasis is a complex genetic disorder reflecting genetic variation in connective tissue disorder genes and TGF-β–related pathways. Some of the results of these studies have been reported previously in the form of an abstract (35).
Methods
The local institutional review board approved the present study. Informed consent and MR imaging safety screening forms were completed in prospectively studied subjects, but informed consent was waived for evaluation of preexisting MR and CT images. All data were recorded in a Health Insurance Portability and Accountability Act–compliant, protected database.
Study Subjects
Measurements were obtained from four groups of adults (≥18 yr of age). Subjects with idiopathic bronchiectasis (n = 71 subjects from 66 families) included individuals with bronchiectasis in two or more lobes; less than a 10–pack-year smoking history; and exclusion of other potential etiologies, including cystic fibrosis, primary ciliary dyskinesia, alpha-1 antitrypsin deficiency, allergic bronchopulmonary aspergillosis, inflammatory bowel disease, autoimmune disease, immune deficiency, prior solid organ transplant, and prior history of tuberculosis. None of the subjects with bronchiectasis met the clinical criteria required for a diagnosis of a heritable connective tissue disorder.
Control subjects (n = 72) without lung or spinal disease or evidence of heritable connective tissue disorders were imaged as previously described (34). Subjects with cystic fibrosis (n = 29) were confirmed to carry two disease-causing mutations in CFTR and served to control for the possibility that coughing might cause increased intracranial and intrathecal pressure and thereby affect dural size.
An additional 24 subjects carried a genetic or clinical diagnosis of Marfan syndrome and had preexisting MR imaging or CT examinations of the lumbosacral spine available for measurement (34). Exclusion criteria for all subjects included severe thoracic or lumbar scoliosis (Cobb angle, ≥30 degrees), thoracic or lumbar spinal surgery, spinal stenosis, or spinal injury.
Imaging Protocol and Analysis
Both preexisting and prospective lumbar MR imaging examinations were performed as described by Daniels and colleagues (34). Additional details are provided in the online supplement.
Determination of Phenotypic Features
Pectus abnormalities were classified as pectus excavatum or pectus carinatum using a corrected Haller index (36) or if there was a sternal tilt resulting in chest wall asymmetry that was noted in a physical examination or by chest imaging. Scoliosis was defined as the presence of a single spinal curve with a Cobb angle of 8 degrees or more or as the presence of two spinal curves, each having a Cobb angle of at least 5 degrees as measured on a posterior–anterior chest radiograph using the Cobb method (37).
Mitral valve prolapse was defined by findings of redundant mitral valve leaflets with prolapse into the left ventricle noted on an echocardiogram, but the presence of mitral regurgitation was not required. Fingers disproportionately long as compared with the palm of the hand were assessed by the (Walker) wrist sign and the (Steinberg) thumb sign (24, 38). A high-arched palate was present when the maximum palate height was greater than twice the height of the teeth (39). Joint hypermobility was determined by applying the Brighton criteria (40). This includes the Beighton score (40), which requires quantified assessment of hyperextension using a goniometer. If joint hypermobility could not be assessed in person, a validated 5-point questionnaire for generalized joint hypermobility (41) was completed by phone or by e-mail.
Methods Used to Determine Clinical Features
Lung function was measured using prebronchodilator spirometric measurements obtained according to American Thoracic Society guidelines (42). Percent predicted values were calculated using published reference values (43). The presence of bronchiectasis was determined for each lobe of the lung; the lingula was considered as the sixth lobe (separate from the left upper lobe). Severity of bronchiectasis was classified as mild, moderate, or severe as defined in Table E1 in the online supplement. The methods we used for defining infection status and family history of bronchiectasis are provided in detail in the online supplement.
Statistical Analysis
Linear regression was performed to compare the effects of covariates, including height, sex, and race, on anterior–posterior dural sac diameter (AP-DSD) and transverse dural sac diameter (TR-DSD) measurements of L1 through L5 and the L1–L5 average. For the effect of the covariate of age, we regressed on the oldest patient per family (n = 66). Pearson’s correlation was used to calculate the unadjusted correlation between readers 1 and 2 for the AP-DSD and TR-DSD measurements. Measurements for AP-DSD and TR-DSD by reader 1 were compared between all four groups using pairwise comparison of means. Tukey’s method was used to adjust for multiple comparisons.
Within the idiopathic bronchiectasis group, comparison of L1–L5 (average) AP-DSD by individual dichotomous clinical characteristic variables was performed using Student’s t test. For comparison of men and women within the idiopathic bronchiectasis group, Student’s t test was used for continuous variables, including FEV1, body mass index (BMI), age, and height. Chi-square and Fisher’s exact tests were used for comparison of categorical variables such as Pseudomonas and mycobacterial positivity, radiographic findings consistent with nontuberculous mycobacterial disease, and family member with bronchiectasis. The Wilcoxon-Mann-Whitney test was used for comparison of lobes with bronchiectasis.
For all tests, P < 0.05 indicated a statistically significant difference. All statistical analysis was performed using STATA release 2013 software (StataCorp, College Station, TX).
Results
Demographics
Demographic features of the IB study subjects are summarized in Table 1. The bronchiectasis group was older (mean age, 57 yr) and predominantly female (75%). As expected, men were taller than women, but there was no significant difference by sex for age (P = 0.99), BMI (P = 0.23), prevalence of Pseudomonas aeruginosa or mycobacterial infection (P = 0.62 and P = 0.22, respectively), lobes with bronchiectasis (P = 0.44), or FEV1 percent predicted (P = 0.7).
Table 1.
Demographics of subjects with idiopathic bronchiectasis
| Female (n = 53) | Male (n = 18) | P Value | |
|---|---|---|---|
| Age, yr, mean (range) | 57.3 (18–89) | 57.3 (19–81) | P = 1 |
| BMI, mean (range) | 23.2 (12.9–47.8) | 24.9 (16.3–34.3) | P = 0.23 |
| Height, cm, mean (range) | 162.7 (149.5–175.5) | 177.0 (156.6–187.6) | P < 0.001 |
| FEV1, % predicted, mean (range) | 74 (19–111) | 72 (29–119) | P = 0.70 |
| Pseudomonas aeruginosa, % (individuals positive/individuals tested) | 48% (25/52) | 41% (7/17) | P = 0.62 |
| NTM, % (individuals positive/individuals tested) | 42% (21/50) | 21% (3/14) | P = 0.22 |
| Lobes with bronchiectasis including lingula, mean (median) | 4.79 (6) | 4.56 (4) | P = 0.44 |
Definition of abbreviations: BMI = body mass index; NTM = nontuberculous Mycobacterium.
The average FEV1 was consistent with mild airway obstruction, but the broad range of FEV1 reflects the diversity of disease severity. Overall, the mean number of lobes affected was 4.7, with the lingula and the right middle lobe being the most severely affected (Table E2). Nearly half of subjects had P. aeruginosa cultured from respiratory samples (46%), and over one-third had environmental mycobacterial respiratory disease (37.5%).
Strikingly, of the 66 families included in the sample, 21% had more than one individual in the family diagnosed with idiopathic bronchiectasis, and this tended to be more common in women (P = 0.051). Scoliosis and pectus abnormalities were present more often in the subjects with bronchiectasis than in the control group and compared with population estimates (5) (Table E3). Pectus abnormalities were present in women significantly more often than in men (P = 0.005).
Subjects with bronchiectasis with a prior echocardiogram had mitral valve prolapse detected much more frequently than in the general population (44). Hypermobility also was more prevalent in the bronchiectasis group as compared with population estimates (45). While a positive wrist sign and high-arched palate were seen more often in the bronchiectasis group than in the general population, the thumb sign reflected population estimates (38). Positive wrist sign tended to be present more often in the women with bronchiectasis subgroup than in men with bronchiectasis (P = 0.091).
Dural Sac Dimensions
We examined dural sac size in patients with idiopathic bronchiectasis and compared measurements with those in the control, Marfan syndrome, and cystic fibrosis groups (Table E4). The subjects in the idiopathic bronchiectasis group tended to be older than those in the other groups and had a significantly lower BMI than those in the control group (P < 0.001). Whereas the height of the subjects with idiopathic bronchiectasis (by sex) was similar to that of control subjects, these subjects were shorter than those with Marfan syndrome (P < 0.001) and taller than those with cystic fibrosis (P = 0.05).
Overall, there was good correlation between the two readers for all groups regarding AP-DSD and TR-DSD (Table E5). Similarly to our prior publication (34), we found greater within-group variability for TR-DSD measurements than for AP-DSD measurements. We focused our present analysis on AP-DSD measurements, based on our prior study data demonstrating a better area under the curve for the receiver operating characteristics (34). Mirroring observations from our prior study (34), L1–L5 (average) AP-DSD measurements for the idiopathic bronchiectasis group and the cystic fibrosis group did not significantly differ by age, sex, height, or race.
The L1–L5 (average) AP-DSD of the idiopathic bronchiectasis group (1.52 ± 0.18 cm) (Figure 1) was significantly larger than those in the control group (1.38 ± 0.15 cm; P < 0.001) and the cystic fibrosis group (1.39 ± 0.15 cm; P = 0.002), and the dural sac diameter at each individual vertebral level in the idiopathic bronchiectasis group was also significantly larger (Table E6). The Marfan syndrome group tended to have a significantly larger dural sac size than that in the idiopathic bronchiectasis group (1.61 ± 0.17 cm; P = 0.084). There was no significant difference between the Marfan syndrome and idiopathic bronchiectasis groups at individual vertebral levels from L1 to L3; however, at the lower vertebral levels (L4 and L5), the Marfan syndrome group had significantly larger AP-DSD measurements.
Figure 1.
Comparison of L1–L5 (average) anterior–posterior dural sac diameter (AP-DSD) by diagnosis. The L1–L5 (average) AP-DSD of the idiopathic bronchiectasis (IB) group is significantly larger than those in the control group (P < 0.001) and the cystic fibrosis (CF) group (P = 0.002). The Marfan syndrome group is trending toward having a significantly larger dural sac size than the IB group (P = 0.094).
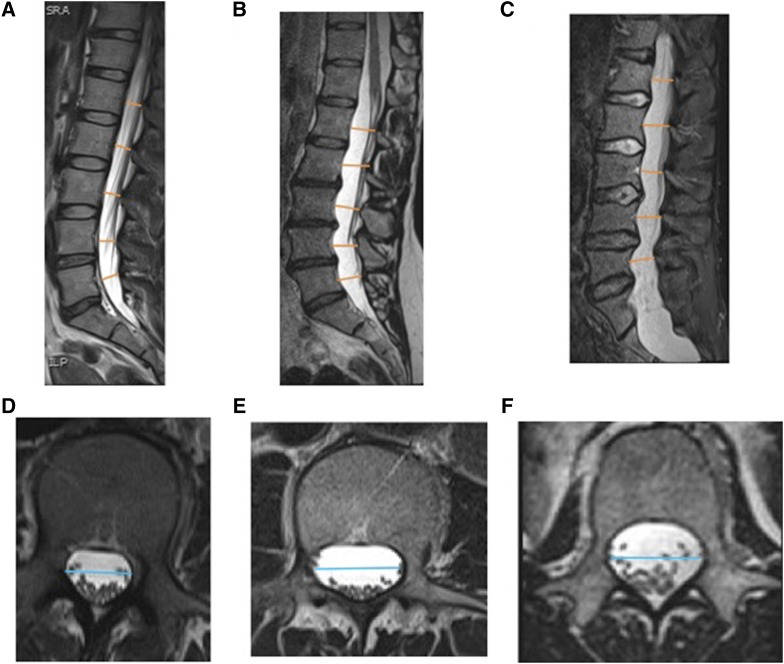
We saw similar results for L1–L5 (average) TR-DSD measurements (Figure 2). The measurement in the idiopathic bronchiectasis group (2.02 ± 0.24 cm) was significantly larger than those in the control (1.89 ± 0.17 cm) and cystic fibrosis groups (1.78 ± 0.19 cm) (P < 0.001). The L1–L5 (average) TR-DSD of the Marfan syndrome group (2.22 ± 0.32 cm) was larger than that in the L1–L5 (average) TR-DSD of the idiopathic bronchiectasis group (P = 0.001). Sagittal and axial MR images of the dural sac in a representative patient with idiopathic bronchiectasis are compared in Figure 3 with images obtained from a healthy control subject and a patient with Marfan syndrome.
Figure 2.
Comparison of L1–L5 (average) transverse dural sac diameter (TR-DSD) by diagnosis. The diameter in the idiopathic bronchiectasis (IB) group is significantly larger than those in the control group (P < 0.001) and the cystic fibrosis (CF) group (P < 0.001), but it is significantly smaller than that in the Marfan syndrome group (P = 0.001).
Figure 3.
Sagittal (A–C) and axial (D–F) T2-weighted three-dimensional turbo spin-echo magnetic resonance images of the lumbosacral spine. Axial images are at the L2 vertebral level. Measurement locations of anterior–posterior dural sac diameter (AP-DSD) (orange lines) and transverse dural sac diameter (TR-DSD) (blue lines) of a healthy control subject (A, D), of a subject with idiopathic bronchiectasis (B, E), and of a patient with Marfan syndrome (C, F).
We saw a robust correlation between increased dural sac diameter and presence of nontuberculous mycobacteria (Figure 4A). Individuals with at least two mycobacterial cultures positive for the same organism had significantly larger L1–L5 (average) AP-DSD than those without pulmonary mycobacterial infection (P = 0.007) (Figure 4A). A positive wrist sign (connoting longer fingers) was also significantly associated with larger L1–L5 (average) AP-DSD measurements (P = 0.003) (Figure 4B). Because only four subjects had a positive thumb sign, no conclusion could be drawn on that basis.
Figure 4.
Comparison of L1–L5 (average) anterior–posterior dural sac diameter (AP-DSD) by feature. Analysis of idiopathic bronchiectasis group based on nontuberculous Mycobacterium (NTM) cultures (A), wrist sign (B), scoliosis (C), and family history of bronchiectasis (BRX) (D). Of the 64 subjects with acid-fast bacilli respiratory culture data, 24 had cultures with positive results for pathogenic species of NTM. Individuals with positive NTM cultures had a significantly larger L1–L5 (average) AP-DSD than that of those with negative culture results (P = 0.007). Of the 62 subjects who were assessed for the wrist sign, the 17 individuals with a positive wrist sign had a significantly larger L1–L5 (average) AP-DSD than that of those with a negative wrist sign (P = 0.003). Of the 71 subjects with idiopathic bronchiectasis (IB) assessed for scoliosis, the 15 individuals with nonsevere scoliosis tended to have a larger L1–L5 (average) AP-DSD than that of those who did not have scoliosis (P = 0.130). Of the 66 IB families included in this analysis, the 14 individuals from families with a history of IB tended to have a larger L1–L5 (average) AP-DSD than that of those who did not have a family history of IB (P = 0.149).
Individuals with nonsevere scoliosis (and therefore not excluded from our study) tended to have larger L1–L5 (average) AP-DSD measurements than those without scoliosis (P = 0.130) (Figure 4C). There was at least one additional family member with bronchiectasis in 14 (21%) of the 66 families. A family history of bronchiectasis tended to be associated with increased dural sac diameter but did not reach statistical significance (P = 0.149) (Figure 4D). No difference was observed in L1–L5 (average) AP-DSD based on presence of sternal abnormalities (P = 0.524), high-arched palate (P = 0.354), hypermobility (as defined by Brighton score) (P = 0.749), or respiratory P. aeruginosa infection (P = 0.45).
As a way to look at the relationship between dural sac diameter and lung disease, we compared L1–L5 (average) AP-DSD with spirometric parameters and severity of bronchiectasis (Table E1). In that comparison, we observed no relationship for FEV1 percent predicted (P = 0.904), FEV1/FVC ratio (P = 0.474), or FEF25–75% predicted (P = 0.971). Although we found no relationship between dural sac size and whole-lung bronchiectasis severity score (P = 0.30), there was a modest association between increased dural sac diameter and a combined right middle lobe and lingular bronchiectasis score (P = 0.049).
Discussion
Bronchiectasis develops in the setting of impaired mucus clearance, which results in recurrent infections, persistent inflammation, and dilation of the airways, reflecting loss of integrity of the supportive connective tissue network of the airways. Although pathophysiological mechanisms for genetic recessive forms of bronchiectasis (cystic fibrosis and primary ciliary dyskinesia) reflect defective mucociliary and cough clearance, the mechanisms involved in idiopathic bronchiectasis are unknown.
The phenotypic features seen in idiopathic bronchiectasis, including a high prevalence of an asthenic body morphotype, pectus deformity, scoliosis, mitral valve prolapse, and hypermobility, are also common in patients with heritable connective tissue disorders, such as Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Moreover, patients with heritable connective tissue disorders have disorganized connective tissue matrices within the lungs that manifest as spontaneous pneumothorax, pneumonia, and bronchiectasis. Autosomal dominant mutations result in abnormal fibrillins in Marfan syndrome and congenital contractural arachnodactyly (Beals syndrome) and in defects in the TGF-β receptor in Loeys-Dietz syndrome and vascular Ehlers-Danlos syndrome. These lead to excessive TGF-β activation and signaling, which play a key pathophysiologic role in the connective tissue abnormalities in these syndromes (6, 7, 46–48), including dilation of the dural sac (dural ectasia), implying reduced integrity of this connective tissue matrix (25, 28, 47).
The morphotypic features seen in idiopathic bronchiectasis (asthenic body morphotype, pectus deformities) overlap with heritable connective tissue disorders, suggesting that genetic variants in the TGF-β1 pathway and signaling cascade may be associated with bronchiectasis in a subset of patients. This observation prompted us to rigorously assess dural sac size in a large cohort of patients with idiopathic bronchiectasis (n = 71) using lumbosacral MR imaging.
In this large cohort of patients with idiopathic bronchiectasis, we saw an increased prevalence of multiple phenotypes that are typically reported in these patients, such as scoliosis, pectus, and mitral valve prolapse (Table E6). In addition, we report novel findings of enlarged dural sac size in patients with idiopathic bronchiectasis compared with control subjects without lung disease (P < 0.001) and with those with cystic fibrosis who had bronchiectasis reflecting mutations in CFTR (P < 0.002). Indeed, dural sac enlargement in idiopathic bronchiectasis approached that seen in Marfan syndrome measured as both AP-DSD and TR-DSD (P = 0.094 and P = 0.001, respectively). These findings support our hypothesis that the development of “idiopathic” bronchiectasis in many people reflects, at least in part, complex genetic variation in connective tissue disorder genes and associated TGF-β–related pathogenic pathway genes.
This concept was reinforced by the presence of some phenotypic features in patients with idiopathic bronchiectasis that were associated with increased dural sac size, including long fingers as assessed by the wrist sign (P = 0.003) and scoliosis (P = 0.130). It should be noted that the association of dural sac diameter with scoliosis would likely have been stronger if we had not had to exclude subjects with severe scoliosis in our quantitation of dural sac size. Strikingly, we discovered in our patients with idiopathic bronchiectasis that respiratory infection with environmental mycobacteria was strongly associated with dural sac size (P = 0.007). It is noteworthy that the subjects with mycobacteria were largely women (21 of 24) and that subjects with mycobacteria were 8.7 years older than those without (62.6 vs. 53.9 yr). Although this older age in subjects with mycobacteria would provide the opportunity for its environmental acquisition, logistic regression including age and nontuberculous mycobacterial status as interaction terms showed that older age alone did not fully explain the association of mycobacteria with larger dural sac size (P = 0.23).
In a recent study of genetic variation in patients with respiratory infection with nontuberculous mycobacteria and bronchiectasis, along with some family members without these organisms, researchers reported results that are congruent with our hypothesis that genetic variants in heritable connective tissue disorders contribute to the development of bronchiectasis (49). Specifically, these 87 individuals (with and without mycobacteria) were exome sequenced, and data were filtered to include only genetic variants that had a population frequency less than 2% and the ability to affect protein structure. Within the 24 “connective tissue” genes surveyed, 99 pulmonary nontuberculous mycobacteria–associated variants passed the filters. As compared with the 1000 Genomes Project, family members with and without nontuberculous mycobacteria infection were found to have more low-frequency, protein-affecting variants in these genes, of which 21 would be considered “heritable connective tissue disorder” genes or in TGF-β–related pathways.
These findings of an increased burden of genetic variants in the heritable connective tissue disorder genes in patients with bronchiectasis and nontuberculous mycobacterial infection, as well as in family members without infection (but 78% of whom had bronchiectasis, scoliosis, pectus excavatum, or joint hypermobility), are congruent with our findings of increased dural sac diameter in patients with idiopathic bronchiectasis with associated phenotypes (including long fingers, aortic root diameter, and scoliosis) and the presence of pulmonary mycobacterial infection. A similar overlap of the features of heritable connective tissue disorders among family members of patients with mycobacteria was reported in an earlier publication (5). Therefore, it is not surprising that we found a trend toward increased prevalence of bronchiectasis among family members of patients with idiopathic bronchiectasis, as they are likely to share similar genetic variants in heritable connective tissue disorder genes. The lack of statistical significance is likely due to limited medical history and diagnostic imaging availability for earlier generations in an older patient population.
Limitations
Our present study has several limitations that should be recognized. As expected on the basis of prior publications, our population of patients with idiopathic bronchiectasis included a relatively small number of men with idiopathic bronchiectasis, and the preponderance of women with idiopathic bronchiectasis largely drove our results. Therefore, our findings may not fully reflect these associations in men with bronchiectasis. The prevalence of mitral valve prolapse in the idiopathic bronchiectasis group could be overestimated because we used the number of echocardiograms, rather than the total number of individuals, as the denominator. Another limitation is that we lacked hypermobility data and some morphometric data (e.g., wrist and thumb sign, mitral valve prolapse) for control subjects. We compared the prevalence of these findings with population estimates when possible, but the prevalence of these findings in our control population might differ from published population estimates.
Implications
In the future, we will extend assessment of the dural sac by lumbar MR imaging to chest CT of the lower thoracic and upper lumbar spine to determine if dural sac enlargement in patients with idiopathic bronchiectasis can be detected by chest CT and whether they correlate with lumbar dural sac measurements by MR imaging. If true, then all patients with idiopathic bronchiectasis can be characterized for dural sac size using already available chest CT scans. This “biomarker” might also identify individuals who present with recurrent airway infections who are at risk for developing progressive bronchiectasis as well as older patients with bronchiectasis who are at higher risk for pulmonary environmental mycobacterial infections.
This concept extends to pertinent family members of patients with idiopathic bronchiectasis who may warrant closer monitoring if they also have dural sac enlargement. We are also performing whole-exome sequencing of a large cohort of patients with idiopathic bronchiectasis and will analyze them for an association between an increased burden of genetic variants in candidate genes associated with the pathophysiology of heritable connective tissue disorders and dural ectasia.
Conclusions
The addition of dural sac size as a phenotypic marker of genetic variation in heritable connective tissue disorder genes in patients with idiopathic bronchiectasis adds further specificity to the phenotypic characterization of patients with idiopathic bronchiectasis and nontuberculous mycobacteria. Characterizing and grouping patients with idiopathic bronchiectasis by heritable connective tissue disorder features, including dural sac size, improves the possibility of detecting genetic variants in these genes and pathways, leading to an improved understanding of the pathogenesis of bronchiectasis. Importantly, the characterization of patients with idiopathic bronchiectasis by dural sac diameter also opens the possibility of developing potential therapies designed to slow or prevent the development of bronchiectasis, such as angiotensin receptor blockers and statins, which have been shown to decrease aortic root dilation in patients with Marfan syndrome (50).
Acknowledgments
Acknowledgment
The authors thank Kelli Sullivan, James Coxe, Veronica Moore, Alison Williams, Katie Paul, Niel Andrews, Kathryn Saba, and Shanah Kirk for study coordination; Jasper Becker for data assistance; and Elizabeth Godwin for administrative support. The authors are indebted to all of the referring physicians and their patients who participated in this study.
Footnotes
This study was supported by a University of North Carolina Clinical and Translational Science Award pilot grant (UL1TR000083), a National Institute of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) fellowship award T32 research training program grant (T32HL007106), and a grant from the Office of Rare Diseases Research (U54HL096458). The Genetic Disorders of Mucociliary Clearance Consortium (U54HL096458) is part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). The RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and NHLBI.
Author Contributions: Study conception: M.L.A.D., K.R.B., and M.R.K.; data acquisition, analysis, and interpretation: M.L.A.D., J.R.L., M.V.P., K.R.B., and M.R.K.; drafting of the manuscript: M.L.A.D., P.G.N., and M.R.K. All authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, Strand MJ, Bai X, Ramamoorthy P, Rothman MS, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187:197–205. doi: 10.1164/rccm.201206-1035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med. 2010;7:5–18. doi: 10.1016/j.genm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 5.Leung JM, Fowler C, Smith C, Adjemian J, Frein C, Claypool RJ, Holland SM, Prevots RD, Olivier K. A familial syndrome of pulmonary nontuberculous mycobacteria infections. Am J Respir Crit Care Med. 2013;188:1373–1376. doi: 10.1164/rccm.201306-1059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyhdalo K, Farver C. Pulmonary histologic changes in Marfan syndrome: a case series and literature review. Am J Clin Pathol. 2011;136:857–863. doi: 10.1309/AJCP79SNDHGKQFIN. [DOI] [PubMed] [Google Scholar]
- 7.Morgan AW, Pearson SB, Davies S, Gooi HC, Bird HA. Asthma and airways collapse in two heritable disorders of connective tissue. Ann Rheum Dis. 2007;66:1369–1373. doi: 10.1136/ard.2006.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nerlich AG, Stöss H, Lehmann H, Krieg T, Müller PK. Pathomorphological and biochemical alterations in Ehlers-Danlos-syndrome type IV. Pathol Res Pract. 1994;190:697–707. doi: 10.1016/S0344-0338(11)80749-1. [DOI] [PubMed] [Google Scholar]
- 9.Burchett ME, Ling IF, Estus S. FBN1 isoform expression varies in a tissue and development-specific fashion. Biochem Biophys Res Commun. 2011;411:323–328. doi: 10.1016/j.bbrc.2011.06.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rand-Hendriksen S, Lundby R, Tjeldhorn L, Andersen K, Offstad J, Semb SO, Smith HJ, Paus B, Geiran O. Prevalence data on all Ghent features in a cross-sectional study of 87 adults with proven Marfan syndrome. Eur J Hum Genet. 2009;17:1222–1230. doi: 10.1038/ejhg.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grahame R, Pyeritz RE. The Marfan syndrome: joint and skin manifestations are prevalent and correlated. Br J Rheumatol. 1995;34:126–131. doi: 10.1093/rheumatology/34.2.126. [DOI] [PubMed] [Google Scholar]
- 12.Brown OR, DeMots H, Kloster FE, Roberts A, Menashe VD, Beals RK. Aortic root dilatation and mitral valve prolapse in Marfan’s syndrome: an ECHOCARDIOgraphic study. Circulation. 1975;52:651–657. doi: 10.1161/01.cir.52.4.651. [DOI] [PubMed] [Google Scholar]
- 13.Van Laer L, Dietz H, Loeys B. Loeys-Dietz syndrome. Adv Exp Med Biol. 2014;802:95–105. doi: 10.1007/978-94-007-7893-1_7. [DOI] [PubMed] [Google Scholar]
- 14.Judge DP, Rouf R, Habashi J, Dietz HC. Mitral valve disease in Marfan syndrome and related disorders. J Cardiovasc Transl Res. 2011;4:741–747. doi: 10.1007/s12265-011-9314-y. [DOI] [PubMed] [Google Scholar]
- 15.Jones KL, Jones MC, del Campo M. Smith’s recognizable patterns of human malformation. 7th ed. Philadelphia, PA: Elsevier Saunders; 2013. Connective tissue disorders; pp. 612–645. [Google Scholar]
- 16.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet. 1998;77:31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Atzinger CL, Meyer RA, Khoury PR, Gao Z, Tinkle BT. Cross-sectional and longitudinal assessment of aortic root dilation and valvular anomalies in hypermobile and classic Ehlers-Danlos syndrome. J Pediatr. 2011;158:826–830.e1. doi: 10.1016/j.jpeds.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Camerota F, Castori M, Celletti C, Colotto M, Amato S, Colella A, Curione M, Danese C. Heart rate, conduction and ultrasound abnormalities in adults with joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. Clin Rheumatol. 2014;33:981–987. doi: 10.1007/s10067-014-2618-y. [DOI] [PubMed] [Google Scholar]
- 19.Ayres JG, Pope FM, Reidy JF, Clark TJ. Abnormalities of the lungs and thoracic cage in the Ehlers-Danlos syndrome. Thorax. 1985;40:300–305. doi: 10.1136/thx.40.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson ML, Olivier KN, Holland SM. Pulmonary non-tuberculous mycobacterial infection in congenital contractural arachnodactyly. Int J Tuberc Lung Dis. 2012;16:561–563. doi: 10.5588/ijtld.11.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang HS, Yi CA, Yoo H, Yang JH, Kim DK, Koh WJ. The prevalence of bronchiectasis in patients with Marfan syndrome. Int J Tuberc Lung Dis. 2014;18:995–997. doi: 10.5588/ijtld.13.0763. [DOI] [PubMed] [Google Scholar]
- 22.Leung JM, Olivier KN, Prevots DR, McDonnell NB. Beyond Marfan: the clinical impact of bronchiectasis and non-tuberculous mycobacteria in connective tissue diseases. Int J Tuberc Lung Dis. 2015;19:1409. doi: 10.5588/ijtld.15.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyeritz RE. Connective tissue in the lung: lessons from the Marfan syndrome. Ann Intern Med. 1985;103:289–290. doi: 10.7326/0003-4819-103-2-289. [DOI] [PubMed] [Google Scholar]
- 24.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 25.Pyeritz RE, Fishman EK, Bernhardt BA, Siegelman SS. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet. 1988;43:726–732. [PMC free article] [PubMed] [Google Scholar]
- 26.Kono AK, Higashi M, Morisaki H, Morisaki T, Naito H, Sugimura K. Prevalence of dural ectasia in Loeys-Dietz syndrome: comparison with Marfan syndrome and normal controls. PLoS One. 2013;8:e75264. doi: 10.1371/journal.pone.0075264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikhzadeh S, Brockstaedt L, Habermann CR, Sondermann C, Bannas P, Mir TS, Staebler A, Seidel H, Keyser B, Arslan-Kirchner M, et al. Dural ectasia in Loeys-Dietz syndrome: comprehensive study of 30 patients with a TGFBR1 or TGFBR2 mutation. Clin Genet. 2014;86:545–551. doi: 10.1111/cge.12308. [DOI] [PubMed] [Google Scholar]
- 28.Söylen B, Singh KK, Abuzainin A, Rommel K, Becker H, Arslan-Kirchner M, Schmidtke J. Prevalence of dural ectasia in 63 gene-mutation-positive patients with features of Marfan syndrome type 1 and Loeys-Dietz syndrome and report of 22 novel FBN1 mutations. Clin Genet. 2009;75:265–270. doi: 10.1111/j.1399-0004.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 29.Isono M, Hori S, Konishi Y, Kinjo H, Kakisako K, Hirose R, Yoshida T. Ehlers-Danlos syndrome associated with multiple spinal meningeal cysts—case report. Neurol Med Chir (Tokyo) 1999;39:380–383. doi: 10.2176/nmc.39.380. [DOI] [PubMed] [Google Scholar]
- 30.Reinhardt R. Scalloping at the lumbosacral canal [in German] Rontgenblatter. 1987;40:229–231. [PubMed] [Google Scholar]
- 31.Brunk I, Stöver B, Ikonomidou C, Brinckmann J, Neumann LM. Ehlers-Danlos syndrome type VI with cystic malformations of the meninges in a 7-year-old girl. Eur J Pediatr. 2004;163:214–217. doi: 10.1007/s00431-004-1407-z. [DOI] [PubMed] [Google Scholar]
- 32.Ahn NU, Sponseller PD, Ahn UM, Nallamshetty L, Rose PS, Buchowski JM, Garrett ES, Kuszyk BS, Fishman EK, Zinreich SJ. Dural ectasia in the Marfan syndrome: MR and CT findings and criteria. Genet Med. 2000;2:173–179. doi: 10.1097/00125817-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Soulen RL, Fishman EK, Pyeritz RE, Zerhouni EA, Pessar ML. Marfan syndrome: evaluation with MR imaging versus CT. Radiology. 1987;165:697–701. doi: 10.1148/radiology.165.3.3685348. [DOI] [PubMed] [Google Scholar]
- 34.Daniels ML, Lowe JR, Roy P, Patrone MV, Conyers JM, Fine JP, Knowles MR, Birchard KR. Standardization and validation of a novel and simple method to assess lumbar dural sac size. Clin Radiol. 2015;70:146–152. doi: 10.1016/j.crad.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniels MLA, Conyers JM, Lowe J, Birchard K, Knowles MR. Idiopathic bronchiectasis and connective tissue fibrillinopathies: dural ectasia as a marker of a distinct bronchiectasis subgroup [abstract] Am J Respir Crit Care Med. 2013;187:A3516. [Google Scholar]
- 36.St Peter SD, Juang D, Garey CL, Laituri CA, Ostlie DJ, Sharp RJ, Snyder CL. A novel measure for pectus excavatum: the correction index. J Pediatr Surg. 2011;46:2270–2273. doi: 10.1016/j.jpedsurg.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Kittleson AC, Lim LW. Measurement of scoliosis. Am J Roentgenol Radium Ther Nucl Med. 1970;108:775–777. doi: 10.2214/ajr.108.4.775. [DOI] [PubMed] [Google Scholar]
- 38.Sponseller PD, Erkula G, Skolasky RL, Venuti KD, Dietz HC., III Improving clinical recognition of Marfan syndrome. J Bone Joint Surg Am. 2010;92:1868–1875. doi: 10.2106/JBJS.I.00892. [DOI] [PubMed] [Google Scholar]
- 39.Hall JG, Allanson JE, Gripp KW, Slavotinek AM. Handbook of physical measurements. 2nd ed. New York; Oxford University Press; 2007. Chapter 7. Craniofacies; pp. 172–173. [Google Scholar]
- 40.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27:1777–1779. [PubMed] [Google Scholar]
- 41.Hakim AJ, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract. 2003;57:163–166. [PubMed] [Google Scholar]
- 42.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 43.Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J. 2010;36:12–19. doi: 10.1183/09031936.00143209. [DOI] [PubMed] [Google Scholar]
- 44.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 45.Fikree A, Aziz Q, Grahame R. Joint hypermobility syndrome. Rheum Dis Clin North Am. 2013;39:419–430. doi: 10.1016/j.rdc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Robbesom AA, Koenders MM, Smits NC, Hafmans T, Versteeg EM, Bulten J, Veerkamp JH, Dekhuijzen PN, van Kuppevelt TH. Aberrant fibrillin-1 expression in early emphysematous human lung: a proposed predisposition for emphysema. Mod Pathol. 2008;21:297–307. doi: 10.1038/modpathol.3801004. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007;17:252–258. doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 49.Szymanski EP, Leung JM, Fowler CJ, Haney C, Hsu AP, Chen F, Duggal P, Oler AJ, McCormack R, Podack E, et al. Pulmonary nontuberculous mycobacterial infection: a multisystem, multigenic disease. Am J Respir Crit Care Med. 2015;192:618–628. doi: 10.1164/rccm.201502-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attenhofer Jost CH, Greutmann M, Connolly HM, Weber R, Rohrbach M, Oxenius A, Kretschmar O, Luscher TF, Matyas G. Medical treatment of aortic aneurysms in Marfan syndrome and other heritable conditions. Curr Cardiol Rev. 2014;10:161–171. doi: 10.2174/1573403X1002140506124902. [DOI] [PMC free article] [PubMed] [Google Scholar]