Abstract
Rationale: Members of racial or ethnic minorities make up an appreciable proportion of patients with cystic fibrosis (CF) and have worse outcomes than non-Latino white individuals. Between 1,999 and 2014, the CF Foundation Patient Registry reported an increase in minorities from 5 to 8.2% for Latinos, from 3 to 4.6% for black individuals and from 1.4 to 3.1% for “Other.”
Objectives: To evaluate the representation of racial and ethnic minorities in pharmacology clinical trials for CF.
Methods: We analyzed pharmacology clinical trials in CF published between 1999 and 2015 by searching PubMed and published study reference lists for qualifying study reports. We examined whether the race and ethnicity of study subjects were reported and, if so, what percentage of subjects represented major minority groups.
Measurements and Main Results: Among 147 pharmacology clinical trials, only 19.7% reported the race or ethnicity of study subjects. Latinos were verified as included in 7.5% of clinical trials, black individuals in 6.8%, and Asians in 2.0%. Inclusion of subjects described as “Other race” was reported in 7.5% of trials. In 29 clinical trials that reported race and ethnicity, the percentage of minorities included as subjects was 2.0% for Latinos, 1.0% for black individuals, and 0.1% for Asians.
Conclusions: Although CF disproportionately affects non-Latino white individuals, members of other racial or ethnic groups are proportionally underrepresented in CF pharmacology clinical trials. Inadequate inclusion of minorities and failure to report the racial or ethnic background of study subjects limits information about factors influencing drug response and may contribute to health disparities for minorities with CF.
Keywords: cystic fibrosis, minority groups, ethnic groups, clinical trials
There have been great advancements in reducing morbidity and mortality in cystic fibrosis (CF) over the past 4 decades. In 1988, only 37% of 18-year-olds with CF had normal lung function. By 2014, the fraction of 18-year-olds with normal lung function had increased to 72% (1). Median predicted survival improved from 14 years in the 1960s to 32 years in 2000 and to 39.3 years in 2014 (1, 2). Improvements in morbidity and mortality are attributable, at least in part, to the development and approval of new pharmaceutical agents targeting pulmonary infections, airway inflammation, and the cystic fibrosis transmembrane conductance regulator (CFTR) abnormality in CF.
Minorities represent an appreciable and increasing proportion of patients with CF included in North American national disease registries. Over the past 15 years, the proportion of patients with CF included in the U.S. CF Foundation Patient Registry who self-identified as black increased from 3.8 to 4.6% (1). Over the past 20 years, the fraction of Latinos doubled in the US registry. In 2014, 8.2% of patients were Latino (1) and in California, more than one-third of babies diagnosed with CF were Latino (3). The fraction of black patients with CF enrolled in the Canadian CF Registry increased from 0.6 to 0.8% between 1998 and 2013. During those same years, Asians increased in the Canadian CF registry from 0.4 to 0.7%. Latino ethnicity was not reported (20). The proportion and composition of minorities represented are not well documented for populations of patients with CF in other countries. The 2014 annual reports of the European CF Society Patient Registry and the Australian CF Data Registry did not report patient race or ethnicity at all (21, 22).
Members of common minority groups tend to suffer worse health outcomes. Black individuals with CF have lower lung function than do white individuals (7). In the U.S. registry, Latinos with CF are 85% more likely to die annually compared with non-Latino white individuals, even though they are less likely to have pancreatic insufficiency (4). Only 75% of Latinos survived 18 years after diagnosis, compared with >90% of non-Latino white individuals, despite no difference in age at the time of diagnosis (5). In contrast, Latinos without CF have a lower overall mortality rate than do non-Latino white individuals (6). These health disparities are not unique to CF. In many areas of medicine, minorities suffer a greater burden from their disease and receive lower-quality healthcare (8).
For many drugs, there are known racial and ethnic differences in therapeutic responses, drug metabolism, and adverse effects (9–11). One-fifth of new drugs approved between 2008 and 2013 for use in the United States were known to have significant racial and ethnic differences (12). In addition, participating in clinical trials may have direct benefits to study subjects, because clinical trial participants often have improved outcomes regardless of treatment allocation (13).
In response to these concerns, Congress and the U.S. National Institutes of Health (NIH) prioritized the inclusion of minority subjects in clinical research with the NIH Revitalization Act (14). Despite the NIH prioritization of minority inclusion, there continue to be racial and ethnic disparities in trial participation (15–18).
Inclusion of minority patients in CF pharmacology clinical trials may be important to understanding differences in clinical responses to drugs. Accordingly, we undertook this study to investigate the reporting of minority subjects and the inclusion of minorities in pharmacology clinical trials for the treatment of CF reported over the past 15 years.
Some of the results of this study have been reported previously in the form of an abstract (19).
Methods
We performed a cross-sectional analysis of all pharmacology clinical trials targeting pulmonary manifestations of CF that were published from 1999 to 2015. We searched PubMed for terms relating to drug therapies for patients with CF, including terms derived from the Cystic Fibrosis Foundation therapeutic pipeline. Initial terms were “cystic fibrosis,” “tobramycin,” “azithromycin,” “gentamicin,” “ibuprofen,” “ataluren,” “PTC124,” “denufosol,” “l-arginine,” “CPX,” “ivacaftor,” “VX-770,” “lumacaftor,” “VX-809,” “CFTR gene therapy,” “tgAAVCF,” “AAV-CFTR,” “clarithromycin,” “aztreonam,” “colisitin,” “hypertonic saline,” “hyaluronic acid,” “pulmozyme,” “rhDNase,” “dornase alfa,” “mannitol,” and “corticosteroid.” In addition, we searched through the reference lists of each clinical trial to identify other relevant articles. We identified additional search terms during this process and searched them as well.
Data extracted from published pharmacology clinical trial reports included whether race and ethnicity of subjects were reported, the percentage of minorities included as study subjects if reported, the source of funding (industry vs. nonindustry), the publication year, the location of the clinical trial (sites in the United States vs. studies performed exclusively in other countries), the size of the clinical trial, and the drug(s) investigated. We translated articles written in a language other than English using an electronic translation program. All phases of drug trials were included. We excluded case reports, Cochrane reviews, cell studies, biomarker studies, and drug delivery studies. Clinical trials published in abstract form only were excluded.
For categorical and continuous variables, we used the chi-square and the Student t test, respectively. Stata 14.2 (StataCorp, College Town, TX) was used for all data analysis.
Results
Overall Minority Inclusion
Between 1999 and 2015, 147 pharmacology clinical trials tested potential treatments for CF. Among these trials, 19.7% (29 of 147) reported the race and/or ethnicity of subjects. Latino subjects were reported as included in 7.5% of clinical trials (11of 147) and black subjects were reported in 6.8% of clinical trials (10 of 147). Only three clinical trials reported Asian subjects (2.0%). In 7.5% of trials (11 of 147), an “other” race/ethnicity category was reported.
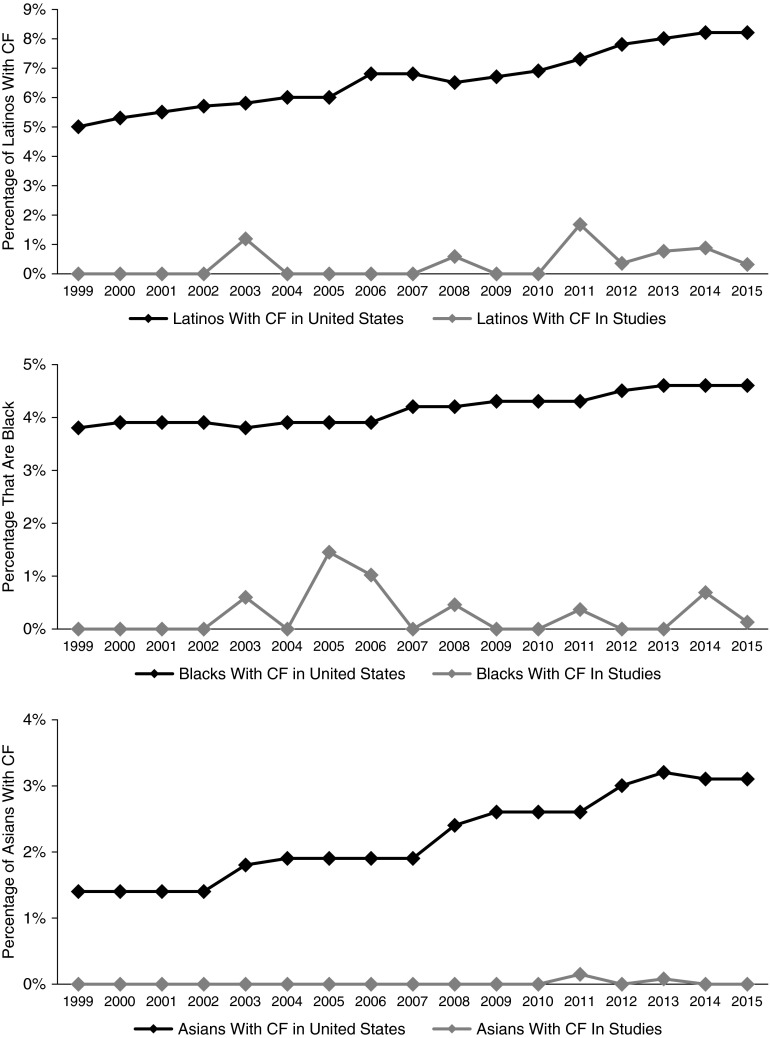
When race and/or ethnicity were reported, the majority of subjects were white (94.4%). Of the 29 clinical trials that reported race and/or ethnicity, 24.1% reported having only white subjects and including no minorities. Only 15.0% of the clinical trials both reported race and/or ethnicity and included minority subjects. In the clinical trials that reported race and/or ethnicity, the percentage of minorities included was low, with 2.0% Latinos, 1.0% black individuals, and 0.1% Asians. Figure 1 shows the overall percentage of Latinos, black individuals, and Asians included in CF clinical trials by year compared with the percentage of the same minority groups listed in the U.S. CF Foundation Patient Registry.
Figure 1.
Percentage of Latinos, blacks, and Asians included in cystic fibrosis (CF) pharmacology clinical trials by year compared with percentages for patients enrolled in the U.S. Cystic Fibrosis Foundation Patient Registry.
Minority Inclusion by Drug Class
Reporting of minority subject inclusion varied greatly by drug or type of drug. Aztreonam trials were the most likely to report the inclusion of minorities; 50% reported race and/or ethnicity, 12.5% reported including Latinos, 25% reported including black individuals, and none reported including Asians. In tobramycin clinical trials, one-third reported race and/or ethnicity, 14.3% reported including Latinos, 21.4% reported including black individuals, and 10.7% reported including Asians. In CFTR modulator clinical trials, 29.2% reported race and/or ethnicity, 12.5% reported including Latinos, 4.2% reported including black individuals, and none reported including Asians. No clinical trials of azithromycin, ataluren, or dornase alfa reported subject race and/or ethnicity.
Minority Inclusion by Clinical Trial Size
Clinical trials with >50 subjects were more likely to report race and/or ethnicity than were those with ≤50 subjects (28.0% vs. 11.1%, P = 0.01) Larger studies were also more likely to include Latino subjects (13.3% vs. 1.4%, P = 0.006). No Asians were included in clinical trials with 50 or fewer subjects. There was no difference in the inclusion of black individuals (2.8% vs. 10.7%, P = 0.06). There was no difference in the percentage of minority subjects included by clinical trial size among trials reporting race and/or ethnicity.
Minority Inclusion by Clinical Trial Location and Funding Source
Inclusion of minority subjects differed between clinical trials conducted exclusively in the United States and those conducted exclusively in other countries. Only one clinical trial conducted exclusively outside the United States reported race, and none reported ethnicity. There was no difference in reporting of race and/or ethnicity in clinical trials supported by industry vs. those supported by nonindustry sources (23.8% vs. 14.9%, P = 0.1). In addition, there was no difference in the number of clinical trials that reported including Latinos, black individuals, Asians, or “other” by funding source, nor was there a difference in the percentage of minority subjects included if race and/or ethnicity were reported.
Discussion
We found that published reports of only 19.7% of pharmacology clinical trials targeting pulmonary manifestations of CF included any description of the race and ethnicity of the study subjects. When race and/or ethnicity were reported, 94.4% of subjects were non-Latino white. Of the 29 clinical trials that reported race and/or ethnicity, 24.1% trials reported having only non-Latino white subjects. Even when racial and ethnic minorities were included in clinical trials, they were included at a much lower rate than in the general U.S. CF Foundation Patient Registry population.
Clinical trials that had >50 subjects or that were conducted exclusively in the United States were more likely to report the inclusion of minority subjects. Inclusion of racial and ethnic minorities also varied by type of drug tested. Studies of some drugs, including azithromycin, ataluren, and dornase alfa, did not report including any minorities.
There are risks to extrapolating trial results to populations not studied in clinical trials (23). In other diseases, some drugs that are effective in non-Latino white individuals are ineffective or dangerous for individuals who identify in other racial or ethnic groups (10, 24–27). By not including minorities in clinical trials, we are potentially losing important information about CF lung disease, especially because members of minority groups tend to have more severe CF lung disease.
Underrepresentation of racial and ethnic minorities in clinical research studies is not unique to the field of CF. It has been reported across many areas of medicine, including cardiovascular diseases (16), oncology (15, 17), and other pulmonary diseases (18). The NIH Revitalization Act in 1993 brought racial and ethnic minority inclusion in clinical research to national attention by requiring the inclusion of minorities as a criterion for NIH funding. The NIH Revitalization Act limited explicit exclusion of minorities but has done little to increase minority participation in clinical trials (15, 28).
To reduce the risk of increasing health disparities, efforts should be made to provide access to and inclusion in clinical research for all people with CF, including minorities. Subject interest in clinical trials is not the cause of this disparity, because minorities are just as willing to participate in clinical trials as are non-Latino white individuals (29).
Pharmaceutical companies and investigators should not only report the race and ethnicity of subjects, but also prioritize the inclusion of minority subjects in therapeutic studies of CF. Investigators should consciously design and conduct clinical trials in a manner that maximizes the participation of minorities to whom new therapies may be prescribed. Study questionnaires should be translated into Spanish or other appropriate languages and should be administered by native-speaking interpreters or study staff. Although stratified randomization or other trial designs to assess subgroup drug responses may not be feasible when the majority of patients with CF are non-Latino white, it is important to include populations that reflect the overall composition of patients with CF (30–33). Journal editors and manuscript reviewers should require the reporting the race and ethnicity of subjects in CF pharmacology trials, even if all subjects are non-Latino white.
Conclusions
Minority patients with CF are underrepresented in CF pharmacology clinical trials. By ignoring the racial and ethnic background of study subjects or by inadequately including minorities, we are potentially losing important information about determinants of drug responses, factors contributing to clinical manifestations of CF, and possible health disparities for minorities with CF. Pharmaceutical companies and investigators should endeavor to enroll members of minority groups, not just to represent the baseline CF population, but also to detect differences in drug response.
Footnotes
Supported by grant NIGMS 5T32GM007546-35 (M.E.M.) and by grant UL1TR001422 (S.A.M.).
The views expressed in this article do not communicate an official position of the authors’ institutions or the National Institutes of Health.
Author Contributions: M.E.M. designed the study, acquired and analyzed the data, drafted the manuscript, and approved the final manuscript. S.A.M. designed the study, drafted the manuscript, and approved the final manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cystic Fibrosis Foundation Patient Registry. 2014 Annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2015. [Google Scholar]
- 2.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Health CDoP Newborn Screening News Newborn Screening NewsVol 152007
- 4.O’Connor GT, Quinton HB, Kahn R, Robichaud P, Maddock J, Lever T, Detzer M, Brooks JG Northern New England Cystic Fibrosis Consortium. Case-mix adjustment for evaluation of mortality in cystic fibrosis. Pediatr Pulmonol. 2002;33:99–105. doi: 10.1002/ppul.10042. [DOI] [PubMed] [Google Scholar]
- 5.Buu MC, Sanders LM, Mayo J, Milla CE, Wise PH. Assessing differences in mortality rates and risk factors between Hispanic and non-Hispanic patients with cystic fibrosis in California. Chest. 2015;149:380–389. doi: 10.1378/chest.14-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias E.United States life tables, 2010. National vital statistics reports; vol 63 no 7. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 7.Hamosh A, FitzSimmons SC, Macek M, Jr, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132:255–259. doi: 10.1016/s0022-3476(98)70441-x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson AR, Smedley BD, Stith AY. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 9.Mega JL, Close SL, Wiviott SD, Man M, Duvvuru S, Walker JR, Sundseth SS, Collet JP, Delaney JT, Hulot JS, et al. PON1 Q192R genetic variant and response to clopidogrel and prasugrel: pharmacokinetics, pharmacodynamics, and a meta-analysis of clinical outcomes. J Thromb Thrombolysis. 2016;41:374–383. doi: 10.1007/s11239-015-1264-9. [DOI] [PubMed] [Google Scholar]
- 10.Carson P, Ziesche S, Johnson G, Cohn JN Vasodilator-Heart Failure Trial Study Group. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5:178–187. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, Cohen M, Dehovitz JA, Sharp GB, Gange SJ, et al. Women’s Interagency HIV Study. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis. 2012;206:1453–1461. doi: 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97:263–273. doi: 10.1002/cpt.61. [DOI] [PubMed] [Google Scholar]
- 13.Baker JR, Vandal AC, Yeoh J, Zeng I, Wong S, Ryan SN. Clinical trial participation improves outcome: a matched historical cohort study. Clin Trials. 2013;10:735–743. doi: 10.1177/1740774513496915. [DOI] [PubMed] [Google Scholar]
- 14.United States Congress. National Institutes of Health Revitalization Act of 1993: Act to Amend the Public Health Service Act to Revise and Extend the Programs of the National Institutes of Health, and for Other Purposes. Public Law 103-43. Washington, DC: US Congress; June 20, 1993. [Google Scholar]
- 15.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 16.Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174:1868–1870. doi: 10.1001/jamainternmed.2014.4758. [DOI] [PubMed] [Google Scholar]
- 17.Tejeda HA, Green SB, Trimble EL, Ford L, High JL, Ungerleider RS, Friedman MA, Brawley OW. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 18.Burchard EG, Oh SS, Foreman MG, Celedón JC. Moving toward true inclusion of racial/ethnic minorities in federally funded studies. A key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med. 2015;191:514–521. doi: 10.1164/rccm.201410-1944PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarry M, McColley S. Minorities with cystic fibrosis are underrepresented in pharmacology clinical trials [abstract] Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.201603-192BC. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Canadian Cystic Fibrosis Registry. 2013 Annual report. Toronto, ON: Cystic Fibrosis Canada; 2014. [Google Scholar]
- 21.Australian Cystic Fibrosis Data Registry. 16th Annual report. Baulkham Hills, NSW, Australia: Cystic Fibrosis Australia; 2014. [Google Scholar]
- 22.European Cystic Fibrosis Society. European Cystic Fibrosis Society Patient Registry annual report, 2013. Karup, Denmark: European Cystic Fibrosis Society; 2014. [Google Scholar]
- 23.Gross CP, Steiner CA, Bass EB, Powe NR. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA. 2000;284:2886–2893. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]
- 24.Mega JL, Simon T, Collet J-P, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Burroughs VJ, Maxey RW, Levy RA. Racial and ethnic differences in response to medicines: towards individualized pharmaceutical treatment. J Natl Med Assoc. 2002;94:1–26. [PMC free article] [PubMed] [Google Scholar]
- 27.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med. 2001;344:1351–1357. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- 28.Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, de Bruin DM, Greenblatt RM, Bibbins-Domingo K, Wu AH, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918. doi: 10.1371/journal.pmed.1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugarman EA, Rohlfs EM, Silverman LM, Allitto BA. CFTR mutation distribution among U.S. Hispanic and African American individuals: evaluation in cystic fibrosis patient and carrier screening populations. Genet Med. 2004;6:392–399. doi: 10.1097/01.gim.0000139503.22088.66. [DOI] [PubMed] [Google Scholar]
- 31.Schrijver I, Ramalingam S, Sankaran R, Swanson S, Dunlop CL, Keiles S, Moss RB, Oehlert J, Gardner P, Wassman ER, et al. Diagnostic testing by CFTR gene mutation analysis in a large group of Hispanics: novel mutations and assessment of a population-specific mutation spectrum. J Mol Diagn. 2005;7:289–299. doi: 10.1016/S1525-1578(10)60557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alper ÖM, Wong L-JC, Young S, Pearl M, Graham S, Sherwin J, Nussbaum E, Nielson D, Platzker A, Davies Z, et al. Identification of novel and rare mutations in California Hispanic and African American cystic fibrosis patients. Hum Mutat. 2004;24:353. doi: 10.1002/humu.9281. [DOI] [PubMed] [Google Scholar]
- 33.Watts KD, Seshadri R, Sullivan C, McColley SA. Increased prevalence of risk factors for morbidity and mortality in the US Hispanic CF population. Pediatr Pulmonol. 2009;44:594–601. doi: 10.1002/ppul.21037. [DOI] [PubMed] [Google Scholar]