In the opening scene of Oscar Wilde’s satirical commentary on late-nineteenth-century societal norms, Lady Windermere refuses to shake hands with Lord Darlington because it would not be proper, as her hands were “wet with roses” (1). This portrayal of fastidious behavior as an analogy for voluntary cough suppression because of societal norms was proposed by Dr. Reich and Dr. Johnson in 1992 to explain the development of a syndrome of lingular and right middle lobe nodular bronchiectasis in women without obvious predisposing pulmonary disease (2).
Although this theatrical eponym has become engrained in the medical vernacular, the analogy is more problematic than just the fact that Wilde used Lady Windermere’s fastidiousness as a mask for flaws in moral character. To begin with, Lady Windermere’s estranged mother (a.k.a. Mrs. Erlynne) would have been a better match for the typical age at presentation. In this month’s issue of AnnalsATS, the study by Daniels and colleagues (pp. 1712–1720) provides yet another piece of an evolving phenotype that further supports a susceptible host model of disease pathogenesis for development of pulmonary disease associated with the ubiquitous environmental mycobacteria (3).
Since Prince and colleagues first described the occurrence of slowly progressive nodular disease predominantly found in older women, there have been multiple reports describing a unique phenotype for many of these patients (4–6). This tall, asthenic body morphotype with a high prevalence of features such as scoliosis, joint hypermobility, chest wall pectus abnormalities, and mitral valve prolapse overlaps considerably with known heritable connective tissue disorders such as Marfan or Beall syndromes.
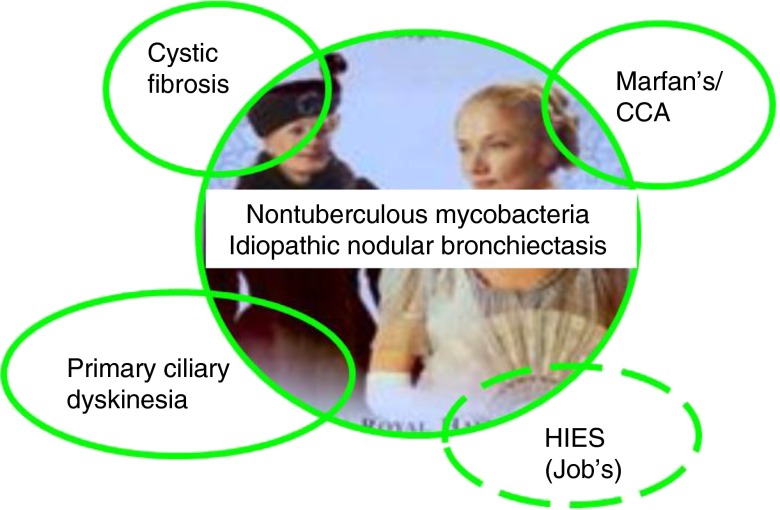
The clustering of these disease manifestations in families has further strengthened the hypothesis of a susceptible host model with potential associated genetic risk variants that may not have protein alterations severe enough to result in the typical gene-associated disease phenotype, but may alter the phenotype enough to predispose to late-onset progressive airway disease and predisposition to nontuberculous mycobacteria (NTM) (7–9). Indeed, there has been considerable overlap in both directions between these patients with “idiopathic” bronchiectasis and NTM and known heritable connective tissue disorders, genetic disorders affecting mucociliary clearance such as cystic fibrosis (CF) and primary ciliary dyskinesia (PCD), and certain primary immune deficiencies such as STAT3 mutated hyper-IgE or Job’s syndrome (Figure 1).
Figure 1.
Overlap with known genetic diseases. Lady Windermere Syndrome, characterized by idiopathic nodular bronchiectasis and nontuberculous mycobacteria, has overlapping features with several known Mendelian transmitted genetic diseases. The inner circle depicts Lady Windermere and Mrs. Erlynne (Lady Windermere’s estranged mother) from the 2002 Theater Royal production (https://www.londontheater.co.uk/reviews/lady-windermeres-fan). Overlapping circles indicate bidirectional relationships, with each of these genetic diseases having an associated high prevalence of nontuberculous mycobacteria and, in turn, Lady Windermere syndrome, having associated genetic or phenotype features from these conditions.
All these genetic disorders have been associated with a high prevalence of NTM lung infection, and genetic or phenotypic features of these disorders have been described in association with idiopathic bronchiectasis or NTM lung infection (10–14). Early studies of these patients noted mono-allelic mutations in CFTR (CF gene) in one-third to one-half of these patients (5, 15). Similarly, we found a significant reduction in ciliary beat frequency in nasal and bronchial epithelial cells obtained from patients with NTM lung infections compared with healthy individuals and CF control subjects (16).
A recent study by our group looked for associated genetic risk loci via whole-exomic sequencing in familial and sporadic cohorts of patients with NTM infection. There was a high prevalence of rare, protein-altering, monoallelic variants in genes associated with each of these 4 areas of overlap, and most patients had variants in more than one gene, and often more than one category of genes (9). In fact, several patients had indels or known deleterious variants in genes such as FBN2, TGFBR1, and COL5A1 associated with congenital contractural arachnodactyly (Beall syndrome), Loeys-Dietz syndrome, and Ehlers Danlos syndrome, respectively.
In the familial cohort, the frequency of variants in heritable connective tissue disorder genes, CFTR, and cilia-related genes was similarly elevated for “affected” and “unaffected” family members, when “affected” was defined as having bronchiectasis and NTM. However, more than 75% of the unaffected family members had one or more of the body morphotype features described here, so they weren’t truly unaffected. Because many of these other individual features, such as joint hypermobility, low body mass index, mitral valve prolapse, pectus abnormalities, and even scoliosis, either affected a relatively small proportion of the patients or were also seen in the background population, it was difficult to correlate them with exomic variants.
Dural ectasia also occurs at a very high frequency in Marfan and Loeys-Dietz syndromes, but has not been described in the general population (17, 18). The defining measurements can be easily obtained from lumbar magnetic resonance imaging scans and are reproducible. In the article by Daniels and coworkers, the average lumbar dural sac diameter in patients with idiopathic bronchiectasis was larger than in both healthy and disease (CF) controls, but not as large as seen in Marfan syndrome. Further, patients with positive respiratory tract cultures for NTM had significantly larger dural sac diameters than patients with bronchiectasis without NTM. A correlation was also seen between increased dural sac diameter and anatomic involvement of bronchiectasis in the anterior regions (right middle lobe and lingula) of the lung, as described in Lady Windermere Syndrome.
In the Wilde play, Lady Windermere is in her early 20s. If she had the syndrome that bears her name, she likely would have had little in the way of associated symptoms or lung findings. One of the limitations of assessing genetic linkage in pedigrees of familial cohorts with idiopathic bronchiectasis and NTM has been the late age of onset of disease and the inability to distinguish family members in younger generations who are likely to develop the disease from those who are not.
If preliminary data from Daniels and coauthors showing a correlation between T10–L1 dural sac diameter assessed by chest computed tomographic (CT) imaging in patients with idiopathic bronchiectasis and the magnetic resonance imaging L1–L5 dural sac diameter is verified in a larger cohort, it may be possible to better segregate family members using readily available bronchiectasis screening CT images in future genetic correlation studies and, as noted, monitor younger family members with dural ectasia for subsequent development of bronchiectasis and chronic NTM infection (19).
In summary, the findings of an association between dural ectasia and patients with idiopathic bronchiectasis and NTM infection provide another important phenotypic link in the pathogenesis of this disease. These findings help better complete the picture of a unique susceptible host with, relative to known genetic diseases such as cystic fibrosis or primary ciliary dyskinesia, delayed onset of respiratory symptoms and detection of disease. Future expanded genotype/phenotype correlative studies that include dural ectasia as part of the phenotype will hopefully have a greater likelihood of elucidating the genetic and biologic pathway origins of this disease.
Footnotes
Supported in part by the Intramural Research Program, National Heart, Lung and Blood Institute, National Institutes of Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wilde O. Lady Windermere’s Fan, Act 1, scene 1. London: Routledge, Chapman, and Hall, 1966.
- 2.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: the Lady Windermere syndrome. Chest. 1992;101:1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- 3.Daniels MLD, Birchard KR, Lowe JR, Patrone MV, Noone PG, Knowles MR. Enlarged dural sac in idiopathic bronchiectasis implicates heritable connective tissue gene variants. Ann Am Thorac Soc. 2016;13:1712–1720. doi: 10.1513/AnnalsATS.201603-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 5.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 7.Colombo RE, Hill SC, Claypool RJ, Holland SM, Olivier KN. Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest. 2010;137:629–634. doi: 10.1378/chest.09-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung JM, Fowler C, Smith C, Adjemian J, Frein C, Claypool RJ, Holland SM, Prevots RD, Olivier K. A familial syndrome of pulmonary nontuberculous mycobacteria infections. Am J Respir Crit Care Med. 2013;188:1373–1376. doi: 10.1164/rccm.201306-1059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szymanski EP, Leung JM, Fowler CJ, Haney C, Hsu AP, Chen F, Duggal P, Oler AJ, McCormack R, Podack E, et al. Pulmonary nontuberculous mycobacterial infection: a multisystem, multigenic disease. Am J Respir Crit Care Med. 2015;192:618–628. doi: 10.1164/rccm.201502-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung JM, Olivier KN, Prevots DR, McDonnell NB. Beyond Marfan: the clinical impact of bronchiectasis and non-tuberculous mycobacteria in connective tissue diseases. Int J Tuberc Lung Dis. 2015;19:1409. doi: 10.5588/ijtld.15.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulson ML, Olivier KN, Holland SM. Pulmonary non-tuberculous mycobacterial infection in congenital contractural arachnodactyly. Int J Tuberc Lung Dis. 2012;16:561–563. doi: 10.5588/ijtld.11.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivier KN, Weber DJ, Wallace RJ, Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 13.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 14.Melia E, Freeman AF, Shea YR, Hsu AP, Holland SM, Olivier KN. Pulmonary nontuberculous mycobacterial infections in hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:617–618. doi: 10.1016/j.jaci.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 16.Fowler CJ, Olivier KN, Leung JM, Smith CC, Huth AG, Root H, Kuhns DB, Logun C, Zelazny A, Frein CA, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med. 2013;187:1374–1381. doi: 10.1164/rccm.201212-2197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyeritz RE, Fishman EK, Bernhardt BA, Siegelman SS. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet. 1988;43:726–732. [PMC free article] [PubMed] [Google Scholar]
- 18.Kono AK, Higashi M, Morisaki H, Morisaki T, Naito H, Sugimura K. Prevalence of dural ectasia in Loeys-Dietz syndrome: comparison with Marfan syndrome and normal controls. PLoS One. 2013;8:e75264. doi: 10.1371/journal.pone.0075264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels MLA, Patrone MV, Conyers JM, Lowe J, Richard KEs, Birchard K, Knowles MR. Dural ectasia on lumbosacral MRI and chest CT in idiopathic bronchiectasis patients: phenotypic overlap with hereditable connective tissue disorders [abstract] Am J Respir Crit Care Med. 2014;189:A5108. [Google Scholar]