Abstract
Objective
To analyze the effect of age on the ECG QT interval, an important predictor of cardiovascular mortality and drug-induced cardiac arrhythmias, and determine whether QT-heart rate correction formulae (QTc) have differential relationships with age and sex.
Methods
Data were examined from the US National Health and Nutrition Examination Survey (NHANES) II and III, civilian population aged 25 to 90 years. QT weighted means and standard deviations were calculated for all ages. The QTc were evaluated for six QTc: proposed by Bazett (QTcBZT), Fridericia (QTcFRD), Hodges (QTcHDG), Dmitrienko (QTcDMT), Rautaharju (QTcRTHa) and Framingham (QTcFRM).
Results
QTc was strongly related to age and gender, for all formulae except for QTcBZT for women. The relationship between QTc and age was significant regardless of whether the relationship was approximated by a linear or non-linear (quadratic or cubic spline) model. QTc increased more dramatically with age in men. There was a significant (P < 0.001) positive relationship between QTc variance and age for each QTc formula for both men and women. There were a greater proportion of individuals with longer QTc with older ages especially age 80 years and above.
Conclusion
QTc and its variance increase with age. Prolonged QTc is more prevalent in older individuals, especially men.
Keywords: Aging, Clinical epidemiology, QT-heart rate adjustment, QT interval
1. Introduction
Measurement of the QT interval on standard ECG is in widespread clinical use because of its established value in identifying a range of clinical conditions from electrolyte abnormalities to drug-induced cardiac toxicity to inherited channelopathies.[1]–[3] Notwithstanding the recognized issues with regards to QT measurement and individual QT variability,[4] the duration of the QT interval also predict the development of cardiac arrhythmias and cardiac sudden death.[5]
Studies that defined the normal duration of the QT interval began many years ago and were conducted mainly on populations of young or middle aged individuals.[6]–[9] The aging population in most countries raises the question whether these previous studies, had sufficient representation of all ages, particularly older individuals. Some investigators have supported the contention that QT interval is influenced by the person's age.[8]–[14] In contrast, other investigators have reported no association between age and QT-heart rate correction formulae (QTc).[15]–[17] While other investigators contend that the magnitude of the impact of age on the QT interval is too small to be meaningful.[18]
An essential first step in the assessment of the QT interval is the process of adjustment of the QT interval for the intrinsic heart rate because of the well-known inverse association between QT interval and heart rate. A plethora of formulae have been suggested to accomplish this goal and to provide the appropriate QTc.[19] QT-heart rate adjustment formulae were initially developed from small patient sample sizes.[20]–[22] Recently, newer formulae have been proposed based on large population samples.[12],[13],[23] The majority of ECGs from large studies were of baseline ECGs from individuals prior to drug testing.[13],[23]
It is important to know whether age should be a consideration in the assessment of the QT interval and whether the QT formulae themselves result in different relationships between age and QT interval. The objectives of this investigation were to examine the relationship between age and corrected QT interval in a population based sample and specifically to compare the strength of this association for the older QTc formulae in widespread clinical usage with the more recently developed QTc. We selected newer statistical modeling tools to examine these relationships. We further sought to examine the proportion of the population with ‘frequently labeled’ prolonged QTc and determine whether it is different in older compared to younger persons.
2. Methods
The US National Health and Nutrition Examination Survey (NHANES) II and III studies, conducted by the Center for Disease Control (CDC) of the US, were chosen to evaluate the QT interval in different age groups. These studies were selected because each NHANES survey was conducted on a representative sample of the civilian US population and importantly weighing factors are available, meaning that the results are more representative than random sample or volunteers in a drug trial. Standard 12-lead resting ECG recordings were performed with signals sampled at 250 samples per second per channel and a representative P-QRS-T cycle was derived by selective averaging.[24] Heart rate and QT intervals were digitally calculated using the Novacode ECG program.[24] NHANES II and III data were downloaded from the CDC.[25]–[27] They were imported and processed in Microsoft Excel 2013 (Redmond, WA, USA). The possibility that the same individuals were selected for both studies was considered but deemed not significant because of the randomness of the participant selection process. The data from NHANES II and III were pooled to create one large data set. Then exclusion criteria were applied to exclude factors from the ECG that are known to affect the duration of the QT interval. The definition of all ECG abnormalities can be found in the NHANES documentation. Briefly, the exclusion criteria included: (1) subjects without valid QT interval duration or heart rate data; (2) subjects with probable myocardial infarction (MI) and possible MI;[26],[27] subjects with major ECG abnormalities;[26],[27] (3) subjects with rhythm not being sinus;[26],[27] (4) subjects with probable left ventricular hypertrophy (LVH);[26],[27] (5) Subjects with Minnesota code 7.1 and 7.2 were excluded because this represented left bundle branch block or right bundle branch block respectively.[26],[27]
Six different QTc were evaluated either because of their long term usage or their recent introduction from large population studies. The formulae include the equations proposed by (i) Bazett (QTcBZT)[20] (QTcBZT = QT/(RR)−1/2, (ii) Fridericia (QTcFRD)[21] (QTcFRD = QT/(RR)−1/3, (iii) Hodges (QTcHDG)[22] (QTcHDG = QT + 105(1/RR – 1), (iv) Framingham (QTcFRM)[18] (QTcFRM = QT + 0.154(1000 – RR), (v) Dmitrienko, et al.[23] (QTcDMT) (QTcDMT = QT/(RR)−0.413, and (vi) Rautaharju, et al.[12] (QTcRTHa) (QTcRTHa = QTx(120 + HR)/180) which were identified by a proposed standardized nomenclature.[19] Two formulae were described in Rautaharju, et al.[12] but only QTcRTHa was included because QTcRTHb already had a correction factor for sex and might have not been comparable to the other formulae.
All data analysis was performed using R statistical software [R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/]. As per the CDC instructions, sample weights were incorporated into summary statistics (means and standard deviations) in order to obtain unbiased estimates for US population parameters. These individual weights are provided by the CDC and are intended to account for non-response, over-sampling, post-stratification and sampling error for individuals with similar demographic profile. In some analyses, individuals were combined to age-groups, formed in 5-year increments (25 to < 30, 30 to < 35, 35 to < 40 years, etc.); all plots utilize the median of the group (i.e.,: 27.5 is plotted for the ≥ 25 to < 30 group). Because the maximum age in the data set is 90, those individuals were included with the 85 to 90 year age group so that a 90–94 group did not contain only 90 year olds.
Relationships between QTc and age were modeled using least-squares regression fits to a linear, quadratic, and spline functions of age. Flexible cubic b-splines with two knots placed at the first and third quantiles were used.[28] A spline was chosen rather than a cubic polynomial to improve performance in the fit at minimum and maximum ages; polynomial regression tends to fit poorly in areas of low curvature near boundaries. A cubic spline was chosen when higher order splines (up to 5-degree with 3 knots) did not provide significant improvement in fit (i.e., the R2 values did not increase) and therefore are not shown.
All regression models were fit to the weighted mean at each age and separately for men and women and themselves were weighted to the sample size at each age. Goodness-of-fit was assessed using an adjusted-R2, to account for model complexity. Comparisons to the null model and between models (Linear vs. Quadratic and Linear vs. Cubic Spline) were performed using an F-test of the residual sum-of-squares. Proportions of individuals with prolonged QTc were estimated using normal distribution theory based on the predicted conditional mean and standard deviation for each age group based on the cubic b-spline model.
3. Results
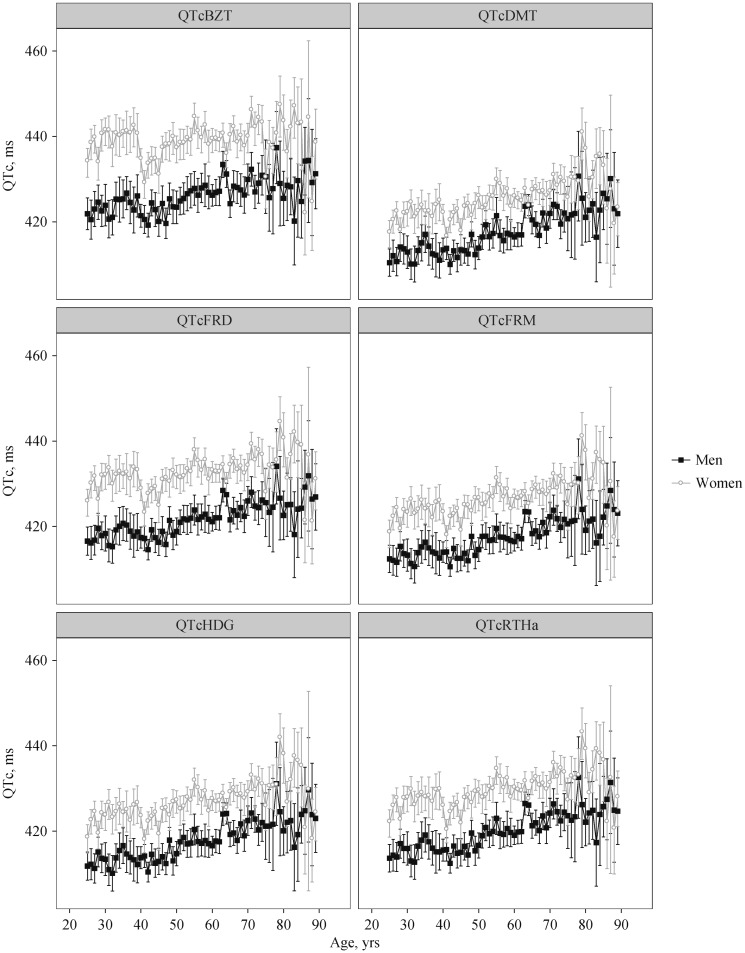
The total utilized sample size was 6173 men and 7454 women. The proportion of men and women over QTc duration threshold for various threshold values for each QTc formula and for age categories are provided in Figure S1 (data online). QTc was significantly shorter for men compared to women for all formulae (all P < 0.001, Figure 1). The average difference between sexes in QTc in order of magnitude were QTcBZT [12.6 ms (95% CI:11.5–13.7 ms)] followed by QTcDMT [10.7 ms (95% CI: 9.8, 11.6)], QTcRTHa [9.5 ms (95% CI: 8.6, 10.4)], QTcFRD [9.0 ms (95% CI: 8.1, 9.8)], QTc FRM [8.8 ms (95% CI: 8.0, 9.7]) and QTc HDG [7.7 ms (95% CI: 6.8, 8.5)]. The difference between men and women decreased with age; the difference between men and women was significantly smaller for those over 50 compared to those aged 50 and under (P < 0.01 for all formulae).
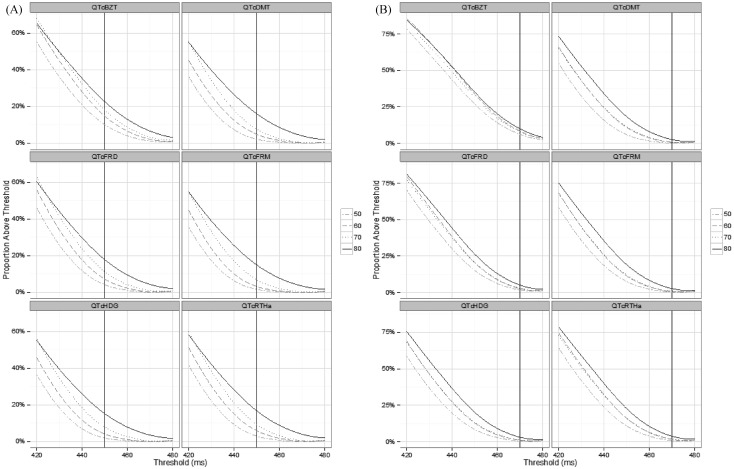
Figure S1. The proportion of men (A) and women (B) over QTc duration threshold for various threshold values for each QTc formula and for age groups 50, 60, 70 and 80 and over years of age.
QT-heart rate correction formula; QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
Figure 1. Weighted means and 95% CIs for all ages, for each QTc formula for both men and women.
QTc: QT-heart rate correction formulae; QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
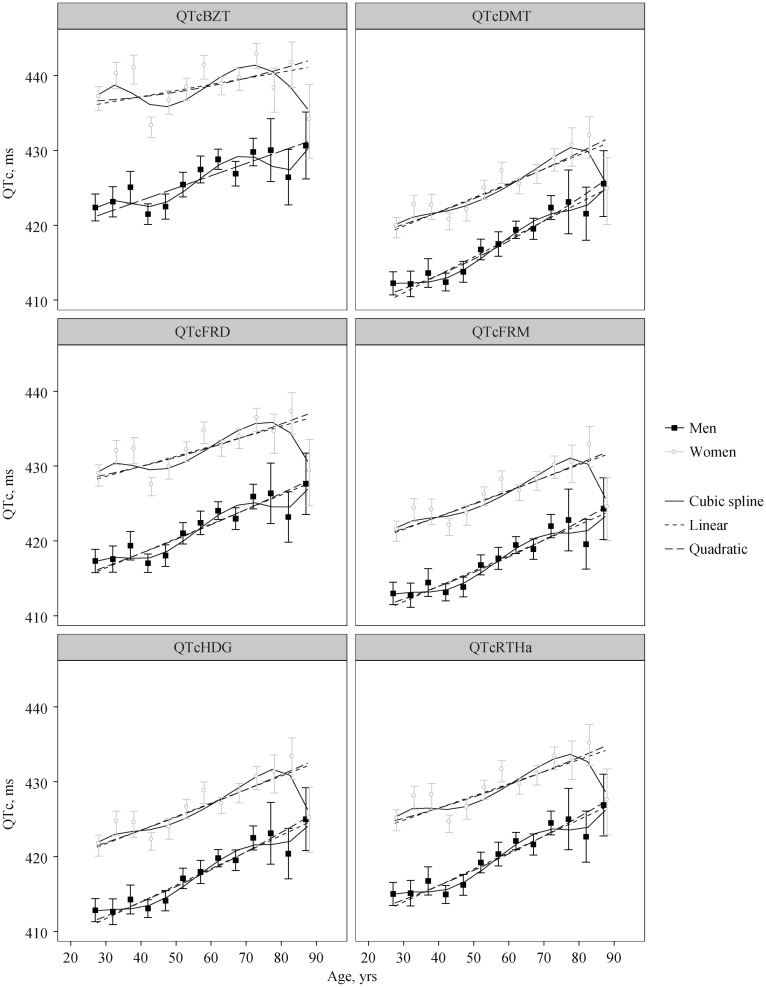
The linear, quadratic and cubic spline model fits are displayed in Figure 2 with model significance and goodness-of-fit statistics in Table 1. Among men, all formulae had significant linear, quadratic and cubic relationships between QTc and age (all P < 0.012). Adjusted-R2 for the quadratic model were not significantly higher than the linear model for any of the formulae (all P > 0.23), while the cubic b-spline had significant increases in adjusted-R2 compared to linear (all P < 0.05) for all formulae except for QTcHDG (P = 0.09). In addition to the significant sigmoid curvature, all formulae exhibited a significant upward trend in QTc with age (all P < 0.001).
Figure 2. Weighted means and 95% CI for age groups for both men and women with the fits of three models overlaid.
QTc: QT-heart rate correction formulae; QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
Table 1. The complexity-adjusted (adjusted-R2) goodness-of-fit for each QTc formulae considering a linear, quadratic and cubic spline models of the relationship between weighted-mean of QTc and age-group.
| Men |
Women |
||||||||||||
| Linear |
Quadratic |
Cubic-Spline |
Linear |
Quadratic |
Cubic-Spline |
||||||||
| Adjusted-R2 | P-Value | Adjusted-R2 | P-Value | Adjusted-R2 | P-Value | Adjusted-R2 | P-Value | Adjusted-R2 | P-Value | Adjusted-R2 | P-Value | ||
| QTcBZT | 0.66 | < 0.001 | 0.62 | 0.003 | 0.72 | 0.012 | 0.12 | 0.128 | 0.05 | 0.318 | 0.13 | 0.339 | |
| QTcDMT | 0.80 | < 0.001 | 0.78 | < 0.001 | 0.84 | 0.002 | 0.48 | 0.005 | 0.43 | 0.023 | 0.49 | 0.078 | |
| QTcFRD | 0.88 | < 0.001 | 0.87 | < 0.001 | 0.91 | < 0.001 | 0.74 | < 0.001 | 0.72 | < 0.001 | 0.74 | 0.008 | |
| QTcFRM | 0.86 | < 0.001 | 0.85 | < 0.001 | 0.88 | < 0.001 | 0.74 | < 0.001 | 0.71 | < 0.001 | 0.75 | 0.008 | |
| QTcHDG | 0.90 | < 0.001 | 0.91 | < 0.001 | 0.94 | < 0.001 | 0.78 | < 0.001 | 0.76 | < 0.001 | 0.77 | 0.006 | |
| QTcRTHa | 0.87 | < 0.001 | 0.86 | < 0.001 | 0.90 | < 0.001 | 0.64 | < 0.001 | 0.61 | 0.004 | 0.63 | 0.026 | |
P-values are F-tests comparing each model to a null model (i.e., no relationship between age and QTc). QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
Among women, there was greater diversity in the QTc -age relationship among the formulae. The Bazett formula did not show any linear, quadratic or cubic relationship between QTc and age (all P > 0.12). QTcDMT showed strong linear and quadratic relationships (P = 0.005 and P = 0.023, respectively), but the sigmoid curve pattern (i.e., cubic model) was not significant (P = 0.078). The remaining formulae, QTcFRD, QTcHDG, QTcFRM and QTcRTHa, showed significant linear, quadratic and cubic relationships (all P < 0.03). Similar to men, the adjusted-R2 for the quadratic model was not superior to the linear model (P > 0.61 for all formulae). However, unlike the men, the cubic spline model also did not show any improvement in adjusted-R2 compared to the linear model (P > 0.07 for all formulae). Also similar to men, there was a significant upward trend for QTc with age (P < 0.005), but the slope of this increase was lower (Figure 2).
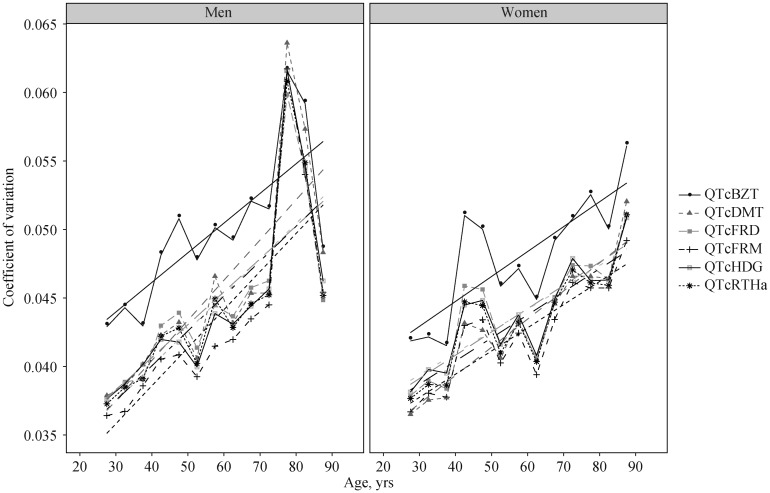
The coefficient of variation, reflecting relative dispersion around the mean. increased with age (Figure 3) for all QTc formulae and for both sexes (P < 0.01 for all formulae). This indicates that even when standardizing by QTc value, there is greater dispersion, or variance, around the mean for older compared to younger individuals.
Figure 3. Coefficient of variation (weighted SD/weighted mean) for men and women for each QTc formula according to age group with linear fits overlaid.
QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
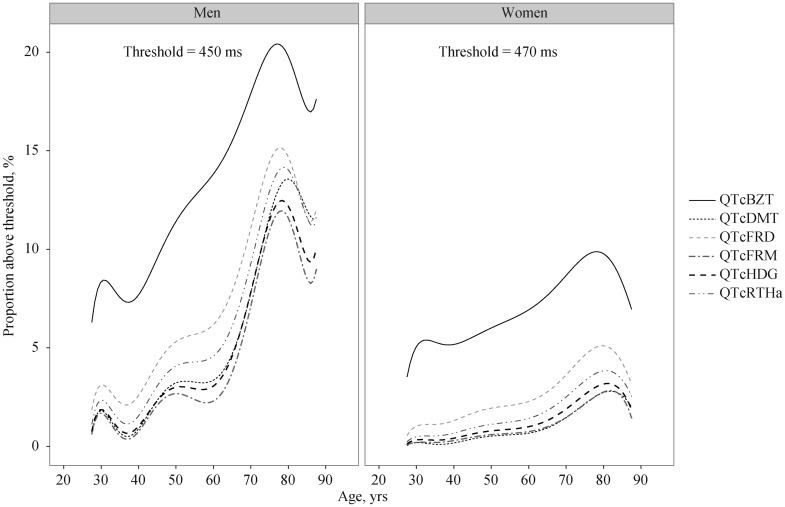
Combining the increasing mean QTc and increasing variance with age, the proportion of individuals with values above a QT prolongation threshold (considering QTc of 450 ms for men and 470 ms for women) increased substantially for both genders (Figure 4). For younger people (≤ 50 years of age) the proportion with longer QTc decreases rapidly to a small fraction of the population. In contrast, the proportion with longer QTc remains a substantial proportion in the oldest age group. The proportions of individuals above threshold were greater in men than in women, although the differences in absolute values need to be recognized. Indeed, there is a compounding effect of increasing mean and variance with age on the proportion of persons at higher QT levels. The relationship among the conversion functions displayed in Figure 4 holds for thresholds above and below the 450/470 ms standards.
Figure 4. The proportion over specific QTc duration thresholds for men and women by age for each QTc formula.
QTcBZT: QTc proposed by Bazett; QTcDMT: QTc proposed by Dmitrienko; QTcFRD: proposed by Fridericia; QTcFRM: QTc proposed by Framingham; QTcHDG: QTc proposed by Hodges; QTcRTHa: QTc proposed by Rautaharju.
4. Discussion
This study is the first to apply rigorous analytical methods to compare six QTc formulae focusing on the changes with age in a population based sample. An increase in QTc with age was observed and suggests that older individuals have a greater QTc which potentially makes them more vulnerable to situations and medications that prolong QTc. There were some differences in the strength of the association depending on the QT formula used and the most commonly used one in clinical practice—QTcBZT showed the greatest difference from the rest.
Our analysis of a sample of the US population— NHANES, showed a significant association between QTc and age in both sexes for all QTc formulae except for QTcBZT for women. The impact of age was evident regardless of whether one considered a linear or non-linear relationship between QTc and age. Indeed, the complexity-adjusted goodness-of-fit analysis found a relatively strong relationship between age and QTc regardless of whether one considers linear, quadratic or cubic spline models of the relationship between QTc and age. The consistent strong relationship between age and QTc with all QTc formulae, other than QTcBZT in women, suggest that QTcBZT is the outlier.
QTc increases with age maybe due to a combination of factors. Aging processes may affect the molecular determinants of the QT interval or alter the myocardium with increased myocardial fibrosis.[29] Aging is also associated with alterations in the amount of sympathetic and parasympathetic tone[30] which can alter myocardial repolarization and the duration of the QTc.[31]
The QTc formulae, that were evaluated, are not encompassing of all QTc formulae, proposed for heart rate correction,[19] but rather they are a combination of frequently used older formulae, based on small clinical patient groups[20]–[22] and more recent proposed ones based on large populations.[12],[18],[23]
Our study provide a potential explanation for reports of the absence an association between age and QTc in some studies.[15]–[17] These negative studies used the Bazett formula. We found that QTcBZT showed the poorest correlation between age and QTc compared to all the other formulae. One study reported the absence of an effect of age used QTcBZT in women.[16] In our comparative analysis, the poorest correlation of QT with age was for QTcBZT in women.
The standard deviation of the QTc formulae increased with age. This observation reinforces the need to be mindful of the QTc at older age groups as there is a greater likelihood of individuals with prolonged QTc at older ages.
The increase in QTc with age means that drugs which induce QT prolongation may more readily reach potentially ‘dangerous’ QTc durations in an older compared to a younger person.[32] An FDA statement is relevant specifically “While the degree of QT prolongation is recognized as an imperfect biomarker for pro-arrhythmic risk, in general there is a qualitative relationship between QT prolongation and the risk of Torsade de Pointe (TdP), especially for drugs that cause substantial prolongation of the QT interval.”[33] The importance of QT and age is underscored by the finding that there is over a threefold increase in cardiovascular events in elderly men with a QTc greater than 420 ms.[34] We show that this is of special concern in persons 70 years of age and older. A Swedish pharmacovigilance database found that the most common risk factor for potentially fatal ventricular tachycardia, TdP, was age over 65 years.[35] The use of antipsychotic drugs, both the older and some of the newer agents, is associated with a greater than two fold increase in sudden death.[36] The recent observation of an increase in risk of death in older men after institution of antipsychotic treatment,[37] would be consistent with our finding of a greater QTc with age in older men. The high use of antipsychotic drugs in institutionalized older persons,[38] raises concerns and suggests the need for examining the QTc interval in older persons before the institution of agents that might prolong QTc.
The differences in QTc between men and women lessen significantly for people over 50. Sex differences in ECG variables have been recognized[11],[20],[39]–[42] and attributed, in part, to differences in sex hormones.[11],[43] In men, QT interval correlates with testosterone levels.[44],[45] Of note, QT interval is longer in castrated men and shorter in women with virilisation.[46] The decline in testosterone levels with age[47],[48] may account for the increase in QTc with age. Hormonal changes, however, are not the entire explanation for the QTc differences between sexes.[49],[50]
Our evaluation found that the correlation between age and QTc was greater in men than in women. This finding is due to the greater prolongation of the QTc observed in older men compared to younger men. The QTc is greater in women than men at younger ages but the difference in QTc between the sexes diminishes at the older age groups, which means the increase in QTc with age is not parallel. The decline in variability in men 80 years of age and older may have several explanations. We can only speculate on them but the explanations include intrinsic biological properties of cardiac ion channels that in men or that survivorship in men is worse than women so that men providing data may represent a healthier sub-cohort of the population.
There has been no clear gold standard for choosing the single equation to correct the QT interval and to compare with age. For men, the rank order of the formulae in descending order, starting from the one with the steepest slope of the relationship with age was QTcHDG, QTcFRD, QTcRTHa, QTcFRM, QTcDMT and QTcBZT. For women, the rank order of the formulae from the one with the strongest association with age was QTcHDG, QTcFRD, QTcFRM, QTcRTHa, QTcDMT and QTcBZT. It is important to emphasize that this ordering does not have any relationship to validity of QT-RR relationship but is solely based on the relationship to age.
There are several limitations of the study that require discussion. First the data are from the NHANES study. While the NHANES study is meant to be representative of the entire US population, the numbers of actual subjects are low for certain ages especially for persons above the age of 80 years. Hence, projections in the form of regression equations can show the trend from which the QTc should follow. We provided both linear and non-linear modeling to test the relationship between QTc and age. In choosing the subjects for this study, we have chosen the NHANES II and NHANES III studies for several reasons. First, each NHANES was conducted on a representative population based sample. Second, weighing factors are available, meaning that the results are more representative than random sample or volunteers in a drug trial. Third, the ECG data is systematically analyzed and interpreted to reduce the degree uncertainty with regards to accuracy. Another issue is that the NHANES data was used to develop one of the QT-heart rate correction equation.[12] However, the data from the other sources was much larger so that the NHANES data contributed only a relatively small proportion to the formulae. Lastly, older subjects, specifically those 85 years of age and older, are potentially a ‘biased’ group in cross sectional studies because they are likely individuals at lower risk than the 50 year olds, as demonstrated by their ability to survive to ages over 80 years.
In conclusion, increasing age is associated with greater prolongation of QTc and the relationship is stronger for men than women. Clinicians should be aware of the strength of this relationship varies somewhat according to the QT heart rate correction formula used. A significant relationship between age and QTc was demonstrated in linear and non-linear analytic models. Men show a steeper relationship between age and QTc than women which should alert clinicians in their prescription of drugs that potentially prolong QTc prolongation to older men. The variance of QTc increases with age. This finding in conjunction with the increasing mean QTc with age indicates that there are older persons than younger persons with prolonged QTc.
References
- 1.Burchell HB. The QT interval historically treated. Pediatr Cardiol. 1983;4:139–148. doi: 10.1007/BF02076339. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 3.Bokil NJ, Baisden JM, Radford DJ, et al. Molecular genetics of long QT syndrome. Mol Genet Metab. 2010;101:1–8. doi: 10.1016/j.ymgme.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Isbister GK, Page CB. Drug induced QT prolongation: the measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol. 2013;76:48–57. doi: 10.1111/bcp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72:23B–25B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 6.Simonson E, Cady LD, Jr, Woodbury M. The normal Q-T interval. Am Heart J. 1962;63:747–753. doi: 10.1016/0002-8703(62)90059-5. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane PW, McLaughlin SC, Devine B, et al. Effects of age, sex, and race on ECG interval measurements. J Electrocardiol. 1994;27(Suppl. 14–19) doi: 10.1016/s0022-0736(94)80039-1. [DOI] [PubMed] [Google Scholar]
- 8.Bachman S, Sparrow D, LK S. Effect of aging on the electrocardiogram. Am J Cardiol. 1981;48:513–516. doi: 10.1016/0002-9149(81)90081-3. [DOI] [PubMed] [Google Scholar]
- 9.Reardon M, Malik M. QT interval change with age in an overtly healthy older population. Clin Cardiol. 1996;19:949–952. doi: 10.1002/clc.4960191209. [DOI] [PubMed] [Google Scholar]
- 10.Mangoni AA, Kinirons MT, Swift CG, et al. Impact of age on QT interval and QT dispersion in healthy subjects: a regression analysis. Age Ageing. 2003;32:326–331. doi: 10.1093/ageing/32.3.326. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju PM, Zhou SH, Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 12.Rautaharju PM, Mason JW, Akiyama T. New age- and sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol. 2014;174:535–540. doi: 10.1016/j.ijcard.2014.04.133. [DOI] [PubMed] [Google Scholar]
- 13.Mason JW, Ramseth DJ, Chanter DO, et al. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Taneja T, BW M, Passman R, et al. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol. 2001;24:16–21. doi: 10.1046/j.1460-9592.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 15.Tran H, White CM, Chow MS, et al. An evaluation of the impact of gender and age on QT dispersion in healthy subjects. Ann Noninvasive Electrocardiol. 2001;6:129–133. doi: 10.1111/j.1542-474X.2001.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavi S, Nevo O, Thaler I, et al. Effect of aging on the cardiovascular regulatory systems in healthy women. Am J Physiol Regul Integr Comp Physiol. 2007;292:R788–R793. doi: 10.1152/ajpregu.00352.2006. [DOI] [PubMed] [Google Scholar]
- 17.Huang JH, Lin YQ, Pan NH, et al. Aging modulates dispersion of ventricular repolarization in the very old of the geriatric population. Hear Vessel. 2010;25:500–508. doi: 10.1007/s00380-010-0026-z. [DOI] [PubMed] [Google Scholar]
- 18.Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 19.Rabkin SW, Cheng XB. Nomenclature, categorization and usage of formulae to adjust QT interval for heart rate. World J Cardiol. 2015;7:315–325. doi: 10.4330/wjc.v7.i6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 21.Fridericia LS. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann Noninvasive Electrocardiol. 2003;8:343–351. doi: 10.1046/j.1542-474X.2003.08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodges M, Salerno D, Erlien D. Bazett's QT correction reviewed: evidence that a linear QT correction for heart rate is better. J Am Coll Cardiol. 1983;1:1983. [Google Scholar]
- 23.Dmitrienko AA, Sides GD, Winters KJ, et al. Electrocardiogram reference ranges derived from a standardized clinical trial population. Drug Inf J. 2005;39:395–405. [Google Scholar]
- 24.Rautaharju P, MacInnis P, Warren J, et al. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Infect Med. 1990;29:362–374. [PubMed] [Google Scholar]
- 25.NHANES II Public Use Data Tape Documentation, 1991 & NHANES III Electrocardiography Data File Documentation, 1998. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/nhanes/nhanesii/5305.pdf; ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes.
- 26.National Health and Nutrition Examination Survey II, Electrocardiogram Data, 2012. Centers for Disease Control and Prevention Web site. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/2A/nh2ecg.dat.
- 27.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey III, Electrocardiogram Data. 2015. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/2A/nh2ecg.dat.
- 28.Hastie TJ. Generalized additive models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S Editors. Wadsworth & Brooks/Cole Advanced Books & Software; 1992. [Google Scholar]
- 29.Liu C, Liu Y, Wu C, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeifer M, Weinberg C, Cook D, et al. Differential changes of autonomic nervous system function with age in man. Am J Med. 1983;75:249–258. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- 31.Can I, Aytemir K, Kose S, et al. Physiological mechanisms influencing cardiac repolarization and QT interval. Card Electrophysiol Rev. 2002;6:278–281. doi: 10.1023/a:1016341311646. [DOI] [PubMed] [Google Scholar]
- 32.Rabkin SW. Aging effects on QT interval: Implications for cardiac safety of antipsychotic drugs. J Geriatr Cardiol. 2014;11:20–25. doi: 10.3969/j.issn.1671-5411.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs, 2005. US Department of Health and Human Services F and D, Administration Web site. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf.
- 34.Dekker J, Schouten E, Klootwijk P, et al. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90:779–785. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 35.Astrom-Lilja C, Odeberg JM, Ekman E, et al. Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2008;17:587–592. doi: 10.1002/pds.1607. [DOI] [PubMed] [Google Scholar]
- 36.Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochon PA, Gruneir A, Gill SS, et al. Older men with dementia are at greater risk than women of serious events after initiating antipsychotic therapy. J Am Geriatr Soc. 2013;61:55–61. doi: 10.1111/jgs.12061. [DOI] [PubMed] [Google Scholar]
- 38.Alanen H, Finne-Soveri H, Noro A, et al. Use of antipsychotics among nonagenarian residents in long-term institutional care in Finland. Age Ageing. 2006;35:508–513. doi: 10.1093/ageing/afl065. [DOI] [PubMed] [Google Scholar]
- 39.Simonson E, Blackburn H, Punchner T, et al. Sex differences in the electrocardiogram. Circulation. 1960;22:598–601. [Google Scholar]
- 40.Merri M, Moss AJ, Benhorin J, et al. Relation between ventricular repolarization duration and cardiac cycle length during 24-hour Holter recordings. Findings in normal patients and patients with long QT syndrome. Circulation. 1992;85:1816–1821. doi: 10.1161/01.cir.85.5.1816. [DOI] [PubMed] [Google Scholar]
- 41.Stramba-Badiale M, Locati EH, Martinelli A, et al. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24-h Holter recordings. Eur Heart J. 1997;18:1000–1006. doi: 10.1093/oxfordjournals.eurheartj.a015357. [DOI] [PubMed] [Google Scholar]
- 42.Adams W. The normal duration of the electrocardiographic ventricular complex. J Clin Invest. 1936;15:335–342. doi: 10.1172/JCI100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pham T, Rosen M. Sex, hormones, and repolarization. Cardiovasc Res. 2002;53:740–751. doi: 10.1016/s0008-6363(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ouyang P, WS P, et al. Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174:403–411. doi: 10.1093/aje/kwr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charbit B, Christin-Maitre S, JL D, et al. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol. 2009;103:887–890. doi: 10.1016/j.amjcard.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Bidoggia H, JP M, Capalozza N, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 47.Feldman H, Longcope C, Derby C, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 48.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 49.Drici MD, Burklow TR, Haridasse V, et al. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 50.Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]