Abstract
Transgenic plants expressing the coat protein (CP) of alfalfa mosaic virus (AIMV) are resistant to infection by AIMV. A mutation was introduced into the second amino acid of the cDNA for the CP of AIMV. Three different transgenic tobacco lines expressing the mutant CP and two different transgenic tobacco lines expressing the wild-type CP at similar levels were challenged with AIMV virions and viral RNA. Whereas the lines expressing the wild-type CP were highly resistant to infection by AIMV virions and viral RNA, the lines expressing the mutant CP were susceptible to infection by both. The binding affinity of the mutant and the wild-type CPs for the 3' terminal protein binding site on AIMV RNAs was similar, as determined by electrophoretic mobility shift assay. A mixture of AIMV genomic RNAs 1-3 was infectious on the plants expressing the mutant CP but not on vector control plants or plants expressing the wild-type CP, indicating that the mutant CP can activate the AIMV genomic RNAs for infection. These results demonstrate that the second amino acid of the AIMV CP is critical for protection from AIMV but not for the initial interaction between the AIMV RNA and CP, suggesting that this initial interaction does not play a major role in CP-mediated protection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bianchi M. E., Beltrame M., Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989 Feb 24;243(4894 Pt 1):1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Bol J. F., van Vloten-Doting L., Jaspars E. M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971 Oct;46(1):73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemenway C., Fang R. X., Kaniewski W. K., Chua N. H., Tumer N. E. Analysis of the mechanism of protection in transgenic plants expressing the potato virus X coat protein or its antisense RNA. EMBO J. 1988 May;7(5):1273–1280. doi: 10.1002/j.1460-2075.1988.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
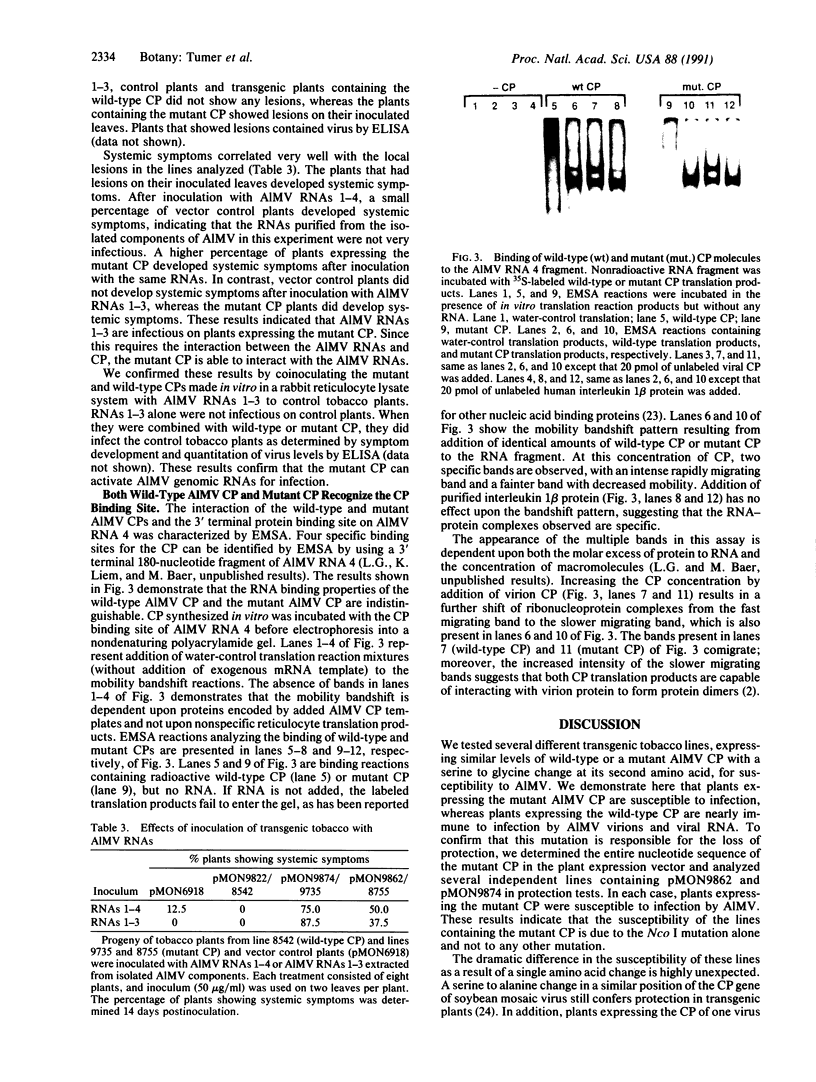
- Houwing C. J., Jaspars E. M. Protein binding sites in nucleation complexes of alfalfa mosaic virus RNA 4. Biochemistry. 1982 Jul 6;21(14):3408–3414. doi: 10.1021/bi00257a025. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Cuthbert C. M., Rogers S. G., Fraley R. T., Gehrke L. In vitro transcription and translational efficiency of chimeric SP6 messenger RNAs devoid of 5' vector nucleotides. Nucleic Acids Res. 1988 May 25;16(10):4483–4498. doi: 10.1093/nar/16.10.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Kruseman J., Kraal B., Jaspars E. M., Bol J. F., Brederode F. T., Veldstra H. Molecular weight of the coat protein of alfalfa mosaic virus. Biochemistry. 1971 Feb 2;10(3):447–455. doi: 10.1021/bi00779a015. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C., Kaniewski W., Haley L., Rozman R., Newell C., Sanders P., Tumer N. E. Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Y in transgenic Russet Burbank. Biotechnology (N Y) 1990 Feb;8(2):127–134. doi: 10.1038/nbt0290-127. [DOI] [PubMed] [Google Scholar]
- Loesch-Fries L. S., Merlo D., Zinnen T., Burhop L., Hill K., Krahn K., Jarvis N., Nelson S., Halk E. Expression of alfalfa mosaic virus RNA 4 in transgenic plants confers virus resistance. EMBO J. 1987 Jul;6(7):1845–1851. doi: 10.1002/j.1460-2075.1987.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B., Flinta C., von Heijne G., Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985 Nov 4;152(3):523–527. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- Register J. C., 3rd, Beachy R. N. Resistance to TMV in transgenic plants results from interference with an early event in infection. Virology. 1988 Oct;166(2):524–532. doi: 10.1016/0042-6822(88)90523-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., O'connell K. M., Nelson R. S., Sanders P. R., Beachy R. N., Fraley R. T., Shah D. M. Expression of alfalfa mosaic virus coat protein gene confers cross-protection in transgenic tobacco and tomato plants. EMBO J. 1987 May;6(5):1181–1188. doi: 10.1002/j.1460-2075.1987.tb02352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vloten-Doting L., Jaspars E. M. The uncoating of alfalfa mosaic virus by its own RNA. Virology. 1972 Jun;48(3):699–708. doi: 10.1016/0042-6822(72)90154-7. [DOI] [PubMed] [Google Scholar]
- van Vloten-Doting L. Coat protein is required for infectivity of tobacco streak virus: biological equivalence of the coat proteins of tobacco streak and alfalfa mosaic viruses. Virology. 1975 May;65(1):215–225. doi: 10.1016/0042-6822(75)90022-7. [DOI] [PubMed] [Google Scholar]