Abstract
Purpose
Numerous studies have assessed the association of SP110 gene variants with tuberculosis (TB), but the results were inconsistent. Through a comprehensive review and meta-analysis, our study aimed to clarify the nature of genetic risks contributed by 11 polymorphisms for the development of TB.
Materials and Methods
Through searching PubMed, web of science, China National Knowledge Infrastructure (CNKI) databases, a total of 11 articles including 13 independent studies were selected. The pooled odd ratios (ORs) along with their corresponding 95% confidence interval (CI) were estimated for allelic comparisons, additive model (homozygote comparisons; heterozygote comparisons), dominant model and recessive model. We also assessed the heterogeneity across the studies and publication bias.
Results
The results of combined analysis revealed a significantly increased risk of TB for single nucleotide polymorphism (SNP) rs9061 in all five comparisons (allelic comparisons: OR=1.28, 95% CI=1.14–1.44, p<0.0001; homozygote comparisons: OR=2.84, 95% CI=1.84–4.38, p<0.00001; heterozygote comparisons: OR=1.23, 95% CI=1.05–1.43, p=0.009; dominant model: OR=1.32, 95% CI=1.14–1.53, p=0.0003; recessive model: OR=2.26, 95% CI=1.18–4.34, p=0.01). In subgroup analysis, the risk of TB associated with SNP rs9061 appeared to be increased. Moreover, increased risk of TB was also found in Asian subgroup of SNP rs11556887, while decreased risk of TB appeared in large sample size subgroup of SNP rs1135791. No significant association was observed between other SNPs and the risk of TB.
Conclusion
Our meta-analysis suggested that the variant of SNP rs9061 might be a risk factor for TB.
Keywords: Tuberculosis, Speckled 110 gene, polymorphism
INTRODUCTION
Tuberculosis (TB) mainly arising from Mycobacterium tuberculosis (M. tuberculosis) infection is respiratory tract infectious disease.1 Although it is curable, TB has become one of the main infectious disease resulting in serious death worldwide.2 Recent World Health Organization report points out that about a third of the population worldwide has infected latent TB, but only 10% of individuals carrying the pathogen will suffer clinical symptoms.3 Moreover, twin studies found that the rate of two people suffering from TB in monozygotic twin pairs was significantly higher than in dizygotic twin pairs.4 Apart from the two evidences above, racial studies and family studies have also shown that genetic factors may have vital effect on the susceptibility of TB in individuals.5,6,7
In 2005, Pan, et al.8 found a new genetic locus on mouse, designated super-susceptibility to TB 1 (sst1), which mediates innate immunity in mouse TB models. Mouse gene, intracellular pathogen resistance-1 (Ipr1), which located in the sst1 region, might promote macrophage activities and enhance the ability of macrophages to fight against M. tuberculosis infection.9,10 The Speckled 110 (SP110) gene in human holds 41% sequence homology to the mouse Ipr1 gene.11 This gene is located on chromosome at 2q37.1 and encodes the SP110 nuclear body protein, which is a component of cellular structures.12,13 By participating in signal transmission processes between nuclear hormone receptors, SP110 protein could regulate the biological activity of macrophages and influence the growth and proliferation of M. tuberculosis, which might be associated with the susceptibility to TB.14,15,16
Certain SP110 polymorphisms result in the change of amino acid, influencing the function of SP110 protein and then having an effect on the development of TB.17 Tosh, et al.6 first found that genetic variants in SP110 gene were associated with TB in West African population in 2006. Since then, increasing numbers of researchers studied the relation between SP110 gene polymorphisms and TB. However, these studies yielded inconsistent consequences. Abhimanyu, et al.,12 Cai, et al.,18 and Cong, et al.19 identified the association of SP110 polymorphisms with TB by a case-control design while irrelevant results were found by Png, et al.20, Fox, et al.21, and Jiang, et al.22 Thus, we performed a comprehensive review and updated meta-analysis with more SP110 polymorphisms, articles and subgroup analysis than before, and attempted to verify and offer an accurate and comprehensive understanding of the association between polymorphisms of SP110 gene and human TB susceptibility.
MATERIALS AND METHODS
Search strategy
We searched the PubMed, web of science, and China National Knowledge Infrastructure (CNKI) databases for articles published from the earliest possible year to October 2015 by using the following keywords: “(tuberculosis or TB), (Speckled 110 gene or SP110), and (polymorphism or variant or genotype)”. Furthermore, we retrieved the bibliography of all available articles to find potential valuable studies. If the article described the analytical results of different ethnicities or diseases, we regarded it as separate studies. We only searched the articles in English and Chinese.
Inclusion/exclusion criteria
The studies, included in the meta-analysis, required to satisfy the following criteria: 1) case-control studies; 2) applications of standardized diagnosis criteria of TB; and 3) providing data on alleles and genotypes for directly calculating the odds ratio (OR) and its 95% confidence interval (CI). The articles meeting one of the following situations were excluded: 1) family studies; 2) reports with data of other SP110 polymorphisms; and 3) patients with other infective disease and immunosuppressive conditions. When multiple publications reported on the same or overlapping data, the most recent or complete study was selected.
Data extraction
Data was extracted independently from qualified articles by two authors according to a pre-determined extraction protocol with any divergence solved by discussion, eventually reaching a consensus in all items. For each study, the following information was collected: the first author’s name, publication year, ethnicity, source of controls, sample sizes, TB diagnosis criteria, genotyping methods, distributions and frequencies of alleles and genotypes for each polymorphism among cases and controls, and results of Hardy-Weinberg equilibrium (HWE) in controls.
Quality score assessment
Two authors assessed independently the quality of each study using the quality scoring criteria modified from previous studies.23,24,25,26,27 The criteria contained nine questions including representative cases, diagnoses of TB cases, tests for HWE, etc. The answers to every problem were divided into three levels, setting as 2 scores, 1 score, and 0 score (Supplementary Table 1, only online). Quality scores appeared in the range of 0 point (worst) to 17 points (best). Studies scoring higher than 14 points were classified as high quality studies. The quality score of each study is shown in Supplementary Table 2, only online.
Statistical analysis
Firstly, HWE test of each polymorphism among studies was performed in control groups using the chi-squared test when genotype data were provided fully and a p value of more than 0.05 was considered significant equilibrium. Secondly, linkage disequilibrium plots were constructed by the Haploview v. 4.2. Thirdly, the association between SP110 gene polymorphisms and the TB risk was evaluated by ORs with 95% CIs for alleles and genotypes comparisons. The pooled ORs along with their corresponding 95% CIs were estimated for allelic comparison (C2 vs. C1), the additive model (homozygote comparisons: C2C2 vs. C1C1; heterozygote comparisons C2C1 vs. C1C1), the dominant model (C2C2+C2C1 vs. C1C1), and the recessive model (C2C2 vs. C2C1+C1C1).
The heterogeneity was checked by chi-square-based Q-test and I-squared test. A random-effect model28 was used to assess the pooled ORs when I2 (%) >50%. Otherwise, a fixed-effect model29 was adopted. Subgroup analyses were performed when necessary, in accordance with the quality assessment score, ethnicity and sample size. In addition, sensitivity analysis was carried out to evaluate the stability of the results through a sequential removal of each study or after excluding those studies that deviated from HWE. Publication bias was assessed formally by Begg’s test30 and Egger’s test.31 All of the above analyses were conducted using RevMan 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration) and STATA 12.0 (Stata, College, TX, USA). The p value of 0.05 for any test or model was considered to be statistically significant.
RESULTS
Eligible articles
A total of 143 relevant articles were primitively searched from PubMed, Web of Science, and CNKI databases. After first selection, 103 articles were excluded because of unsuitable data. Next, we carefully reviewed the full text of remaining studies. Among these articles, 18 articles were designed on animal models; 6 articles were excluded because of the duplicated use of data; 5 articles were excluded because of no case-control design. Two of the ultimately selected articles included data from 4 independent studies.12,21 Therefore, 11 articles including 13 studies were included in our meta-analysis, with 9 articles written in English and 2 in Chinese.12,18,19,20,21,22,32,33,34,35,36 The flowchart of article sifting is shown in Fig. 1, and the characteristics of all included studies are summarized in Table 1.
Fig. 1. Flow diagram of the study search and selection.
Table 1. Characteristics of Selected Articles for Evaluating Associations of SP110 Gene Polymorphisms and TB Risk.
| No. | Author | Country | Ethnicity | Study size | Type | SNPs included | Quality score* |
|---|---|---|---|---|---|---|---|
| 1 | Liang, et al.34 | China | Asian | 308/628 | PTB | rs11556887, rs9061, rs1135791 | 16 |
| 2 | Abhimanyu, et al.12† | India | Asian | 110/78 | PTB | rs11556887, rs9061, rs3948464, rs1346311, rs6436915, rs2114591 | 13 |
| 3 | Abhimanyu, et al.12 | India | Asian | 32/78 | LNTB | rs11556887, rs9061, rs1135791, rs1346311 | 12 |
| 4 | Cai, et al.18 | China | Asian | 702/452 | PTB | rs11556887, rs9061, rs1135791, rs3948464, rs11679983, rs1365776 | 16 |
| 5 | Png, et al.20 | Indonesia | Asian | 351/364 | PTB | rs9061, rs1135791, rs3948464, rs11679983, rs1365776, rs2114592, rs6436915, rs2114591 | 16 |
| 6 | Fox, et al.21† | Vietnam | Asian | 530/566 | PTB | rs11556887, rs1135791, rs3948464, rs1346311, rs1365776, rs2114592, rs6436915 | 16 |
| 7 | Fox, et al.21 | Vietnam | Asian | 133/566 | EPTB | rs11556887, rs1135791, rs3948464, rs1346311, rs1365776, rs2114592, rs6436915 | 15 |
| 8 | Babb, et al.35 | South Africa | African | 381/417 | PTB | rs1135791, rs3948464, rs1365776, rs2114592 | 13 |
| 9 | Cong, et al.19 | China | Asian | 100/106 | PTB | rs9061, rs1135791, rs3948464, rs11679983, rs722555 | 14 |
| 10 | Szeszko, et al.33 | Russia | European | 1912/2104 | PTB | rs11556887, rs3948464, rs1346311, rs11679983, rs1365776, rs722555 | 14 |
| 11 | Ying, et al.36 | China | Asian | 198/195 | PTB | rs1135791, rs722555 | 16 |
| 12 | Thye, et al.32 | West Africa | African | 1055/1409 | PTB | rs11556887, rs3948464, rs1346311, rs1365776, rs2114591 | 16 |
| 13 | Jiang, et al.22 | China | Asian | 424/424 | PTB | rs9061, rs1135791 | 16 |
PTB, pulmonary tuberculosis; EPTB, extra pulmonary tuberculosis; LNTB, lymph node tuberculosis; SNPs, single nucleotide polymorphisms; TB, tuberculosis; SP110, Speckled 110.
*Quality scores ranged from 0 points (worst) to 17 points (best), studies scoring more than 14 points were classified as high quality, †We treat the article as two independent studies on account of having data of two kinds of TB.
Study characteristics
If we could obtain the data of alleles and genotypes distributions from three or more unduplicated studies for a certain polymorphism, a meta-analysis was worth conducting. Therefore, a total of 11 polymorphisms in SP110 gene were studied, each of those included three or more studies. Their valuable data were abstracted (Supplementary Table 3, only online) and analyzed completely in this meta-analysis. In control groups, allele distributions of single nucleotide polymorphisms (SNPs) rs1135791, rs9061, rs1346311, and rs11679983, respectively deviated the HWE in just one study.19,32,34 In terms of linkage disequilibrium plot (Supplementary Fig. 1, only online), we found that just two single nucleotide polymorphisms (SNPs rs722555 and rs6436915) were in strong linkage disequilibrium (r2=0.85 in Chinese population; r2=0.67 in European population) and formed a haplotype block in both Chinese and European populations. Therefore, we selected one of them, namely SNP rs6436915, to conduct further analysis.
Comprehensive and subgroup analyses: rs9061 polymorphisms
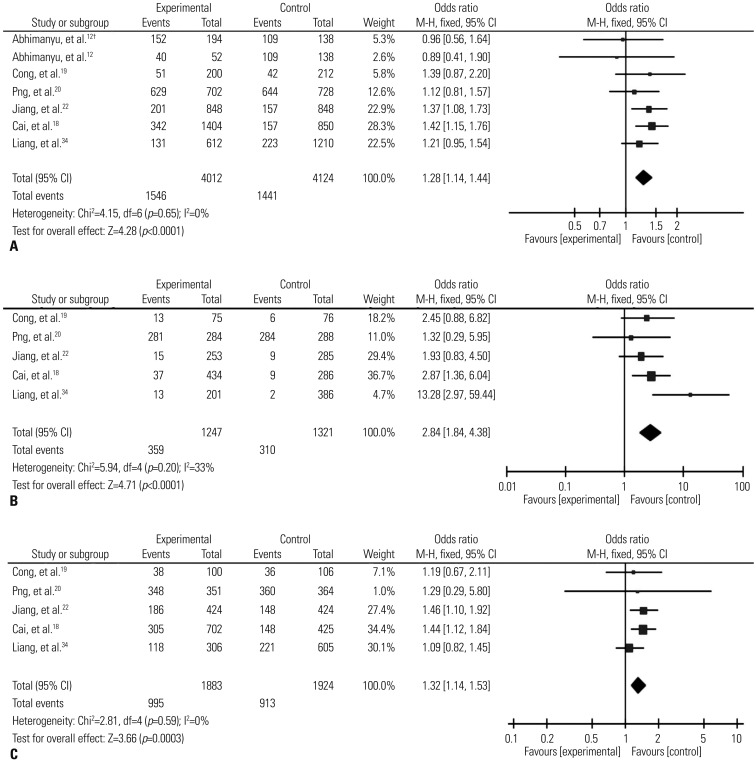
The results of combined analyses revealed a significantly increased risk of TB for SNP rs9061 in all the five comparisons (allelic comparison: OR=1.28, 95% CI=1.14–1.44, p<0.0001; homozygote comparisons: OR=2.84, 95% CI=1.84–4.38, p<0.00001; heterozygote comparisons: OR=1.23, 95% CI=1.05–1.43, p=0.009; the dominant model: OR=1.32, 95% CI=1.14–1.53, p=0.0003; and the recessive model: OR=2.26, 95% CI=1.18–4.34, p=0.01). Particularly, it should be pointed out that individuals carrying the mutant homozygote (AA) have as nearly three times risk of TB as ones carrying the wild homozygote (Table 2, Fig. 2).
Table 2. Overall and Subgroup Analyses and Heterogeneity of SP110 Gene Polymorphisms with the Risk of TB.
| Variables | n† | Allelic models | Homozygote comparisons | Heterozygote comparisons | Dominant models | Recessive models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)* | I2 (%) | p value | OR (95% CI)* | I2 (%) | p value | OR (95% CI)* | I2 (%) | p value | OR (95% CI)* | I2 (%) | p value | OR (95% CI)* | I2 (%) | p value | ||
| rs9061 | A vs. G | AA vs. GG | AG vs. GG | AA+AG vs. GG | AA vs. AG+GG | |||||||||||
| Overall | 7 (5) | 1.28 (1.14, 1.44) | 0 | <10−4 | 2.84 (1.84, 4.38) | 33 | <10−5 | 1.23 (1.05, 1.43) | 13 | 0.009 | 1.32 (1.14, 1.53) | 0 | <10−3 | 2.26 (1.18, 4.34) | 71 | 0.01 |
| All in HWE | 6 (4) | 1.31 (1.15, 1.49) | 0 | <10−4 | 2.32 (1.46, 3.69) | 0 | <10−3 | 1.34 (1.11, 1.60) | 0 | 0.002 | 1.42 (1.19, 1.69) | 0 | <10−4 | 1.47 (1.11, 1.95) | 44 | 0.008 |
| High quality | 5 (5) | 1.31 (1.17, 1.48) | 0 | <10−5 | 2.84 (1.84, 4.38) | 33 | <10−5 | 1.23 (1.05, 1.43) | 13 | 0.009 | 1.32 (1.14, 1.53) | 0 | <10−3 | 2.26 (1.18, 4.34) | 71 | 0.01 |
| Sample size | ||||||||||||||||
| >500 | 4 (4) | 1.31 (1.16, 1.48) | 0 | <10−4 | 2.93 (1.81, 4.73) | 49 | <10−4 | 1.25 (1.06, 1.46) | 22 | 0.006 | 1.33 (1.14, 1.55) | 0 | <10−3 | 2.28 (1.04, 4.98) | 77 | 0.04 |
| <500 | 3 (1) | 1.13 (0.82, 1.55) | 0 | 0.46 | 2.45 (0.88, 6.82) | NA | 0.09 | 0.94 (0.50, 1.77) | NA | 0.85 | 1.19 (0.67, 2.11) | NA | 0.55 | 2.49 (0.91, 6.83) | NA | 0.08 |
| rs1135791 | C vs. T | CC vs. TT | CT vs. TT | CC+CT vs. TT | CC vs. CT+TT | |||||||||||
| Overall | 10 (7) | 0.99 (0.84, 1.18) | 70 | 0.95 | 0.76 (0.57, 1.02) | 29 | 0.07 | 0.95 (0.75, 1.21) | 71 | 0.69 | 0.94 (0.74, 1.19) | 73 | 0.61 | 0.80 (0.64, 1.07) | 28 | 0.13 |
| All in HWE | 9 (6) | 1.04 (0.87, 1.24) | 67 | 0.68 | 0.75 (0.55, 1.02) | 40 | 0.07 | 1.02 (0.81, 1.30) | 64 | 0.84 | 1.01 (0.79, 1.30) | 69 | 0.94 | 0.78 (0.58, 1.06) | 38 | 0.11 |
| High quality | 8 (6) | 1.01 (0.82, 1.25) | 7 | 0.92 | 0.71 (0.49, 1.02) | 37 | 0.06 | 0.96 (0.72, 1.28) | 75 | 0.78 | 0.95 (0.71, 1.27) | 77 | 0.72 | 0.76 (0.53, 1.09) | 38 | 0.14 |
| Ethnicity | ||||||||||||||||
| Asian | 9 (6) | 1.01 (0.82, 1.23) | 74 | 0.94 | 0.71 (0.49, 1.02) | 37 | 0.06 | 0.96 (0.72, 1.28) | 75 | 0.78 | 0.95 (0.71, 1.27) | 77 | 0.72 | 0.76 (0.53, 1.09) | 38 | 0.14 |
| Sample size | ||||||||||||||||
| >500 | 7 (5) | 0.91 (0.77, 1.09) | 71 | 0.31 | 0.73 (0.47, 1.14) | 51 | 0.17 | 0.81 (0.71, 0.92) | 29 | 0.001 | 0.80 (0.70, 0.90) | 34 | <10−3 | 0.76 (0.52, 1.09) | 50 | 0.13 |
| <500 | 3 (2) | 1.46 (1.07, 1.98) | 0 | 0.02 | 1.12 (0.16, 8.04) | 0 | 0.91 | 1.80 (1.23, 2.63) | 0 | 0.003 | 1.80 (1.23, 2.63) | 0 | 0.003 | 0.88 (0.56, 1.41) | 0 | 0.60 |
| rs11556887 | T vs. C | TT vs. CC | TC vs. CC | TT+TC vs. CC | TT vs. TC+CC | |||||||||||
| Overall | 8 (4) | 1.10 (0.90, 1.34) | 70 | 0.35 | 1.32 (0.93, 1.85) | 6 | 0.12 | 1.26 (0.85, 1.87) | 81 | 0.26 | 1.32 (0.88, 2.00) | 83 | 0.18 | 1.23 (1.02, 1.48) | 0 | 0.03 |
| High quality | 6 (4) | 1.16 (0.94, 1.44) | 75 | 0.17 | 1.32 (0.93, 1.85) | 6 | 0.12 | 1.26 (0.85, 1.87) | 81 | 0.26 | 1.32 (0.88, 2.00) | 83 | 0.18 | 1.23 (1.02, 1.48) | 0 | 0.03 |
| Ethnicity | ||||||||||||||||
| Asian | 6 (2) | 1.11 (0.83, 1.50) | 66 | 0.47 | 2.76 (0.97, 7.89) | 0 | 0.06 | 1.56 (1.20, 2.02) | 0 | <10−3 | 1.61 (1.25, 2.07) | 0 | <10−3 | 2.59 (0.91, 7.39) | 0 | 0.07 |
OR, odds ratio; CI, confidence interval; TB, tuberculosis; HWE, Hardy-Weinberg equilibrium; SP110, Speckled 110.
I2, value of I2 for heterogeneity test; p value, p value of the Z test for overall effect.
*A random-effect model was used when I2 >50%; otherwise, a fixed-effect model was used, †Figure before the bracket is the number of studies for allele model; figure in the bracket is the number of studies for other four model.
Fig. 2. Forest plot of pooled OR with 95% CI for association between SNP rs9061 and TB risk under the allelic model (A), homozygote comparisons (B), and the dominant model (C). The squares and horizontal lines correspond to the study-specific OR and 95% CI, respectively; the box size is proportional to the meta-analysis study weight; the diamond represents the pooled OR and 95% CI. †We treat the article as two independent studies on account of having data of two kinds of TB. OR, odds ratio ; CI, confidence interval; SNP, single nucleotide polymorphisms; TB, tuberculosis.
When groups were stratified according to the sample size, significantly increased associations were found for large sample size subgroups in all five models: (the allelic comparison: OR=1.31, 95% CI=1.16–1.48, p<0.0001; the homozygote comparison: OR=2.93, 95% CI=1.81–4.73, p<0.0001; the heterozygote comparison: OR=1.25, 95% CI=1.06–1.46, p=0.006; the dominant model: OR=1.33, 95% CI=1.14–1.55, p=0.0003; and the recessive model: OR=2.28, 95% CI=1.04–4.98, p=0.04). All data are shown in Table 2.
In the subgroup analyses by the result of HWE test, significantly elevated risks were associated with SP110 polymorphisms for allelic comparisons (OR=1.31, 95% CI=1.15–1.49, p<0.0001), the homozygote comparison (OR=2.32, 95% CI=1.46–3.69, p=0.0001), the heterozygote comparison (OR=1.34, 95% CI=1.11–1.60, p=0.002), the dominant model (OR=1.42, 95% CI=1.19–1.69, p<0.0001), and the recessive model (OR=1.47, 95% CI=1.11–1.95, p=0.008) (Table 2).
Additionally, we also found significantly elevated TB risks in high quality score subgroups in all genetic models. In addition, moderate heterogeneity existed in the overall analysis of the recessive model but disappeared in the HWE subgroups of the model. No significant heterogeneity was found in other four comparisons.
Comprehensive analysis: rs1135791 and rs11556887 polymorphisms
In this meta-analysis, no evident association between SNP rs1135791 polymorphisms and susceptibility of TB was identified in all genetic models. Meanwhile, however, a significant positive correlation, was reached for the SNP rs1135791 in studies with large sample sizes for the dominant model (OR=0.80, 95% CI=0.70–0.90, p=0.0004) and heterozygote comparison (OR=0.81, 95% CI=0.71–0.92, p=0.001). However, in smaller sample sizes, opposite but equally meaningful results were found in the dominant model (OR=1.80, 95% CI=1.23–2.63, p=0.003) and the heterozygote comparison (OR=1.80, 95% CI=1.23–2.63, p=0.003) (Table 2). For SNP rs11556887, the recessive model revealed a relation between this polymorphism and TB risk (OR=1.23, 95% CI=1.02–1.48, p=0.03). We further performed a stratified analysis among Asian subjects, and the synthesized results highlighted significant associations between this polymorphism and TB risk in the heterozygote comparison (OR=1.56, 95% CI=1.20–2.02, p=0.0008) and the dominant model (OR=1.61, 95% CI=1.25–2.07, p=0.0002) (Table 2). In addition, for SNPs rs1135791 and rs11556887, we identified moderate heterogeneity in the overall analyses of the allelic model, the heterozygote comparison and the dominant model. Nevertheless, heterogeneity decreased in HWE subgroups.
Meta-analysis: other seven SNP polymorphisms
We find no evidence that could confirm the correlation of TB risk with seven other SNP polymorphisms in all genetic models and subgroup analysis. The analytical results are shown in Supplementary Table 4, only online.
Sensitivity and publication bias analysis
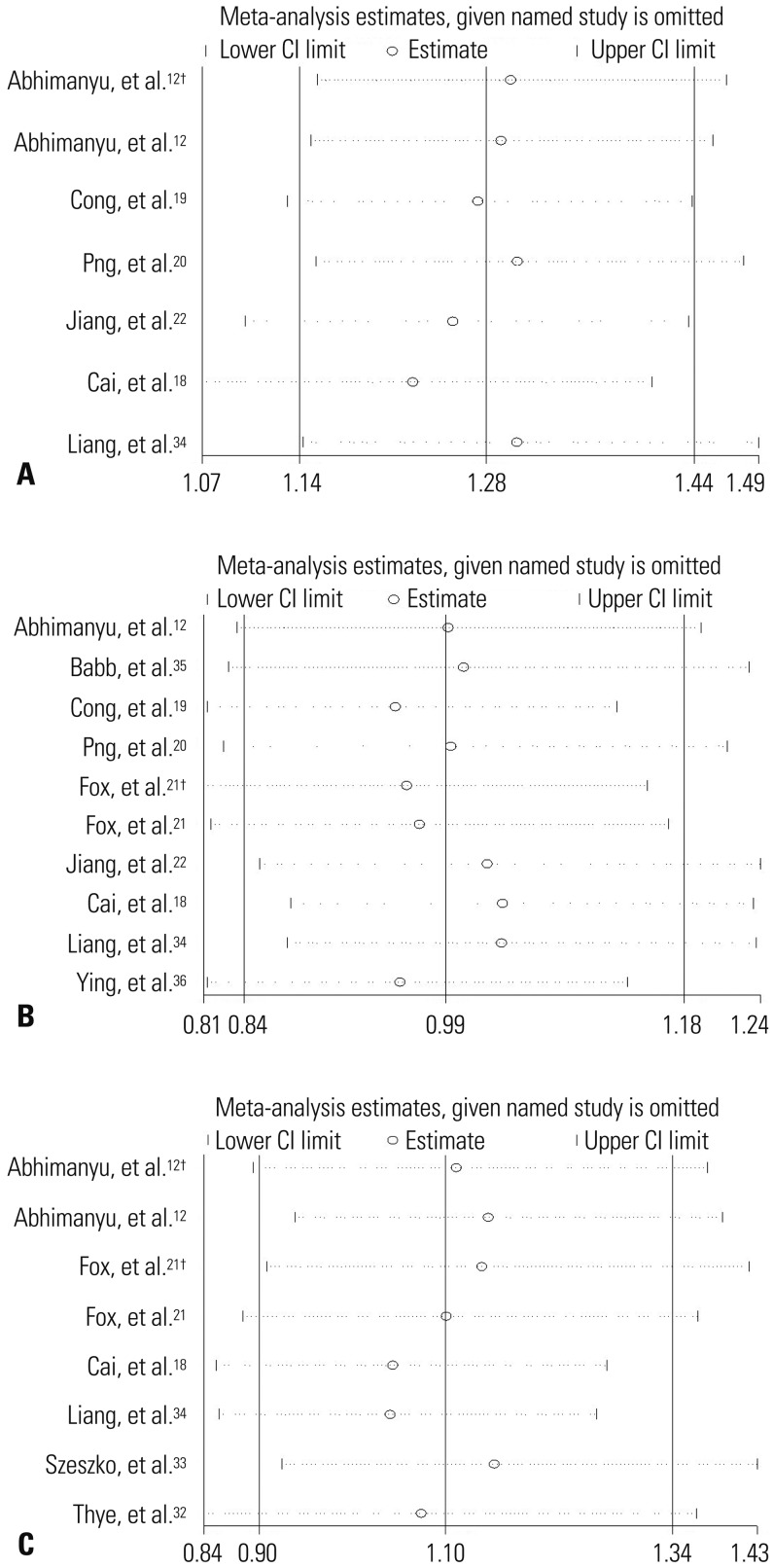
On the basis of allelic comparison, sensitivity analysis showed that there is just one article respectively influencing the result of this meta-analysis for SNPs rs3948464, rs11679983, and rs643 6915. For other SNPs, any selected study failed to observably change the pooled ORs, indicating the stability and reliability of our meta-analysis results. The results of the sensitivity analysis for SNPs rs9061, rs1135791, and rs11556887 are shown in Fig. 3. Publication bias was evaluated by carrying out Begg’s and Egger’s test. All p values for the Begg’s and Egger’s tests were greater than 0.05, proving the inexistence of publication bias.
Fig. 3. Sensitivity analyses on associations between rs9061 (A), rs1135791 (B), rs11556887 polymorphisms (C), and TB risk. Results were computed by omitting each study (left column) in turn. †We treat the article as two independent studies on account of having data of two kinds of TB. CI, confidence interval; TB, tuberculosis.
DISCUSSION
TB is a complex disease, in which both genetic and environmental factors have a dramatically vital important effect.37 SP110 could affect the evolution of the body infected with TB by participating in signal transmission processes between nuclear hormone receptors and adjusting the biological activity of macrophages, which could protect against TB. Concerning the effect of SP110 polymorphisms on TB, contradictory results were observed by previous studies. In the present study, meta-analysis was the priority selection in order to more accurately assess the effect of SNPs of SP110 on TB risk. Our meta-analysis identified that SNP rs9061 in SP110 gene could increase the susceptibility of TB, which may be a risk factor for the immunity of the body to TB. Recently, Lei, et al.38 collected data from 6 articles on five SP110 gene polymorphisms, and found no association between SP110 polymorphisms and TB risk. However, our present study is more comprehensive and persuasive in the following aspects: first, we focused on a broader range of SP110 gene polymorphisms (n=11); second, owing to relatively large study number, we conducted more subgroup analyses based on the result of HWE, ethnicities, quality assessment scores and sample sizes; and third, our study doubled the number of relevant studies compared to previous meta-analysis. It is highly possible that there are some flaws in the process of previous meta-analysis, such as the accuracy of data extraction method and the exact number of articles included.
In the present meta-analysis, we observed that the minor allele A was associated with a 28% increased TB risk compared with the G allele. Considering that SNP rs9061 induces an amino acid change from glutamic acid to lysine at codon position 207 of SP110 protein, we hypothesized that this locus, transforming acidic amino acid to basic amino acid, may have a potential to alter the protein structure. Subsequent bioinformatics using Anthe-2000 software (Institute of Biology and Chemistry of Proteins, France).39 suggested that the A allele may result in obvious changes of α-helix and β-sheet in the secondary structure of SP110 protein compared with the G allele. By predicting the potential functions of SNP rs6091 in the Mutation Taster database40 (http://www.mutationtaster.org/), we found that the minor allele A of SNP rs9061, which increased TB risk in this meta-analysis, may also lead to a splicing change of SP110. These findings partially supported the above hypothesis. Functional studies are required to elucidate the exact effects of SNP rs9061 on SP110 gene and TB risk.
When we assessed the associations of susceptible loci with complex disease like TB, large sample size in a study can increase statistical power, avoid selection bias and consequently make the results more stable. In subgroup analyses stratified by sample size, statistically significant increase of TB risk relevant to the SNP rs9061 polymorphisms was identified in the large sample size subgroup. For SNP rs1135791 polymorphisms, a protective role of the T allele on the development of TB was discovered in the large sample size subgroup under the heterozygote comparison and the dominant model, which resembled the overall analytical result. However, an almost completely opposite outcome was discerned in the small sample size subgroup with a pooled OR of 1.80. A small sample study with finite participants might have the probability to falsely estimate the association between SP110 variants and TB due to the selection bias of patients and controls.41 On the other hand, participants of studies were unable to represent overall situation of the population, because both two studies of small sample size failed to detect the homozygote CC genotype. Furthermore, the result of subgroup analysis by ethnicities revealed that SNP rs11556887 polymorphism could increase the susceptibility to TB in Asian populations. Therefore, although it is well agreed that there exist differences of polymorphisms between ethnicities, this result might also due to relatively small number of study.
In the present meta-analysis, we performed a sensitivity analysis for each study under the allele comparison of 11 SNPs. The results showed that most studies failed to clearly change the pooled OR, suggesting the higher stability of our analytical results. However, two studies reported by Cong, et al.19 and Abhimanyu, et al.12 showed a great effect on the pooled OR of several SNPs. Specifically, in the study of Cong, et al.19, the calculation of the pooled OR of SNPs rs3948464 and rs11679983 were transformed from a fixed-effect model. Similar influence was also identified in the study of Abhimanyu, et al.12 on the SNP rs6436915. Our careful review uncovered that, in the former study, SNPs rs3948464 and rs11679983 were genotyped by PCR-RFLP and ASA-PCR, respectively, while other six SNP polymorphisms were detected by NASP-PCR and DNA sequencing. The difference of genotyping method might affect the reliability of the result. The latter study selected only female patients with cervical tubercular lymphadenopathy to assess the association of SP110 polymorphisms with TB, thus not representing general situation of whole population. Selection of reliable genotyping method and widely representative population play a vital effect on the quality of experimental result.
Although we obtained credible results by designing strict inclusion and exclusion criteria, and also by collecting comprehensive literatures. However, our study has certain limitations. First, we explored 11 polymorphisms of SP110, far less than the number of reported SNPs associated with TB, thus being unable to fully profile the association of the entire SP110 gene and the risk of TB. Second, in addition to genetic factors, psychosocial factors and environmental factors also play an important role in the occurrence of TB. However, because of the limitation of data source, we did not analyze the interaction between these factors and genetic factors. Third, although we performed subgroup analyses, moderate heterogeneity still existed in some subgroups.
In conclusion, our present results provide strong evidence that the variant of SNP rs9061 may be a dangerous factor to the development of TB. The result may be exploited to provide genetic advice for the prevention and treatment of TB. On the other hand, however, the occurrence of TB is a complex process, in which multiple genes interact with environmental factors. To fully elucidate the effect of SP110 genes on its susceptibility to TB, we should further analyze the interaction of SP110 gene with other genes as well as environmental factors in the future.
Footnotes
The authors have no financial conflicts of interest.
Supplementary Material
Scale for Quality Assessment
Quality Assessment Scores of Studies Selected to Evaluate the Association of SP110 Polymorphisms and the Risk of TB
Data Extraction of SNP Polymorphisms of SP110 (m/n: m represent the number of case; n represent the number of control)
Overall and Subgroup Analysis and Heterogeneity of Other Seven SP110 Gene Polymorphisms with the Risk of TB
Linkage disequilibrium plots of SNPs of SP110 gene with tuberculosis in Chinese population (A) and European population (B). r2 values are displayed within each diamond. High r2 values are dark, and low r2 values are light. SNP, single nucleotide polymorphism.
References
- 1.Sakamoto K. The pathology of Mycobacterium tuberculosis infection. Vet Pathol. 2012;49:423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–486. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 4.Comstock GW. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–624. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 5.Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 6.Tosh K, Campbell SJ, Fielding K, Sillah J, Bah B, Gustafson P, et al. Variants in the SP110 gene are associated with genetic susceptibility to tuberculosis in West Africa. Proc Natl Acad Sci U S A. 2006;103:10364–10368. doi: 10.1073/pnas.0603340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möller M, Hoal EG. Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberculosis (Edinb) 2010;90:71–83. doi: 10.1016/j.tube.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Liu P, Wang L, Liu J, Yuan X, Meng W, et al. Effect of Ipr1 on expression levels of immune genes related to macrophage anti-infection of mycobacterium tuberculosis. Int J Clin Exp Med. 2015;8:3411–3419. [PMC free article] [PubMed] [Google Scholar]
- 10.Kramnik I. Genetic dissection of host resistance to Mycobacterium tuberculosis: the sst1 locus and the Ipr1 gene. Curr Top Microbiol Immunol. 2008;321:123–148. doi: 10.1007/978-3-540-75203-5_6. [DOI] [PubMed] [Google Scholar]
- 11.Apt AS. Are mouse models of human mycobacterial diseases relevant? Genetics says: ‘yes!’. Immunology. 2011;134:109–115. doi: 10.1111/j.1365-2567.2011.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abhimanyu, Jha P, Jain A, Arora K, Bose M. Genetic association study suggests a role for SP110 variants in lymph node tuberculosis but not pulmonary tuberculosis in north Indians. Hum Immunol. 2011;72:576–580. doi: 10.1016/j.humimm.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Wang Y, Zhang Y, Yang M, Lv J, Liu J, et al. TALE nickase-mediated SP110 knockin endows cattle with increased resistance to tuberculosis. Proc Natl Acad Sci U S A. 2015;112:E1530–E1539. doi: 10.1073/pnas.1421587112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Li G. Nuclear body Sp110 and its biological functions. J Med Mol Biol. 2006;4:271–274. [Google Scholar]
- 15.Bloch DB, Nakajima A, Gulick T, Chiche JD, Orth D, de La Monte SM, et al. Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol Cell Biol. 2000;20:6138–6146. doi: 10.1128/mcb.20.16.6138-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy R. Genetic susceptibility to tuberculosis. Clin Chest Med. 2005;26:233–246. doi: 10.1016/j.ccm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Cai L, Deng SL, Liang L, Pan H, Zhou J, Wang MY, et al. Identification of genetic associations of SP110/MYBBP1A/RELA with pulmonary tuberculosis in the Chinese Han population. Hum Genet. 2013;132:265–273. doi: 10.1007/s00439-012-1244-5. [DOI] [PubMed] [Google Scholar]
- 19.Cong J, Li G, Zhou D, Tao Y, Xiong Y. [Study on relation between Sp110 gene polymorphism and tuberculosis genetic susceptibility of Chongqing Han People] Wei Sheng Yan Jiu. 2010;39:540–544. [PubMed] [Google Scholar]
- 20.Png E, Alisjahbana B, Sahiratmadja E, Marzuki S, Nelwan R, Adnan I, et al. Polymorphisms in SP110 are not associated with pulmonary tuberculosis in Indonesians. Infect Genet Evol. 2012;12:1319–1323. doi: 10.1016/j.meegid.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Fox GJ, Sy DN, Nhung NV, Yu B, Ellis MK, Van Hung N, et al. Polymorphisms of SP110 are associated with both pulmonary and extrapulmonary tuberculosis among the Vietnamese. PLoS One. 2014;9:e99496. doi: 10.1371/journal.pone.0099496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang SY, Li LL, Yue J, Chen WZ, Yang C, Wan CL, et al. The effects of SP110’s associated genes on fresh cavitary pulmonary tuberculosis in Han Chinese population. Clin Exp Med. 2016;16:219–225. doi: 10.1007/s10238-015-0339-4. [DOI] [PubMed] [Google Scholar]
- 23.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: a HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–270. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu XC, Yu W, Tao Y, Zhao PL, Li K, Tang LJ, et al. Contribution of transforming growth factor α polymorphisms to nonsyndromic orofacial clefts: a HuGE review and meta-analysis. Am J Epidemiol. 2014;179:267–281. doi: 10.1093/aje/kwt262. [DOI] [PubMed] [Google Scholar]
- 25.Liang J, Lin C, Hu F, Wang F, Zhu L, Yao X, et al. APC polymorphisms and the risk of colorectal neoplasia: a HuGE review and meta-analysis. Am J Epidemiol. 2013;177:1169–1179. doi: 10.1093/aje/kws382. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Liu J, Zhou Y, Ying J, Zou H, Guo S, et al. Predictive value of XRCC1 gene polymorphisms on platinum-based chemotherapy in advanced non-small cell lung cancer patients: a systematic review and meta-analysis. Clin Cancer Res. 2012;18:3972–3981. doi: 10.1158/1078-0432.CCR-11-1531. [DOI] [PubMed] [Google Scholar]
- 27.Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162:201–211. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thye T, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, et al. No associations of human pulmonary tuberculosis with Sp110 variants. J Med Genet. 2006;43:e32. doi: 10.1136/jmg.2005.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szeszko JS, Healy B, Stevens H, Balabanova Y, Drobniewski F, Todd JA, et al. Resequencing and association analysis of the SP110 gene in adult pulmonary tuberculosis. Hum Genet. 2007;121:155–160. doi: 10.1007/s00439-006-0293-z. [DOI] [PubMed] [Google Scholar]
- 34.Liang L, Zhao YL, Yue J, Liu JF, Han M, Wang H, et al. Association of SP110 gene polymorphisms with susceptibility to tuberculosis in a Chinese population. Infect Genet Evol. 2011;11:934–939. doi: 10.1016/j.meegid.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Babb C, Keet EH, van Helden PD, Hoal EG. SP110 polymorphisms are not associated with pulmonary tuberculosis in a South African population. Hum Genet. 2007;121:521–522. doi: 10.1007/s00439-007-0335-1. [DOI] [PubMed] [Google Scholar]
- 36.Ying X, Hui L, Yu HD, Jie L, Jing S, Long W, et al. Interaction of SP110 and VDR gene polymorphisms with environmental factors in tuberculosis. J Reg Anat Oper Sorg. 2013;04:377–379. [Google Scholar]
- 37.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei X, Zhu H, Zha L, Wang Y. SP110 gene polymorphisms and tuberculosis susceptibility: a systematic review and meta-analysis based on 10 624 subjects. Infect Genet Evol. 2012;12:1473–1480. doi: 10.1016/j.meegid.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Deléage G, Combet C, Blanchet C, Geourjon C. ANTHEPROT: an integrated protein sequence analysis software with client/server capabilities. Comput Biol Med. 2001;31:259–267. doi: 10.1016/s0010-4825(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 41.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304. doi: 10.1136/bmj.f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scale for Quality Assessment
Quality Assessment Scores of Studies Selected to Evaluate the Association of SP110 Polymorphisms and the Risk of TB
Data Extraction of SNP Polymorphisms of SP110 (m/n: m represent the number of case; n represent the number of control)
Overall and Subgroup Analysis and Heterogeneity of Other Seven SP110 Gene Polymorphisms with the Risk of TB
Linkage disequilibrium plots of SNPs of SP110 gene with tuberculosis in Chinese population (A) and European population (B). r2 values are displayed within each diamond. High r2 values are dark, and low r2 values are light. SNP, single nucleotide polymorphism.