Abstract
Purpose
Adequate hemostasis is important for postoperative outcomes of abdominal surgery. This study evaluated the hemostatic effects and accompanying histopathological changes of a novel oxidized regenerated cellulose, SurgiGuard®, during abdominal surgery.
Materials and Methods
Ten pigs underwent wedge resection of the spleen (1×1 cm) and liver (1.5×1.5 cm). The resected surface was covered with Surgicel® fabric or fibril type (Group A) or SurgiGuard® fabric or fibril type (Group B). Surgicel® and SurgiGuard® were randomized for attachment to the resected surface by fabric type (n=5) or fibril type (n=5). Blood loss was measured 5, 7, and 9 min after resection. Pigs were necropsied 6 weeks postoperatively to evaluate gross and histopathological changes.
Results
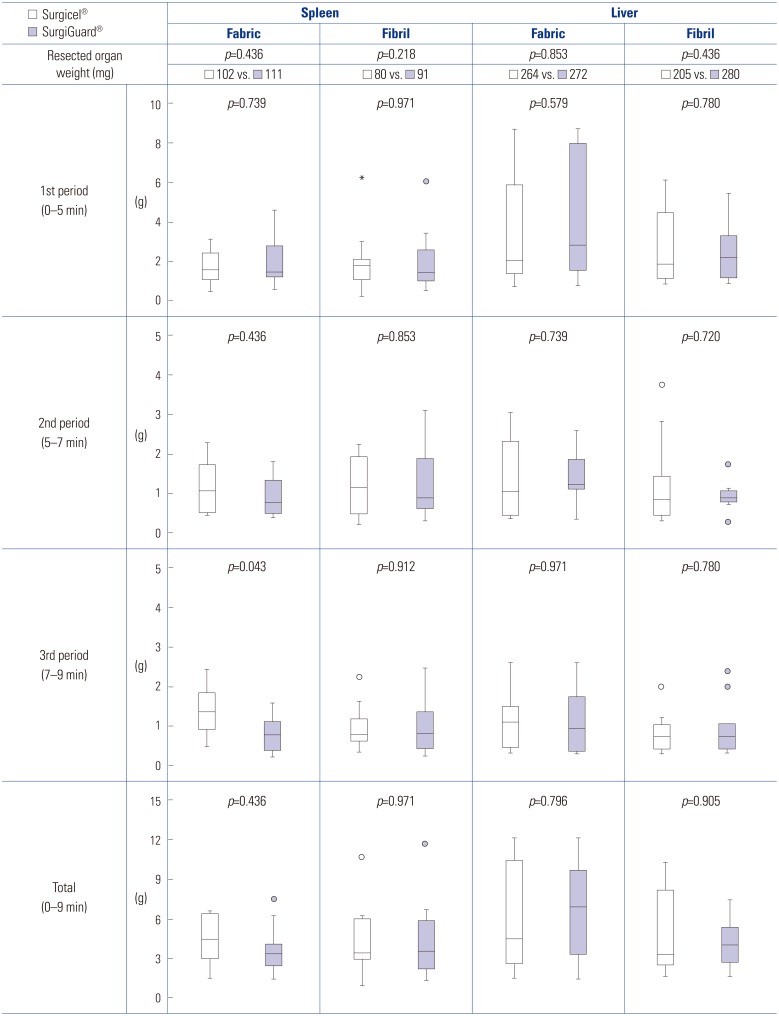
There was no significant difference in total blood loss between groups [spleen fabric: Group A vs. Group B, 4.38 g (2.74–6.43) vs. 3.41 g (2.46–4.65), p=0.436; spleen fibril: Group A vs. Group B, 3.44 g (2.82–6.07) vs. 3.60 g (2.03–6.09), p=0.971; liver fabric: Group A vs. Group B, 4.51 g (2.67–10.61) vs. 6.93 g (3.09–9.95), p=0.796; liver fibril: Group A vs. Group B, 3.32 g (2.50–8.78) vs. 3.70 g (2.32–5.84), p=0.971]. Histopathological analysis revealed no significant difference in toxicities related to Surgicel® or SurgiGuard® [inflammation, fibrosis, foreign bodies, and hemorrhage (spleen: p=0.333, 0.127, 0.751, and 1.000; liver: p=0.155, 0.751, 1.000, and 1.000, respectively)].
Conclusion
SurgiGuard® is as effective and non-toxic as Surgicel® in achieving hemostasis after porcine abdominal surgery.
Keywords: Animal model, blood loss, hemostatics, histopathology, oxidized regenerated cellulose
INTRODUCTION
Postoperative intra-abdominal bleeding is associated with significant morbidity and mortality.1 There are several hemostatic techniques, such as mechanical means, thermal devices, and topical hemostatic agents, for preventing postoperative hemorrhage.2,3,4 Since each technique has advantages and disadvantages, surgeons use them selectively depending on the operation field situation. However, despite efforts to prevent postoperative bleeding, unwanted bleeding does occur. Due to continuous improvement in hemostatic techniques, nonetheless, the incidence of postoperative bleeding has decreased, with significant events reported in less than 1% of surgeries.5,6,7
Minor postoperative bleeding likely occurs frequently, but goes undetected. Though this minor bleeding does not affect hemodynamic instability, it can cause complications, such as infection, inflammation, and adhesion.8 Moreover, these complications may affect the recurrence of cancer in cancer surgery,9,10 making reduction of blood loss during surgery an important issue.
Unlike mechanical means and thermal devices, topical hemostatic agents can have a hemostatic effect after surgery, as they remain in the intra-abdomen. Topical hemostatic agents are also used before completion of surgery, and several are available in a range of configurations. Among topical hemostatic agents, oxidized regenerated cellulose (ORC) has been in use for several decades. ORC conducts hemostatic action through blood absorption, surface interaction with platelets (PLTs) and proteins, and coagulation cascade activation.11 Since its introduction in 1943, several commercial ORC products have been developed,12 and are now frequently used in most gastrointestinal and non-gastrointestinal operations.13,14
A novel ORC system, SurgiGuard® (Samyang Biopharmaceuticals Corp., Seoul, Korea), has received approval from the Korean FDA (product license no. 47, 30/09/2014 KFDA). The present study was performed to evaluate the hemostatic and pathologic effects of SurgiGuard® in abdominal surgery using a reproducible and clinically relevant porcine model.
MATERIALS AND METHODS
SurgiGuard® fabric and SurgiGuard® fibrillar
SurgiGuard® is an ORC designed to assist in the control of capillary, venous, and small arterial hemorrhage. Fundamentally, it is almost the same material as Surgicel®. SurgiGuard® fabric is a densely knit material similar to Surgicel® Nu-Knit, and SurgiGuard® fibrillar is a soft, layered material similar to Surgicel® Fibrillar. Experimental animals were divided into two groups: Group A [Surgicel® (Ethicon, Cincinnati, OH, USA)] and Group B [SurgiGuard® (Samyang Biopharmaceuticals Corp.)].
Experimental animals and preoperative laboratory parameters
This study was approved by Yonsei University College of Medicine, Institutional Animal Care and Use Committee (Approval No. IACUC 2015-0084). Ten female pigs (25–30 kg) (XP Bio pig supplies and services, Ansung, Korea) were used in the study. All animals were kept according to Association of Assessment and Accreditation of Laboratory Animal Care International (AAALAC) standards in the mid-large sized animal facility in Avison Biomedical Research Center (AAALAC accreditation No. 1071) with regular water and food supply under constant temperature (22℃) and moisture level (55%). Before surgery, all pigs were weighed and blood samples were taken to determine the complete blood cell count, including white blood cell (WBC), hemoglobin (Hb), PLT, and lymphocyte counts, C-reactive protein (CRP), and liver profiles, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, albumin, alkaline phosphatase, and total bilirubin.
Surgery preparation
Anesthesia was induced with Zoletil® (Grovet, Utrecht, the Netherlands) and Rompun® (Bayer, Santa Ana, CA, USA), and the hair in the surgical region (upper abdomen) was removed. Endotracheal intubation was performed and anesthesia was maintained by isoflurane inhalation. The pigs were monitored during the procedure by pulse and oxygen saturation. The surgical region was disinfected with povidone iodine, followed by the opening of the upper abdominal cavity for liver and spleen exposure.
Spleen wedge resection
After exposure of the spleen, resection points were marked on each side at 6 and 12 cm proximal to the distal end of the spleen. Parenchymal wedge resection of the spleen was performed using Mayo scissors. The resected volume was 1×1 cm at each site. The ORC (either Surgicel® or SurgiGuard®) was then applied to the resection margin and covered by a cotton gauze sheet, and the resected spleen was weighed.
Blood loss was measured at the time of the exposure of the resection margin. Blood loss was measured three times for 9 minutes after the resection. The 1st period was up to 5 minutes after resection, the 2nd between 5 and 7 minutes, and the 3rd between 7 and 9 minutes. For each period, the cotton gauze sheet and ORC were weighed and exchanged for new ones. If complete hemostasis was not achieved after the 3rd period (at 9 minutes after resection), a mechanical or thermal method was performed to stop the bleeding.
Liver wedge resection
Parenchymal wedge resection of the liver was performed in a similar way to the spleen. After exposure of the liver, resection points were marked on the left lateral and left medial lobes. The resected volume was 1.5×1.5 cm at each site. Each ORC was then applied to the resection margin and covered by a cotton gauze sheet. Measurement of the resected organs and blood loss were performed as described in next section.
Quantification of bleeding
Bleeding was quantified as follows. One sheet of a sterilized waterproof surgical drape was placed below the region of resection, and blood was absorbed by sterilized gauze immediately after the organ resection. The blood in the surgical field was also absorbed by sterilized gauze. The wet gauze was then weighed, and blood loss was calculated as the difference between the weight of the wet gauze and the premeasured weight of the dry gauze.
After quantification of blood loss, the surgical region was arranged to avoid adhesion formation with the resection margin of the spleen and liver. The muscle and skin were then sutured. The time of every procedure of the operation was recorded.
Postoperative care and follow-up laboratory parameters
Following surgery, the pigs were permitted to consume food and water as normal upon recovery from anesthesia. They were subsequently monitored on a regular basis once per day. If any abnormality was found, the type and date of occurrence and severity of symptoms were recorded for each abnormality. All pigs were weighed once per week throughout the experimental period. One week after the operation, a blood sample was taken to measure the same parameters that were determined preoperatively.
Pathology
Necropsy was performed 6 weeks after surgery. All pigs were fasted before necropsy for at least 12 hours. Prior to receiving anesthesia, another set of blood samples was obtained from each pig to analyze the same parameters as the preoperative samples. The animals were then deeply anesthetized with Zoletil® and Rompun® and euthanized by exsanguination from the carotid artery.
The resected spleens and livers were examined grossly, and then, resection margins of the spleens and livers were marked using dye and fixed in a 10% neutral formalin solution. After 2 days, the resected spleens and livers were embedded in paraffin, and microsections with a thickness of 4–5 µm were obtained. Hematoxylin and eosin-stained slides were prepared, and the specimens were examined with an optical microscope and scored by an expert pathologist using the 0–4 Ehrlich and Hunt numerical scale as modified by Phillips, et al.15 Inflammation, foreign body reaction, fibrosis, and hemorrhage were graded from 0–4 as follows: 0, no evidence; 1, occasional evidence; 2, light scattering; 3, abundant evidence; and 4, confluent cells or fibers.
Statistical analyses
Differences in blood loss and histopathological changes between the two groups were evaluated by the Mann-Whitney test adding Bonferroni's method for correction of type I errors to perform pair-wise comparisons between groups. Changes in preoperative and postoperative laboratory parameters were evaluated using the Wilcoxon signed-rank test. The criterion for statistical significance was a p value <0.05. SPSS 20.1 software (IBM, Armonk, NY, USA) were used for the analyses. The data were presented using the nonparametric method, except where otherwise indicated.
RESULTS
Hemostasis during wedge resection and changes in laboratory parameters
There was no significant difference in the weight of the resected spleen and liver between the two groups. There was also no significant difference in total blood loss between the two groups. Upon subgroup analysis, no significant differences in blood loss were found, except in the subgroup of blood loss in the spleen at 7–9 minutes for fabric type [Group A vs. Group B, 1.35 g (0.85–1.94) vs. 0.81 g (0.42–1.12), p=0.043] (Fig. 1).
Fig. 1. Weight of the resected organs and blood loss in accordance with time and type.
When preoperative laboratory parameters were compared to those on postoperative day (POD) 7, the PLT count was significantly increased [preoperative vs. POD 7, 182 103/µL (72–309) vs. 245 103/µL (182–305), p=0.049]. However, this significant difference was not present on POD 42. On the other hand, the weight of the pigs significantly increased [preoperative vs. POD 42: 27.3 kg (26.5–28.9) vs. 31.1 kg (28.2–32.7), p=0.002], and significant differences were found for AST and ALT. Despite these differences, all values were within normal range (Table 1).
Table 1. Changes between Preoperative and Postoperative Laboratory Parameters.
| Paremeter | Preoperative | POD 7 | p value | POD 42 | p value |
|---|---|---|---|---|---|
| Weight | 27.3 (26.5–28.9) | 27.5 (26.2–29.1) | 0.787 | 31.1 (28.2–32.7) | 0.002 |
| WBC | 17.52 (12.60–20.56) | 16.58 (13.97–21.36) | 1.000 | 15.29 (12.40–15.89) | 0.105 |
| Hb | 8.1 (7.1–10.9) | 7.5 (7.0–8.0) | 0.176 | 7.0 (6.3–7.7) | 0.059 |
| PLT | 182 (72–309) | 245 (182–305) | 0.049 | 258 (189–315) | 0.322 |
| CRP | 0.2 (0.2–0.3) | 0.25 (0.2–0.3) | 1.000 | 0.2 (0.2–0.3) | 1.000 |
| Lymphocyte | 8.73 (7.35–11.09) | 9.56 (6.30–10.36) | 0.695 | 9.33 (6.95–9.72) | 0.625 |
| AST | 32 (25–34) | 31 (28–37) | 0.426 | 20 (17–28) | 0.023 |
| ALT | 27 (25–30) | 24 (22–33) | 0.682 | 39 (32–46) | 0.027 |
| TP | 6.0 (5.5–6.4) | 6.1 (5.7–6.7) | 0.430 | 6.1 (5.7–6.4) | 0.791 |
| ALB | 3.0 (2.7–3.3) | 2.8 (2.5–3.1) | 0.086 | 3.2 (3.0–3.4) | 0.215 |
| ALP | 320 (236–353) | 221 (174–371) | 0.492 | 270 (207–302) | 0.322 |
| TBIL | 0.1 (0.1–0.3) | 0.1 (0.1–0.2) | 1.000 | 0.1 (0.1–0.1) | 0.250 |
POD, postoperative day; WBC, white blood cells; Hb, hemoglobin; PLT, platelet; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TP, total protein; ALB, albumin; ALP, alkaline phosphatase; TBIL, total bilirubin.
Necropsy and histopathological findings
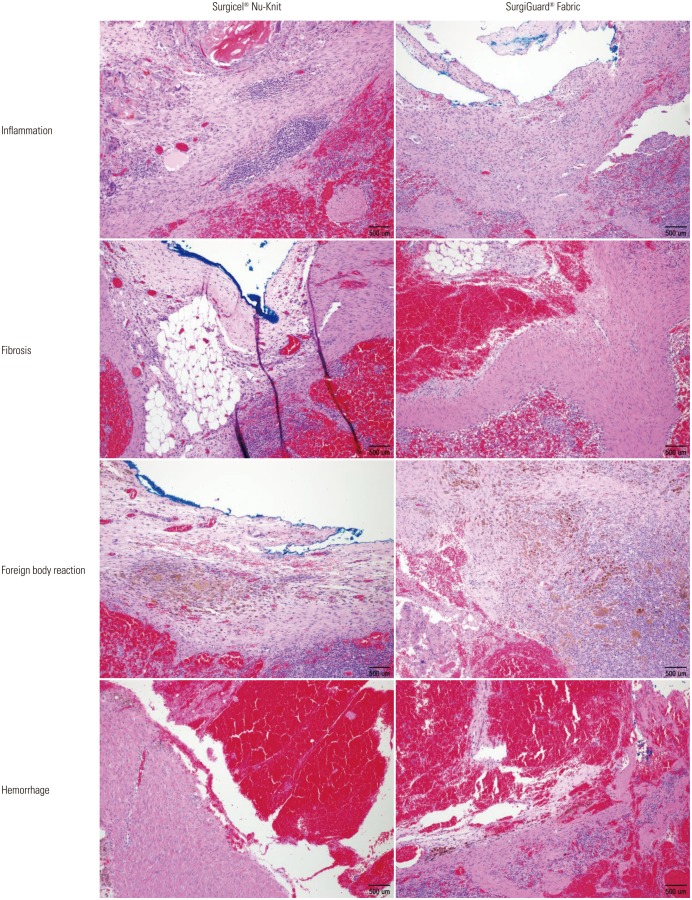
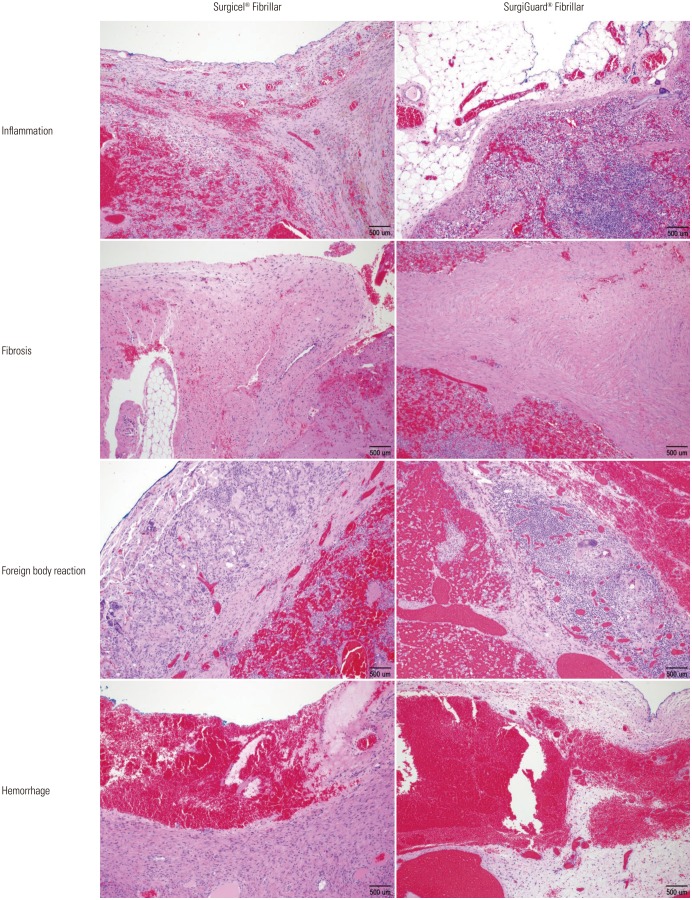
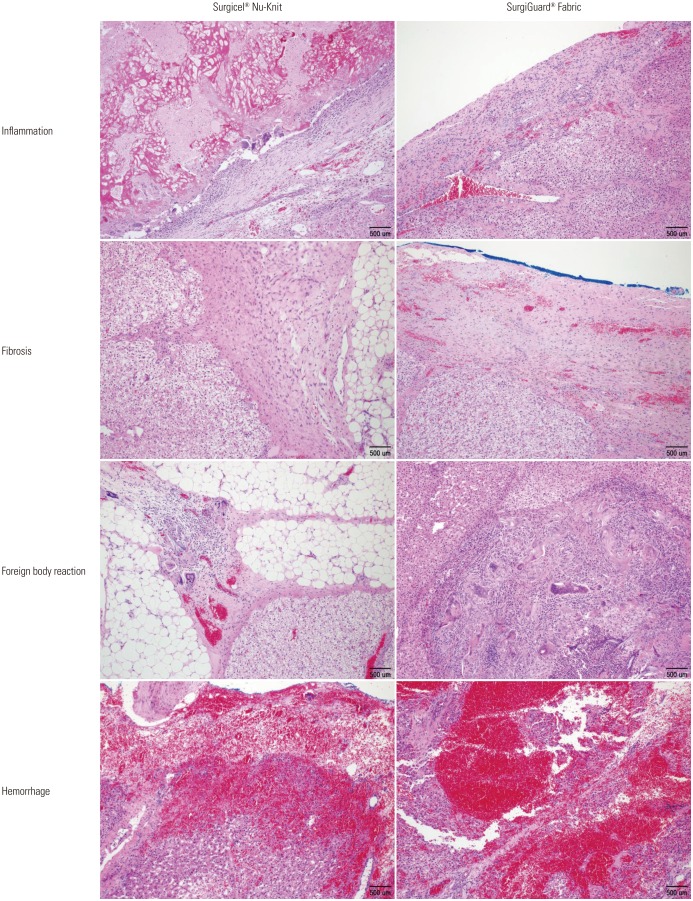
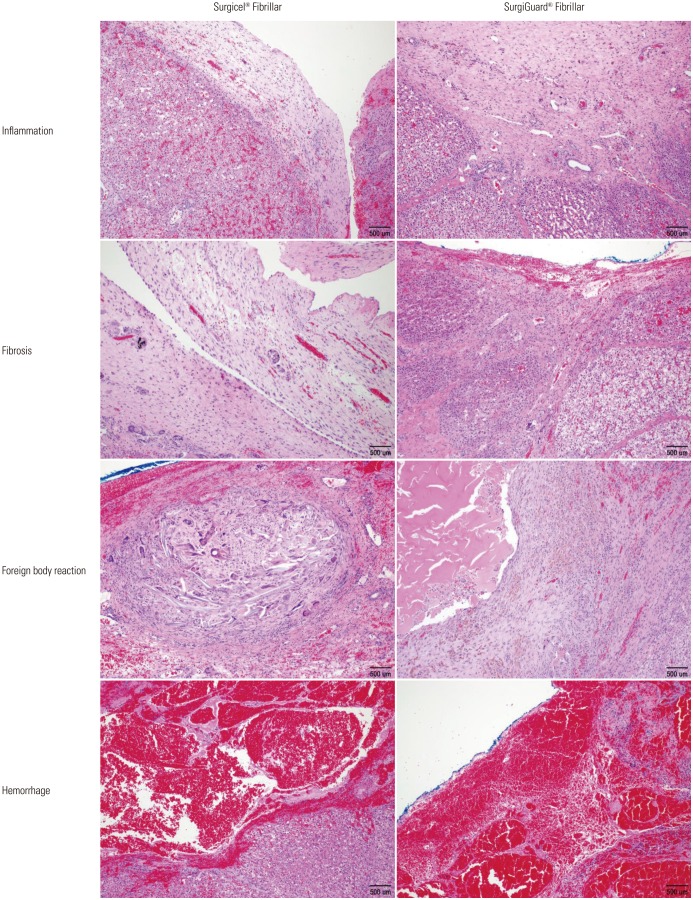
No hematoma, granuloma, or severe adhesion was grossly observed in any of the pigs at necropsy. Histopathological analysis of the spleen revealed no significant differences in inflammation, fibrosis, foreign bodies, or hemorrhage between the two groups in accordance with the grading system (Table 2, Figs. 2 and 3). Similar results were found upon histopathological analysis of the liver (Table 3, Figs. 4 and 5).
Table 2. Histopathological Findings of Resected Spleen.
| Type | Finding | Surgicel® (n=10) | SurgiGuard® (n=10) | p value |
|---|---|---|---|---|
| Fabric | Inflammation | 0.481 | ||
| G=0 | 9 | 7 | ||
| G=1 | 1 | 3 | ||
| G≥2 | 0 | 0 | ||
| Fibrosis | 0.063 | |||
| G=0 | 1 | 5 | ||
| G=1 | 7 | 5 | ||
| G≥2 | 2 | 0 | ||
| Foreign body reaction | 0.280 | |||
| G=0 | 5 | 8 | ||
| G=1 | 5 | 2 | ||
| G≥2 | 0 | 0 | ||
| Hemorrhage | 0.739 | |||
| G=0 | 8 | 7 | ||
| G=1 | 2 | 3 | ||
| G≥2 | 0 | 0 | ||
| Fibril | Inflammation | 0.481 | ||
| G=0 | 5 | 3 | ||
| G=1 | 5 | 7 | ||
| G≥2 | 0 | 0 | ||
| Fibrosis | 0.218 | |||
| G=0 | 1 | 2 | ||
| G=1 | 6 | 8 | ||
| G≥2 | 3 | 0 | ||
| Foreign body reaction | 0.912 | |||
| G=0 | 5 | 4 | ||
| G=1 | 4 | 6 | ||
| G≥2 | 1 | 0 | ||
| Hemorrhage | 0.739 | |||
| G=0 | 9 | 10 | ||
| G=1 | 1 | 0 | ||
| G≥2 | 0 | 0 |
Fig. 2. Microscopic findings of resected spleen surface (Fabric type, hematoxylin and eosin stain, ×100).
Fig. 3. Microscopic findings of resected spleen surface (Fibril type, hematoxylin and eosin stain, ×100).
Table 3. Histopathological Findings of Resected Liver.
| Type | Finding | Surgicel® (n=10) | SurgiGuard® (n=10) | p value |
|---|---|---|---|---|
| Fabric | Inflammation | 0.280 | ||
| G=0 | 9 | 6 | ||
| G=1 | 1 | 4 | ||
| G≥2 | 0 | 0 | ||
| Fibrosis | 0.393 | |||
| G=0 | 5 | 7 | ||
| G=1 | 4 | 3 | ||
| G≥2 | 1 | 0 | ||
| Foreign body reaction | 0.631 | |||
| G=0 | 7 | 6 | ||
| G=1 | 3 | 3 | ||
| G≥2 | 0 | 1 | ||
| Hemorrhage | 1.000 | |||
| G=0 | 2 | 2 | ||
| G=1 | 6 | 6 | ||
| G≥2 | 2 | 2 | ||
| Fibril | Inflammation | 0.481 | ||
| G=0 | 8 | 6 | ||
| G=1 | 2 | 4 | ||
| G≥2 | 0 | 0 | ||
| Fibrosis | 0.739 | |||
| G=0 | 5 | 5 | ||
| G=1 | 3 | 5 | ||
| G≥2 | 2 | 0 | ||
| Foreign body reaction | 0.739 | |||
| G=0 | 7 | 8 | ||
| G=1 | 3 | 2 | ||
| G≥2 | 0 | 0 | ||
| Hemorrhage | 0.165 | |||
| G=0 | 2 | 3 | ||
| G=1 | 2 | 5 | ||
| G≥2 | 6 | 2 |
Fig. 4. Microscopic findings of resected liver surface (Fabric type, hematoxylin and eosin stain, ×100).
Fig. 5. Microscopic findings of resected liver surface (Fibril type, hematoxylin and eosin stain, ×100).
DISCUSSION
The ideal topical hemostatic material should have ample hemostatic action and minimal tissue reactivity. It should also be inexpensive, biodegradable, and nonantigenic.16 Many topical hemostatic materials have been developed over the past several decades taking these parameters into account, and their clinical benefits and risks have been evaluated. Since ORC was first developed in 1943, numerous studies in various fields have described its hemostatic effects.11,12,14,17 Currently, ORC is used not only in laparotomy surgery, but also in endoscopic procedures.18 The results of our current study are similar to these previous reports.
Many protocols have been established to evaluate ORC materials.19,20,21 Most of these protocols, however, involve application of a topical hemostatic material to separate animals, introducing the possibility that the different hemostatic capacity of each animal could affect bleeding. In order to avoid this bias, we evaluated different ORCs in the same animal, and used pigs due to their reproducible size and similarity to human anatomy, which enabled us to perform several experiments. In addition, because blood loss and spontaneous hemostasis differs between organs, studies were carried out on both the spleen and liver to more accurately evaluate the hemostatic capacity of the ORCs.16
We found no statistically significant difference in total blood loss between the two groups. The only statistically significant difference in blood loss was in the spleen in the 7–9 minute subgroup of the fabric type. This result may indicate that the hemostatic effect of SurgiGuard® Fabric occurred earlier than that of Surgicel® Nu-Knit on the spleen. However, because there were no statistically significant differences in blood loss in other subgroups, we cannot state that the hemostatic effect of SurgiGuard® occurred earlier than Surgicel®.
Postoperative minor bleeding is rarely detected. However, it is important due to its relationship with complications,8 and ORC is also occasionally used in breast cancer surgery to prevent minor bleeding for aesthetic reasons.22 In our study, we found no significant difference in preoperative and postoperative Hb values, and no gross hematoma was detected at necropsy. Upon microscopic evaluation, several specimens displayed evidence of hemorrhage at the resected margin. However, most of this hemorrhage was grade I, and there was no statistically significant difference between the two groups. These data suggest that there is no significant difference in hemostatic capacity between the two ORCs.
As the presence of artificial materials increases susceptibility to inflammation and infection and is toxic to adjacent organs, most local hemostatic agents should not be used in an infected wound.23 However, due to its acidic pH, ORC is resistant to bacterial infection,24 and several studies have found that ORC actually prevents infection.25,26 In the current study, none of the animals exhibited significant preoperative to postoperative changes in WBC and CRP values, the presence of which may suggest infection. Although the PLT count significantly increased on POD 7 in this study, we believe this was due not to the toxicity of the material, but to temporary hyposplenism due to the impaired splenic function. In addition, this significance was not present on POD 42.27,28 Although there were also significant changes between preoperative and postoperative AST and ALT levels, these values were within the normal range, and thus not clinically relevant. In addition, there was no evidence of abscesses or other infection-related changes found at necropsy, and severe inflammation was not apparent upon microscopic evaluation. Therefore, the absence of significant differences between the two groups suggests that SurgiGuard® is not toxic to host cells.
ORC dissolves promptly at various sites, with complete dissolution by 6 weeks,12,29 which is particularly important in cancer surgery, because remnant ORC has occasionally been mistaken for abscess formation or recurrent mass.30,31 Although several remnant foreign bodies were found upon microscopic examination, there were no significant differences between the two groups and none were observed at POD 42 necropsy. These results suggest that SurgiGuard® has a suitable capacity for dissolving within a reasonable time frame.
As adhesion is the most common complication of abdominal surgeries, it is a concern when ORC is evaluated in abdominal surgery.32 Several studies have described the anti-adhesive effectiveness of ORC;33,34 Interceed® (Ethicon, Cincinnati, OH, USA) was developed to improve upon this aspect and is used in various types of surgery.35 In our study, the animals' weight constantly increased, and they showed no symptoms that suggested bowel obstruction. At necropsy, several pigs displayed minimal adhesion formation around the resected margin. However, it did not interfere with the necropsy, and microscopic examinations showed no significant differences in fibrosis between the two groups.
We designed our study to use the same animal for each group in order to establish equivalent experimental conditions. However, a limitation of our study is the small size of the experimental groups, which potentially influenced the evaluation of statistically significant outcomes. Moreover, although we strove to perform the wedge resection identically in each animal, there was an average error depending on the experimenters. Nevertheless, SurgiGuard® showed an efficacy on par with the reference material, Surgicel®, in hemostatic effects and postoperative pathologic changes.
In conclusion, our study suggests that SurgiGuard® could be as effective and non-toxic as Surgicel® in achieving hemostasis after porcine spleen and liver resection. In order to overcome the limitations of this study, future studies should be performed to provide more data regarding a systemic environment, as well as to obtain more similar resection margins.
ACKNOWLEDGEMENTS
This study was supported by a research grant of Samyang Biopharmaceuticals Corp. (SYM-1151).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Dagi TF. The management of postoperative bleeding. Surg Clin North Am. 2005;85:1191–1213. doi: 10.1016/j.suc.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Zwischenberger JB, Brunston RL, Jr, Swann JR, Conti VR. Comparison of two topical collagen-based hemostatic sponges during cardiothoracic procedures. J Invest Surg. 1999;12:101–106. doi: 10.1080/089419399272656. [DOI] [PubMed] [Google Scholar]
- 3.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 4.Tan SR, Tope WD. Effectiveness of microporous polysaccharide hemospheres for achieving hemostasis in mohs micrographic surgery. Dermatol Surg. 2004;30:908–914. doi: 10.1111/j.1524-4725.2004.30261.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanizawa Y, Bando E, Kawamura T, Tokunaga M, Ono H, Terashima M. Early postoperative anastomotic hemorrhage after gastrectomy for gastric cancer. Gastric Cancer. 2010;13:50–57. doi: 10.1007/s10120-009-0535-6. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Serrano MA, Parés D, Pera M, Pascual M, Courtier R, Egea MJ, et al. Management of lower gastrointestinal bleeding after colorectal resection and stapled anastomosis. Tech Coloproctol. 2009;13:49–53. doi: 10.1007/s10151-009-0458-6. [DOI] [PubMed] [Google Scholar]
- 7.Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB (Oxford) 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones S, May AK. Postoperative gastrointestinal hemorrhage. Surg Clin North Am. 2012;92:235–242. doi: 10.1016/j.suc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash GF, Chopada A, Patel H, Kakkar AK. Stored blood products stimulate cancer growth. Br J Surg. 2002;89:19. [Google Scholar]
- 11.Kanko M, Liman T, Topcu S. A low-cost and simple method to stop intraoperative leakage-type bleeding: use of the vancomycin-oxidized regenerated cellulose (ORC) sandwich. J Invest Surg. 2006;19:323–327. doi: 10.1080/08941930600889425. [DOI] [PubMed] [Google Scholar]
- 12.Frantz VK. Absorbable cotton, paper and gauze: (oxidized cellulose) Ann Surg. 1943;118:116–126. doi: 10.1097/00000658-194311810-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simo KA, Hanna EM, Imagawa DK, Iannitti DA. Hemostatic agents in hepatobiliary and pancreas surgery: a review of the literature and critical evaluation of a novel carrier-bound fibrin sealant (tachoSil) ISRN Surg. 2012;2012:729086. doi: 10.5402/2012/729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabel M, Stummer W. The use of local agents: surgicel and surgifoam. Eur Spine J. 2004;13(Suppl 1):S97–S101. doi: 10.1007/s00586-004-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am J Surg. 1992;163:71–77. doi: 10.1016/0002-9610(92)90255-p. [DOI] [PubMed] [Google Scholar]
- 16.Chvapil M, Owen JA, DeYoung DW. A standardized animal model for evaluation of hemostatic effectiveness of various materials. J Trauma. 1983;23:1042–1047. doi: 10.1097/00005373-198312000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Koea JB, Batiller J, Patel B, Shen J, Hammond J, Hart J, et al. A phase III, randomized, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgery. HPB (Oxford) 2013;15:61–70. doi: 10.1111/j.1477-2574.2012.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velázquez-Aviña J, Mönkemüller K, Sakai P, Sulbaran M, Chávez-Vargas C, Montalvo Javé E, et al. Hemostatic effect of oxidized regenerated cellulose in an experimental gastric mucosal resection model. Endoscopy. 2014;46:878–882. doi: 10.1055/s-0034-1365494. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson RW, Broughton D, Barbolt TA, Poandl T, Muench T, Rockar R, et al. Hemostatic effectiveness of Fibrin pad after partial nephrectomy in swine. J Surg Res. 2011;167:e291–e298. doi: 10.1016/j.jss.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Matonick JP, Hammond J. Hemostatic efficacy of EVARREST™, Fibrin Sealant Patch vs. TachoSil® in a heparinized swine spleen incision model. J Invest Surg. 2014;27:360–365. doi: 10.3109/08941939.2014.941444. [DOI] [PubMed] [Google Scholar]
- 21.Muench TR, Kong W, Harmon AM. The performance of a hemostatic agent based on oxidized regenerated cellulose--polyglactin 910 composite in a liver defect model in immunocompetent and athymic rats. Biomaterials. 2010;31:3649–3656. doi: 10.1016/j.biomaterials.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 22.Giuliani M, Fubelli R, Patrolecco F, Rella R, Borelli C, Buccheri C, et al. Mammographic and ultrasonographic findings of oxidized regenerated cellulose in breast cancer surgery: a 5-year experience. Clin Breast Cancer. 2015;15:e249–e256. doi: 10.1016/j.clbc.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs. 2005;8:137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 24.Dineen P. The effect of oxidized regenerated cellulose on experimental intravascular infection. Surgery. 1977;82:576–579. [PubMed] [Google Scholar]
- 25.Gottrup F, Cullen BM, Karlsmark T, Bischoff-Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013;21:216–225. doi: 10.1111/wrr.12020. [DOI] [PubMed] [Google Scholar]
- 26.Alfieri S, Di Miceli D, Menghi R, Quero G, Cina C, Pericoli Ridolfini M, et al. Role of oxidized regenerated cellulose in preventing infections at the surgical site: prospective, randomized study in 98 patients affected by a dirty wound. Minerva Chir. 2011;66:55–62. [PubMed] [Google Scholar]
- 27.Bullen AW, Losowsky MS. Consequences of impaired splenic function. Clin Sci (Lond) 1979;57:129–137. doi: 10.1042/cs0570129. [DOI] [PubMed] [Google Scholar]
- 28.Khan PN, Nair RJ, Olivares J, Tingle LE, Li Z. Postsplenectomy reactive thrombocytosis. Proc (Bayl Univ Med Cent) 2009;22:9–12. doi: 10.1080/08998280.2009.11928458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katti DS, Lakshmi S, Langer R, Laurencin CT. Toxicity, biodegradation and elimination of polyanhydrides. Adv Drug Deliv Rev. 2002;54:933–961. doi: 10.1016/s0169-409x(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 30.Behbehani S, Tulandi T. Oxidized regenerated cellulose imitating pelvic abscess. Obstet Gynecol. 2013;121(2 Pt 2 Suppl 1):447–449. doi: 10.1097/aog.0b013e318276ce3f. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Chen P. Surgicel® (oxidized regenerated cellulose) granuloma mimicking local recurrent gastrointestinal stromal tumor: a case report. Oncol Lett. 2013;5:1497–1500. doi: 10.3892/ol.2013.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postoperative adhesion. evaluation at early second-look procedures. Operative Laparoscopy Study Group. Fertil Steril. 1991;55:700–704. [PubMed] [Google Scholar]
- 33.Raftery AT. Absorbable haemostatic materials and intraperitoneal adhesion formation. Br J Surg. 1980;67:57–58. doi: 10.1002/bjs.1800670118. [DOI] [PubMed] [Google Scholar]
- 34.Ates U, Ata B, Ortakuz S, Seyhan A, Urman B. Prevention of adhesion formation following ovarian surgery in a standardized animal model: comparative study of Interceed and double layer Surgicell. J Obstet Gynaecol Res. 2008;34:12–17. doi: 10.1111/j.1447-0756.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad G, O'Flynn H, Hindocha A, Watson A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2015;(4):CD000475. doi: 10.1002/14651858.CD000475.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]