Abstract
Purpose
MicroRNAs are small non-coding RNAs that play important roles in vascular smooth muscle cell (VSMC) function. This study investigated the role of miR-379 on proliferation, invasion, and migration of VSMCs and explored underlying mechanisms thereof.
Materials and Methods
MicroRNA, mRNA, and protein levels were determined by quantitative real-time PCR and western blot. The proliferative, invasive, and migratory abilities of VSMCs were measured by CCK-8, invasion, and wound healing assay, respectively. Luciferase reporter assay was used to confirm the target of miR-379.
Results
Platelet-derived growth factor-bb was found to promote cell proliferation and suppress miR-379 expression in VSMCs. Functional assays demonstrated that miR-379 inhibited cell proliferation, cell invasion, and migration. Flow cytometry results further showed that miR-379 induced apoptosis in VSMCs. TargetScan analysis and luciferase report assay confirmed that insulin-like growth factor-1 (IGF-1) 3'UTR is a direct target of miR-379, and mRNA and protein levels of miR-379 and IGF-1 were inversely correlated. Rescue experiments showed that enforced expression of IGF-1 sufficiently overcomes the inhibitory effect of miR-379 on cell proliferation, invasion, and migration in VSMCs.
Conclusion
Our results suggest that miR-379 plays an important role in regulating VSMCs proliferation, invasion, and migration by targeting IGF-1.
Keywords: Vascular smooth muscle cells, miR-379, cell proliferation, invasion, migration, IGF-1
INTRODUCTION
Cardiovascular diseases, such as coronary artery disease, atherosclerosis, congestive heart failure, hypertension, stroke, and myocardial infarction, are a major cause of death in the world.1,2,3 Studies have demonstrated that abnormal proliferation of vascular smooth muscle cells (VSMCs) play a critical role in the pathogenesis of cardiovascular diseases.4,5,6 However, to our best knowledge, the underlying molecular mechanisms thereof remain unclear.
Platelet-derived growth factor-bb (PDGF-bb) is released primarily by vascular endothelial cells and platelets at the sites of vascular injury, and it has been shown to potently stimulate VSMCs proliferation and migration by modulating several key molecular signaling pathways.7,8 Several studies have demonstrated that increased expression of signaling proteins in the PDGF-bb pathway is associated with cardiovascular diseases, including restenosis and atherosclerosis,9 and inhibition of PDGF-bb-related pathways has been shown to exert beneficial effects on these cardiovascular disorders.10 However, the underlying mechanisms by which PDGF-bb modulates VSMC proliferation and migration are not fully understood. Therefore, it is of great scientific interest to explore the molecular mechanisms involved in the modulation of PDGF-bb-dependent VSMC proliferation and migration.
MicroRNAs (miRNAs) are a class of small non-coding RNAs, by targeting the 3' untranslated region (3'UTR) of mRNA, miRNAs can induce mRNA degradation, which in turn results in the translation repression of target genes.11 Various studies have showed that miRNAs are important in cell development, growth, differentiation, proliferation, and apoptosis.12,13,14 Increasing evidence has also demonstrated that aberrant expression of miRNAs is involved in the development and progression of many types of cancers, including colon cancer, liver cancer, gastric cancer, lung cancer, breast cancer, and so on.15,16,17 Meanwhile, recent studies have found that miRNAs also regulate the functions of VSMCs: for example, Xu, et al.18, demonstrated that miR-135b and miR-499a promote cell proliferation and migration in the atherosclerosis by directly targeting myocyte enhance factor 2C; Liu, et al.19, reported that miR-1 regulates the proliferation of VSMCs by targeting insulin-like growth factor-1 (IGF-1); and Xie, et al.20, showed that miR-599 inhibits VSMCs proliferation and migration by targeting transforming growth factor beta 2.20 However, the role of miRNAs in VSMC functions has not been fully explored.
Xu, et al.18, reported that miR-379 was down-regulated in circulating blood from patients with atherosclerotic coronary artery disease. miR-379 has also been found to inhibit cell growth in certain types of cancers including breast cancer, malignant mesothelioma, and liver cancer.21,22,23 In this study, we investigated the functional role of miR-379 in the growth of VSMCs. Herein, PDGF-bb treatment promoted proliferation of VSMCs and down-regulated the expression of miR-379. Functional assays demonstrated that miR-379 inhibits cell proliferation, cell invasion, and migration. Flow cytometry results showed that miR-379 induces apoptosis in VSMCs. Using a bioinformatic analytic tool (Targetscan; http://www.targetscan.org), the 3'UTR of IGF-1 gene was found to be a target of miR-379. Luciferase report assay further confirmed that IGF-1 3'UTR is a direct target of miR-379, and the expression of miR-379 and IGF-1 at both mRNA and protein levels were inversely correlated. Finally, rescue experiments showed that enforced expression of IGF-1 sufficiently overcomes the inhibitory effect of miR-379 on cell proliferation in VSMCs.
MATERIALS AND METHODS
Cell culture
VSMC lines (Cell bank of Chinese Academy of Science, Shanghai, China) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, GE Health Care, Chicago, IL, USA) supplemented with 10% fetal bovine serum (FBS). VSMCs were stored in an incubator with 5% CO2 at 37℃.
miRNAs and plasmids
miR-379 mimics, miR-379 inhibitors, and scrambled miRNA were purchased from GenePharma (Shanghai, China). The empty vectors pcDNA3.1 and pcDNA3.1-IGF-1 were synthesized by Ribobio (Guangzhou, China).
Transfection and PDGF-bb treatment
Lipofectamine 2000 (Invitrogen, Waltham, MA, USA) was used to perform miRNA transfection according to the manufacturer's instructions. Co-transfection of miR-379 mimics and pcDNA-3.1-IGF-1 were performed according to the similar procedures above. PDGF-bb (Sigma, St. Louis, MO, USA) treatments were carried at a concentration of 10–30 ng/mL, and the CCK-8 assay were performed at 24, 48, and 72 h.
Preparation of RNA and quantitative real-time PCR
Total RNAs were extracted using Trizol reagent (Invitrogen). The TaqMan MicroRNA Reverse Transcription Kit (Takara, Dalian, China) and the TaqMan Reverse Transcription Kit (Takara) were used to reverse transcribe RNA for miRNA detection and to obtain cDNA for mRNA detection, respectively. For miR-379 and IGF-1 mRNA, quantitative real-time PCR (qRT-PCR) was performed using miRscript SYBR Green PCR Kit and SYBR Green PCR Kit (Takara), respectively. The internal controls for miR-379 and IGF-1 mRNA were U6 and GAPDH, respectively. The primers for miR-379 were forward,5'-GCTACATGATAC AGTGCAAA-3' and reverse, 5'-AGTTTGCTTGATCCCTCTTC AG-3'; the primers for IGF-1 mRNA were forward, 5'-CTTCA GTTC GTGTGT GGA GAC AG-3' and reverse, 5'-CGCC CTCC GACTGCT-3'. Data are expressed as fold changes normalized to U6 or GAPDH based on the following formula: RQ=2-ΔΔCt.
Western blot
Proteins extraction was performed using RIPA lysis buffer (Bio-Rad, Milan, Italy). The extracted protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to a polyvinylidene difluoride membrane (PVDF, Millipore, Billerica, MA, USA). Membranes were blocked with 5% bovine serum albumin, followed by overnight incubation with primary antibody at 4℃: rabbit anit-IGF-1 (1:2500; Abcam, Cambrigde, MA, USA) and mouse anti-β-actin (1: 7000; Abcam, Cambridge, MA, USA). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:4000; Abcam). The membranes were exposed using a ChemoDoc XRS detection system (Bio-Rad, Milan, Italy).
Cell proliferation assay
For the CCK-8 assay, cells were seeded in 96-well plates (5×103 cells/well), and incubated for 24 h. Cells were then treated with PDGF-bb; transfected with miR-379 mimics, miR-379 inhibitor, or their respective controls; or co-transfected with miR-379 and pcDNA3.1-IGF-1. At 24, 48, and 72 h after PDGF-bb treatment or at 48 h after transfection, cell proliferation indices were measured using a CCK-8 kit (Beyotime, Beijing, China).
Cell invasion assay
For invasion assay, cells were seeded on 24-well transwell plates (5×104 cells/well), cultured for 24 h, and then transfected with miR-379 mimics, miR-379 inhibitor, or their respective controls. Cells were grown in the upper chambers of the plates in serum-free DMEM separated by a Matrigel-coated membrane (BD Bioscience, San Jose, CA, USA), and 500 µL of DMEM supplemented with 10% FBS was added to the bottom of the chambers. Cells were then incubated for 24 h at 37℃, and were stained with 0.1% crystal violet.
Wound healing assay
For the wound healing assay, cells were seeded in six-well plates (5×105 cells/well). Twenty-four hours after transfection, a scratched wound was created using a pipette tip. Cells migrated into the wound surface, and the average distance of the migrating cells was determined under an inverted microscopy at 0 h and 24 h.
Luciferase reporter assay
The constructed wild type or mutant miR-379 binding site of IGF-1 3'UTR pmirGLO vectors were purchased from Ribobio (Guangzhou, China). VSMCs were cultured on 24-well plates 24 h before co-transfected with 50 ng of wild type or mutant luciferase vector and 20 µM miR-379 mimics, miR-379 inhibitor, or their respective controls. After 48 h, luciferase activity was measured by the Dual-luciferase Reporter Assay System (Promega, Madison, WI, USA).
Cell apoptosis assay
For cell apoptosis analysis, cells were seeded on a six-well plate, and 24 h post-transfection, cells were harvested and washed with phosphate buffered saline (PBS). Cell apoptosis was then detected using an Annexin V-FITC/PI apoptosis detection kit according to the manufacturer's instructions.
Statistical analysis
GraphPad Prism version 6 (GraphPad Prism version 6.0, Inc., La Jolla, CA, USA) was used to perform all statistical analyses. Significant differences between groups were determined by t-test or one-way ANOVA followed by Bonferroni's multiple comparison tests. All data are expressed as the mean values of three independent replicates±SEM; differences were considered significant when p<0.05.
RESULTS
PDGF-bb promotes cell proliferation and inhibits miR-379 expression in VSMCs
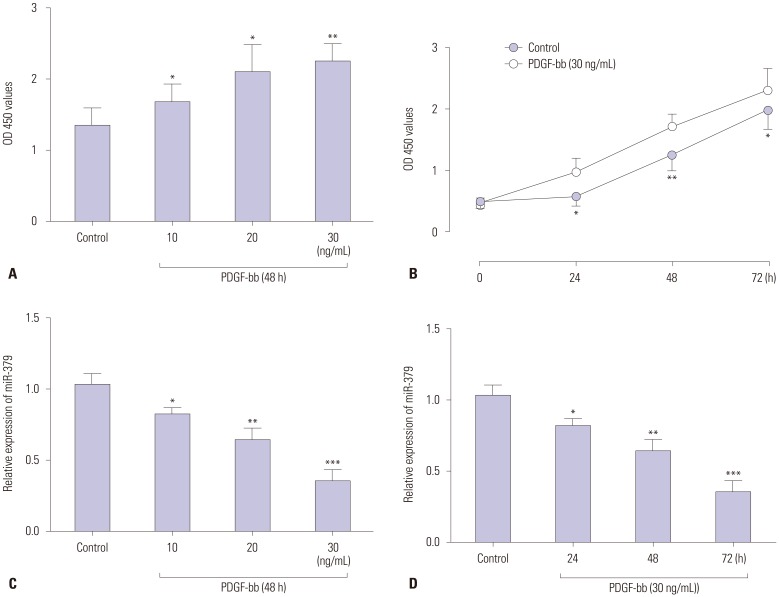
The effect of PDGF-bb on cell proliferation of VSMCs was assessed by CCK-8 assay. As shown in Fig. 1A and B, PDGF-bb promoted proliferation of VSMCs in a concentration-dependent and time-dependent manner. The expression of miR-379 in VSMCs was determined by qRT-PCR, and PDGF-bb treatment significantly suppressed the expression level of miR-379 in VSMCs (Fig. 1C and D).
Fig. 1. The effect of PDGF-bb on cell proliferation and miR-379 expression in VSMCs. (A) PDGF-bb promotes cell proliferation of VSMCs in a concentration-dependent (10–30 ng/mL) manner as measured by CCK-8 assay. (B) PDGF-bb promotes cell proliferation of VSMCs in a time-dependent (24–72 h) manner as measured by CCK-8 assay. (C) Expression of miR-379 on qRT-PCR is down-regulated after treatment with different concentrations of PDGF-bb. (D) Expression of miR-379 on qRT-PCR is down-regulated after treatment with different durations of PDGF-bb. Data represent the mean values of three independent replicates±SEM; significant differences relative to control group are shown as *p<0.05, **p<0.01, ***p<0.001. PDGF-bb, platelet-derived growth factor-bb; VSMCs, vascular smooth muscle cells; qRT-PCR, quantitative real-time PCR; SEM, standard error of the mean.
miR-379 inhibits cell proliferation, invasion and migration of VSMCs
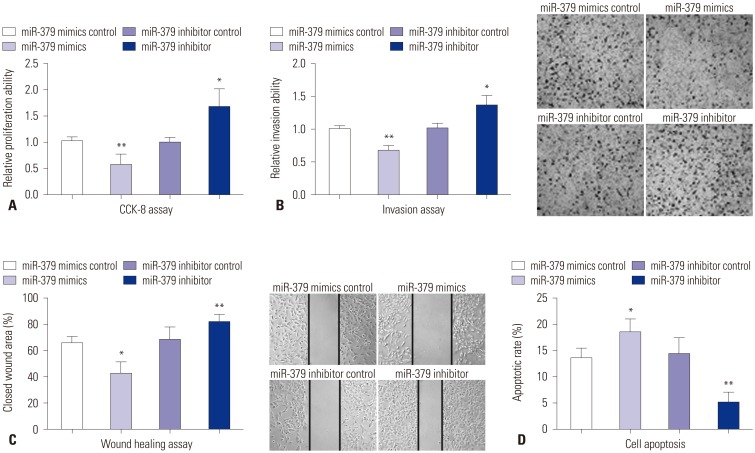
To validate the inhibitory role of miR-379 on proliferation of VSMCs, cell proliferation assay was performed by using CCK-8 kit. Compared to their respective control groups, the proliferative ability of miR-379 mimics-transfected VSMCs were significantly inhibited, while the proliferative ability of miR-379 inhibitor-transfected VSMCs were significantly enhanced (Fig. 2A). Cell invasion assay was performed to further examine if the differential expression of miR-379 was correlated with cell invasion. As shown in Fig. 2B, miR-379 mimics transfection in VSMCs caused lower invasion ability when compared to that transfected with control; meanwhile, miR-379 inhibitor transfection enhanced the invasive ability of VSMCs (Fig. 2B). Wound healing assay was conducted to determine the effect of miR-379 on the motility of VSMCs. Transfection of miR-379 mimics in VSMCs caused a decrease in wound healing cell migration, compared to its control group; transfection of miR-379 inhibitor in VSMCs increased wound healing cell migration, compared to its control group (Fig. 2C).
Fig. 2. The effect of miR-379 on cell proliferation, invasion, migration, and apoptosis of VSMCs. Twenty-four hours after cells were transfected with miR-379 mimics, miR-379 inhibitor, or their respective controls, (A) cell proliferation of VSMCs was measured by CKK-8 assay; (B) cell invasion ability of VSMCs was measured by transwell assay (40×); (C) migration ability of VSMCs was measured by wound healing assay (40×); and (D) cell apoptosis of VSMCs was measured by flow cytometry. Data represent the mean values of three independent replicates±SEM; significant differences relative to control group are shown as *p<0.05, **p<0.01. VSMCs, vascular smooth muscle cells; SEM, standard error of the mean.
miR-379 induced cell apoptosis in VSMCs
To examine if miR-379 induces apoptosis in VSMCs, we further performed flow cytometry experiment. As shown in Fig. 2D, transfection with miR-379 mimics increased the cell apoptotic rate in VSMCs, compared to its respective control, while miR-379 inhibitor treatment caused a decrease in the cell apoptotic rate of VSMCs, compared to its respective control (Fig. 2D).
IGF-1 is a direct target of miR-379
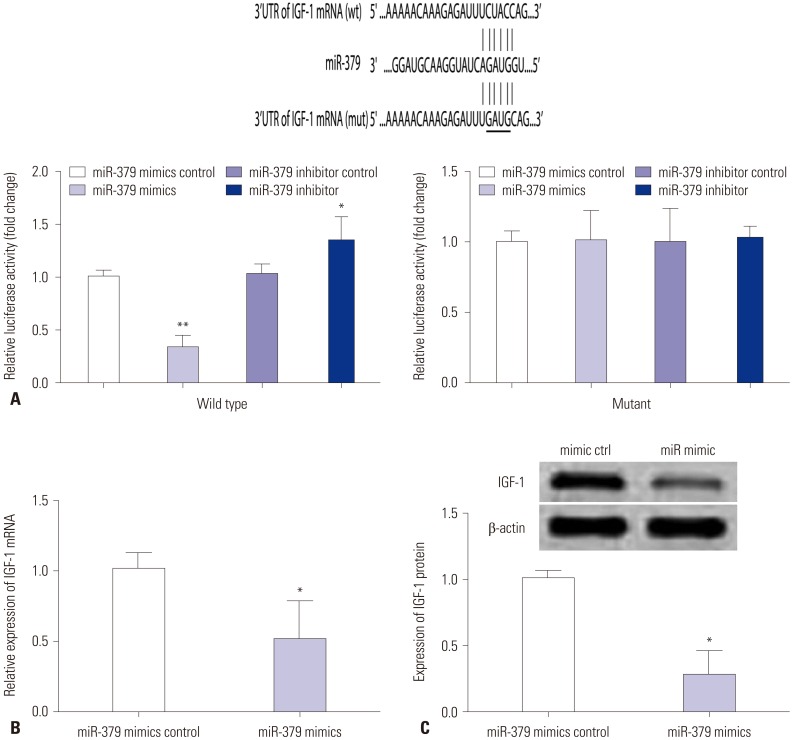
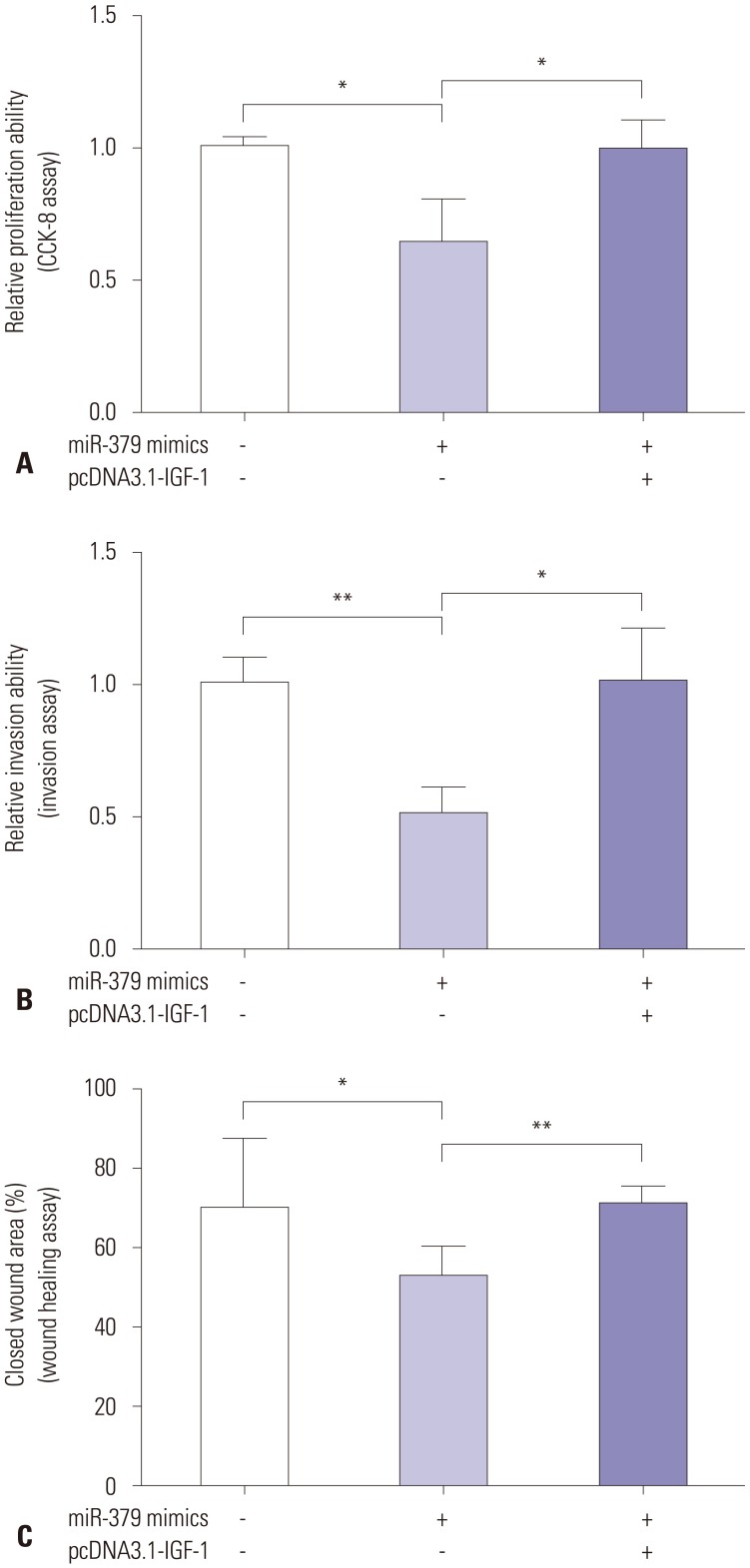
The downstream mediator of miR-379 was predicted by TargetScan (Supplementary Table 1, only online), and there were complementarities between miR-379 and IGF-1 3'UTR (Fig. 3A). Co-transfection with miR-379 mimics and the wild type reporter plasmid significantly suppressed the relative luciferase activity of the reporter containing wild type IGF-1 3'UTR. Co-transfection of miR-379 inhibitor and the report plasmid significantly increased the luciferase activity of the reporter containing wild type IGF-1 3'UTR, while the luciferase activity of the reporter containing mutant IGF-1 3'UTR was unaffected (Fig. 3A). Further, qRT-PCR and western blot results showed that the expressions of miR-379 and IGF-1 at both the mRNA and protein level were inversely correlated (Fig. 3B and C). To further investigate the role of IGF-1 in cell proliferation, invasion, and migration of VSMCs, pcDNA3.1-IGF-1 and miR-379 mimics were co-transfected into VSMCs, and CCK-8 assay, invasion assay, and wound healing assay were performed, respectively. As shown in Fig. 4, enforced expression of IGF-1 was able to sufficiently overcome the inhibitory effect of miR-379 on the proliferation, invasion, and migration of VSMCs (Fig. 4).
Fig. 3. IGF-1 is a direct target of miR-379. (A) VSMCs were co-transfected with miR-379 mimics or its control with wild type (wt) or mutant (mut) 3'UTR of IGF-1, and luciferase activity was detected. (B) The mRNA expression levels of IGF-1 in VSMCs were determined by qRT-PCR at 24 h after cells were transfected with miR-379 mimics or its control. (C) Protein expression of IGF-1 in VSMCs was measured by western blot at 24 h after cells were transfected with miR-379 mimics or its control. Data represent the mean values of three independent replicates±SEM; significant differences relative to control group are shown as *p<0.05, **p<0.01. IGF-1, insulin-like growth factor-1; VSMCs, vascular smooth muscle cells; 3'UTR, 3' untranslated region; qRT-PCR, quantitative real-time PCR; SEM, standard error of the mean.
Fig. 4. The effect of enforced expression of IGF-1 on cell proliferation, invasion, and migration of VSMCs. Twenty-four hours after cells were co-transfected with miR-379 mimics and pcDNA3.1-IGF-1 or their respective controls, (A) cell proliferation of VSMCs was measured by CKK-8 assay; (B) cell invasion of VSMCs was measured by cell invasion assay; and (C) cell migration of VSMCs was measured by wound healing assay. Data represent the mean values of three independent replicates±SEM; significant differences relative to control group are shown as *p<0.05, **p<0.01. IGF-1, insulin-like growth factor-1; VSMCs, vascular smooth muscle cells; SEM, standard error of the mean.
DISCUSSION
In the present study, we observed that PDGF-bb promotes VSMCs proliferation, and the expression of miR-379 was suppressed in VSMCs by PDGF-bb treatment. Functional assays confirmed that miR-379 inhibited cell proliferation, cell invasion, and migration. Flow cytometry results demonstrated that miR-379 induces apoptosis in VSMCs. Reporter gene assay, qRT-PCR, and western blot showed that translation suppressed by miR-379 was mediated by IGF-1 mRNA 3'UTR dependent on the presence of a single miR-379 cognate binding site, and enforced expression of IGF-1 overcame the inhibitory effect of miR-379 on the proliferation, invasion, and migration of VSMCs. The above results suggest that miR-379 plays a crucial role in controlling VSMC proliferation.
miRNAs have been implicated in various cell functions, including differentiation, proliferation, death, metabolism, and carcinogenesis.12,13,14 Deregulation of miRNAs has been found in many types of human cancers.15,16,17 Recent studies have found that miRNAs also regulated the functions of VSMSCs.18,19,20 As PDGF-bb treatment promotes VSMC proliferation and migration, changes in miRNA expression profiles by PDGF-bb may be of great interest to identify novel miRNAs, which may regulate proliferation and migration. In our preliminary studies, we treated VSMCs with PDGF-bb and examined the expression levels of several miRNAs potentially involved in the regulation of VSMCs (Supplementary Table 2, only online). The preliminary results showed that miR-379 is down-regulated by PDGF-bb stimulation (Supplementary Table 2, only online); therefore, miR-379 was chosen for further functional study. To date, the role of miR-379 in VSMC function has not been investigated. One study found that miR-379 levels are lower in circulating blood from patients with atherosclerotic coronary artery disease.18 In cancer studies, miR-379 was found to inhibit breast cancer cell proliferation by regulating cyclin B1 expression;21 Yamamoto reported that miR-379 inhibited cell growth of malignant pleural mesothelioma by targeting interleukin 18.23 In addition, miR-379 also exhibited a tumor-suppressive function in liver cancer.22 Consistently, our results showed that miR-379 inhibited cell proliferation, cell invasion, and migration of VSMCs.
IGF-1 is a growth factor and has been found to regulate cell proliferation and migration of VSMCs.24 Studies have demonstrated that over-expression of IGF-1 promotes neointimal formation after carotid artery injury, which results from an increase in the proliferation and migration of VSMCs.25 IGF-1 exerts its functions by acting on IGF-1 receptor, which is expressed in VSMCs.26 In the present study, the 3'UTR of IGF-1 gene was a target of miR-379, as predicted by TargetScan, and this predicted target was further confirmed by luciferase report assay. Furthermore, miR-379 and IGF-1 expressions at both the mRNA and protein level were inversely correlated. In addition, rescue experiment showed that enforced expression of IGF-1 overcame the inhibitory effect of miR-379 on cell proliferation of VSMCs. Collectively, these results may suggest that IGF-1 potentiates the proliferation of VSMCs, which can be suppressed by overexpression of miR-379. It has been demonstrated that miRNA may target more than one gene; it is likely that miR-379 targets both IGF-1 and other genes to regulate cell proliferation, invasion, and migration of VSMCs. However, in the present study, we did not show whether miR-379 targets other genes (Supplementary Table 1, only online) that modulate cell proliferation, migration, and invasion, and thus, further study may be required to confirm the role of other genes targeted by miR-379 in VSMCs.
In conclusion, the present study indicates that miR-379 plays a key role in regulating VSMCs proliferation, invasion, and migration via targeting IGF-1.
ACKNOWLEDGEMENTS
This project is supported by Shaanxi Science Research Program (No.2013SX6598).
Footnotes
The authors have no financial conflicts of interest.
Supplementary materials
Targets of miR-379 as Predicted by TargetScan (A List of 50 Genes are Shown)
Differential Expression of miRNAs after PDGF-bb Treatment in VSMCs
References
- 1.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 2.Ma S, Ma CC. Recent development in pleiotropic effects of statins on cardiovascular disease through regulation of transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 2011;22:167–175. doi: 10.1016/j.cytogfr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiong M, Cartes-Saavedra B, Norambuena-Soto I, Mondaca-Ruff D, Morales PE, García-Miguel M, et al. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol. 2014;2:72. doi: 10.3389/fcell.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 2015;214:33–50. doi: 10.1111/apha.12466. [DOI] [PubMed] [Google Scholar]
- 6.Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 7.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006;16:25–28. doi: 10.1016/j.tcm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getachew R, Ballinger ML, Burch ML, Reid JJ, Khachigian LM, Wight TN, et al. PDGF beta-receptor kinase activity and ERK1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology. 2010;151:4356–4367. doi: 10.1210/en.2010-0027. [DOI] [PubMed] [Google Scholar]
- 11.Nouraee N, Mowla SJ. miRNA therapeutics in cardiovascular diseases: promises and problems. Front Genet. 2015;6:232. doi: 10.3389/fgene.2015.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 13.Costa FF. Non-coding RNAs: new players in eukaryotic biology. Gene. 2005;357:83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Lin J, Kong D, Huang M, Xu C, Kim TK, et al. Current state of circulating MicroRNAs as cancer biomarkers. Clin Chem. 2015;61:1138–1155. doi: 10.1373/clinchem.2015.241190. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka M, Ling H, Doki Y, Mori M, Calin GA. MicroRNA processing and human cancer. J Clin Med. 2015;4:1651–1667. doi: 10.3390/jcm4081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuna M, Machado AS, Calin GA. Genetic and epigenetic alterations of microRNAs and implications for human cancers and other diseases. Genes Chromosomes Cancer. 2016;55:193–214. doi: 10.1002/gcc.22332. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y, et al. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci Rep. 2015;5:12276. doi: 10.1038/srep12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Ying Z, Qi X, Shi Y, Tang Q. MicroRNA-1 regulates the proliferation of vascular smooth muscle cells by targeting insulin-like growth factor 1. Int J Mol Med. 2015;36:817–824. doi: 10.3892/ijmm.2015.2277. [DOI] [PubMed] [Google Scholar]
- 20.Xie B, Zhang C, Kang K, Jiang S. miR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2. PLoS One. 2015;10:e0141512. doi: 10.1371/journal.pone.0141512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Khan S, Brougham CL, Ryan J, Sahrudin A, O'Neill G, Wall D, et al. miR-379 regulates cyclin B1 expression and is decreased in breast cancer. PLoS One. 2013;8:e68753. doi: 10.1371/journal.pone.0068753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigalli JP, Ciriaci N, Arias A, Ceballos MP, Villanueva SS, Luquita MG, et al. Regulation of multidrug resistance proteins by genistein in a hepatocarcinoma cell line: impact on sorafenib cytotoxicity. PLoS One. 2015;10:e0119502. doi: 10.1371/journal.pone.0119502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto K, Seike M, Takeuchi S, Soeno C, Miyanaga A, Noro R, et al. MiR-379/411 cluster regulates IL-18 and contributes to drug resistance in malignant pleural mesothelioma. Oncol Rep. 2014;32:2365–2372. doi: 10.3892/or.2014.3481. [DOI] [PubMed] [Google Scholar]
- 24.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 25.Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA. Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology. 2001;142:3598–3606. doi: 10.1210/endo.142.8.8331. [DOI] [PubMed] [Google Scholar]
- 26.Delafontaine P. Insulin-like growth factor I and its binding proteins in the cardiovascular system. Cardiovasc Res. 1995;30:825–834. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Targets of miR-379 as Predicted by TargetScan (A List of 50 Genes are Shown)
Differential Expression of miRNAs after PDGF-bb Treatment in VSMCs