Abstract
Purpose
Decitabine, a DNA hypomethylating agent, was recently approved for use in Korea for older adults with acute myeloid leukemia (AML) who are not candidates for standard chemotherapy. This study aimed to evaluate the role of decitabine as a first-line treatment for older adults with AML.
Materials and Methods
Twenty-four patients with AML who received at least one course of decitabine (20 mg/m2/d intravenously for 5 days every 4 weeks) as a first-line therapy at Severance Hospital were evaluated retrospectively.
Results
The median age of the patients was 73.5 years. The longest follow-up duration was 502 days. A total of 113 cycles of treatment were given to 24 patients, and the median number of cycles was four (range, 1–14). Thirteen patients dropped out because of death, no or loss of response, patient refusal, or transfer to another hospital. Twenty-one (87.5%) and 12 (50%) patients completed the second and fourth cycles, respectively, and responses to treatment were evaluated in 17. A complete response (CR) or CR with incomplete blood-count recovery was achieved in six (35.3%) patients, and the estimated median overall survival was 502 days. Ten patients developed grade >2 hematologic or non-hematologic toxicities. In univariate analysis, bone marrow blasts, lactate dehydrogenase, serum ferritin level, and bone marrow iron were significantly associated with response to decitabine.
Conclusion
Five-day decitabine treatment showed acceptable efficacy in older patients with AML who are unfit for conventional chemotherapy, with a CR rate 35.3% and about a median overall survival of 18 months.
Keywords: Decitabine, elderly, AML, treatment
INTRODUCTION
In 2012, more than 1800 patients were newly diagnosed with acute myeloid leukemia (AML) in Korea, and about 33.7% of those patients were 65 years of age or older.1 Due to comorbidities, poor performance status, and elevated risks associated with older age in patients with AML, such patients are often ineligible for intensive chemotherapy or participation in clinical trials. Therefore, their treatment options are limited, and treatments that are less intensive than conventional chemotherapy have been proposed. Decitabine, or 5-aza-2'-deoxycytidine, is a cytosine analogue that was first synthesized in 1968 by Sorm and Veselý.2 The potential activity of decitabine against leukemia was first reported in 1968.2 Decitabine was originally developed to be used at high doses as an antimetabolite, although low-dose therapy is now more commonly used than high-dose therapy.3 When used at low doses, decitabine is incorporated into DNA and degrades DNA methyltransferases (DNMTs) by covalent trapping,4 resulting in hypomethylation of DNA and reactivation of silenced gene expression, which can reactivate tumor-suppressor genes and promote apoptosis of leukemic blasts in the absence of DNMTs.5 However, the exact anti-leukemic mechanism of decitabine is still unclear, as correlations between hypomethylation, gene re-expression, and clinical response have not yet been determined.6,7 Other potential mechanisms, such as the generation of intracellular reactive oxygen species, have been suggested.8,9
So far, several studies have demonstrated that decitabine is effective against AML and shows tolerable toxicity profiles in patients of older age.10,11 However, the United States Food and Drug Administration did not approve decitabine for the treatment of newly diagnosed AML based on the results of a clinical trial reported by Kantarjian, et al.12 in 2012. In that study, decitabine treatment for older adults with newly diagnosed AML did not provide a statistically significant survival improvement, compared with supportive care or low-dose cytarabine therapy at the pre-planned, prospectively defined time point. However, in the same study, decitabine treatment did show a survival benefit over standard therapies in an unplanned survival analysis (median survival 7.7 months vs. 5.0 months; p=0.037). Based on that result, the European Medicines Agency Committee for Medicinal Products for Human Use approved decitabine as a first-line treatment for older adults with AML in 2012. Although the exact role and proper indications of decitabine for older adults with AML are still controversial, the Korean Food and Drug Administration (KFDA) recently approved decitabine as a first-line treatment for the same indications as those of European Union in December 2013. Since gaining KFDA approval, the number of older Korean patients with AML who are not candidates for standard induction chemotherapy and are instead treated with decitabine as the first-line therapy has been increasing. As national data relating to the clinical outcomes of decitabine treatment for older adults with newly diagnosed AML have not been reported, we conducted a retrospective study to evaluate safety, clinical efficacy, and factors affecting treatment responses using data from older adults with AML who were treated with decitabine as the first-line therapy at Severance Hospital in Seoul, Korea.
MATERIALS AND METHODS
Patients
Clinical data from patients diagnosed with de novo or secondary AML, excluding acute promyelocytic leukemia and isolated myeloid sarcoma, between December 1, 2013 and May 31, 2015 at Severance Hospital in Korea were retrospectively reviewed. Twenty-four patients treated with decitabine as the first-line therapy for remission induction were included. AML was diagnosed according to the 2008 World Health Organization criteria.13 Patients with a prior history of other malignancy were included, while patients who were treated with hypomethylating agents, such as decitabine or azacitidine, before being diagnosed with AML were excluded. The end point of the study was July 23, 2015 or the time of death or loss from follow-up. Treatment response, overall survival (OS), progression-free survival (PFS), treatment toxicity, and factors affecting the treatment response were analyzed. The study was approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine in Seoul, South Korea.
Treatment, response, and toxicity
All patients were treated with at least one course of low-dose decitabine at an initial dose of 20 mg/m2 intravenously daily for 5 days over 28-day cycles. Responses after decitabine treatment were assessed according to the 2003 International Working Group criteria.14 Specifically, a complete response (CR) was defined as bone marrow (BM) blasts less than 5%, platelet count greater than 100000/µL, white blood cell (WBC) count greater than 1000/µL, and no extramedullary leukemia. CR with incomplete blood-count recovery (CRi) was defined as CR status without a platelet count greater than 100000/µL or a WBC count greater than 1000/µL. Partial remission (PR) was defined as at least a 50% decrease in the percentage of BM blasts to 5–25%. No response (NR) was defined as a failure to achieve CR or PR or as death before day 28 of the first cycle of decitabine therapy. Loss of response (LOR) was defined as an initial CR or PR with a subsequent increase of BM blasts of more than 25% relative to the previous BM blast percentage. To analyze factors affecting treatment response, we divided the patients into two groups: responders and non-responders. Responders were defined as patients who achieved CR or CRi after decitabine treatment. Non-responders were defined as patients who did not achieve CR or CRi after decitabine treatment. The treatment response was not defined for patients who did not receive a response evaluation after a minimum of four cycles of treatment, unless there was clear evidence of a lack of response before the fourth cycle. Patients showing CR, CRi, or PR continued receiving cyclic decitabine treatment, and those showing NR or LOR could be considered as having treatment failure and received secondline treatment according to the physician's decision. Treatment toxicity was assessed according to the Common Terminology Criteria for Adverse Events version 4.0.
Assessment of clinical outcomes
OS was measured from the starting date of the first decitabine treatment to date of death (any cause). PFS was measured from the starting date of the first decitabine treatment to the first documented date of NR, LOR, or death (any cause). The duration of the treatment response was measured from the first documented date of a treatment response of at least PR to the first documented date of LOR or death.
Statistical analysis
The Kaplan-Meier method was used to describe OS and PFS. Frequencies were compared between groups using chi-square or Fisher's exact tests. The Mantel-Haenszel chi-square test was used to compare linear trends in ordinal variables between groups. Mann-Whitney U-tests were applied for continuous variables. Two-tailed p values less than 0.05 were considered significant. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
A total of 24 patients (12 males and 12 females) diagnosed with AML received decitabine as a first-line treatment between December 1, 2013 and May 31, 2015 at Severance Hospital, Seoul, Korea. The baseline characteristics of the patients are shown on Table 1. Eleven patients (45.8%) were between 70 and 74 years of age, four (16.7%) were less than 70 years of age, and nine (37.5%) were 75 years of age or older. Twenty-two patients (91.7%) had de novo AML, and two had secondary AML; one patient had therapy-related AML with a prior history of prostate cancer and thyroid cancer, and the other had AML progressed from myelodysplastic syndromes. Until July 2015, a total of 113 cycles of decitabine treatment were given to the 24 patients, and the median number of cycles was four (mean, 4.7; range, 1–14). Twenty-one (87.5%) and 12 (50%) patients completed the second and the fourth cycles, respectively. A total of 13 patients were dropped from decitabine treatment because of death (n=2), NR or LOR (n=6), patient refusal (n=4), or transfer to another hospital (n=1).
Table 1. Baseline Characteristics of All Patients, the Responders and the Non-Responders.
| Characteristics | Total (n=24) | Responders (n=6) | Non-responders (n=11) | p value |
|---|---|---|---|---|
| Age (yrs)* | 73.5 (66–82) | 70.5 (68–74) | 73.0 (70–76) | 0.103 |
| Gender, M/F† | 12/12 | 3/3 | 7/4 | 0.622 |
| Performance status | ||||
| 0–1/2† | 20/4 | 5/1 | 7/4 | NS |
| Cytogenetic risk group† | NS | |||
| Better/intermediate/poor | 3/16/3 | 1/4/1 | 2/6/1 | |
| Unknown | 2 | 0 | 2 | |
| Type of AML | ||||
| de novo/secondary† | 22/2 | 5/1 | 10/1 | NS |
| CCI, 0/1–2/3–4/≥5† | 12/10/1/1 | 4/1/0/1 | 7/3/1/0 | 0.849 |
| BM iron stain | 2.0 (0–6) | 2 (0–2) | 3 (2–6) | 0.033‡ |
| Laboratory values* | ||||
| WBC (×103/µL) | 6.74 (0.68–366.15) | 5.68 (1.46–14.73) | 14.45 (1.05–157.77) | 0.272 |
| Hemoglobin (g/dL) | 7.65 (3.9–13.4) | 8.05 (6.6–13.4) | 7.2 (4.2–10.5) | 0.328 |
| Platelets (×103/µL) | 42.5 (5–170) | 81.0 (22–170) | 36.0 (13–153) | 0.224 |
| PB blast count (/µL) | 741.15 (0–227, 013) | 58.95 (0–3639) | 4570 (0–70996.5) | 0.061 |
| BM blasts (%) | 47.1 (2.4–88.7) | 35.5 (22.2–70.5) | 71.4 (27.6–88.7) | 0.027 |
| LDH (IU/L) | 337.5 (161–9361) | 274.5 (161–438) | 448.5 (251–9361) | 0.049 |
| Creatinine (mg/dL) | 0.855 (0.5–1.6) | 0.72 (0.6–1.1) | 0.82 (0.6–1.1) | 0.475 |
| Ferritin (ng/mL) | 620.8 (71.4–15000) | 337.7 (71.4–362.0) | 933.3 (273.7–15000) | 0.036 |
| CRP (mg/L) | 20.7 (0.6–329.2) | 7.3 (0.6–85.6) | 70.75 (3.3–329.2) | 0.162 |
| Cycles of Tx, median (range) | 4 (1–14) | 8 (6–14) | 2 (1–7) | 0.001 |
M, male; F, female; AML, acute myeloid leukemia; CCI, Charlson comorbidity index; WBC, white blood cell; PB, peripheral blood; BM, bone marrow; LDH, lactate dehydrogenase, CRP, C-reactive protein; Tx, decitabine treatment.
*Median (range), †Number, ‡Mantel-Haenszel chi-square test for linear trends.
Treatment responses
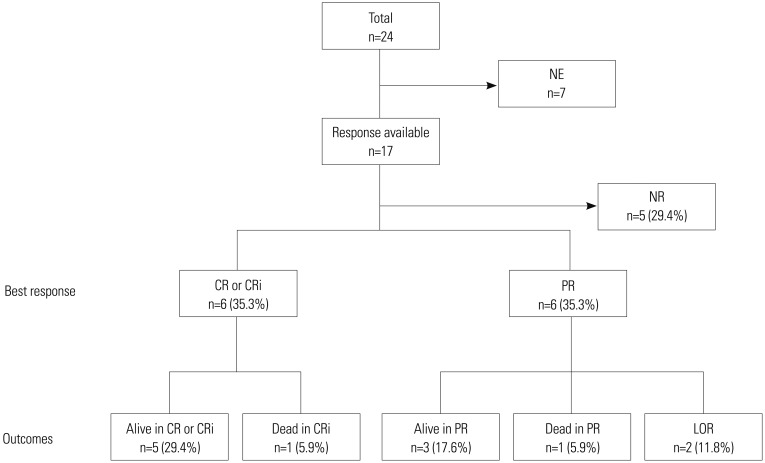
After the fourth cycle of decitabine treatment, 17 patients were evaluated by BM study to determine treatment responses (Fig. 1). Until completion of the fourth cycle of treatment, five patients discontinued decitabine treatment because of a lack of response. The treatment response could not be evaluated in seven patients: three refused the follow-up BM study, one transferred to another hospital shortly after treatment, and three had not yet finished the fourth cycle of treatment and showed no evidence of a lack of response. Overall, 12 of 17 patients (70.6%) had shown a response to decitabine. The best response was CR or CRi in six patients (35.3%) and PR in the other six patients (35.3%). Eight patients (CR or CRi, 3; PR, 5) achieved their best response after two cycles of treatment, and four patients (CR or CRi, 3; PR, 1) achieved their best response after four cycles of treatment (median 3.0 cycles). All six patients who initially achieved CR or CRi maintained their treatment response until the end of the study period, for a median 9.0 cycles of treatment (range, 6–13 cycles). One patient died from pneumonia, however, despite achieving CRi status after the eighth cycle. Among the six patients that achieved PR as their best response, four maintained their treatment response until the end of the study, for a median 22.5 days (range, 4–189 days), although two finally lost their response after receiving two more and five more further cycles of treatment, respectively. One of the four patients who maintained PR status until the end of the study ultimately died from sepsis after the fifth cycle of treatment. Five patients who experienced failure of decitabine treatment received a second-line therapy. The second-line regimens were FLAG-IDA (composed of fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor) for three patients, standard 7+3 regimen (composed of cytarabine and idarubicin) for one patient, and low-dose cytarabine for one patient. No patients received any type of hematopoietic stem cell transplantation.
Fig. 1. Overall treatment responses and outcomes diagram for older patients with AML. NE, not evaluable; NR, no response; CR, complete remission; CRi, CR with incomplete blood-count recovery; PR, partial remission; LOR, loss of response; AML, acute myeloid leukemia.
Factors affecting the treatment response
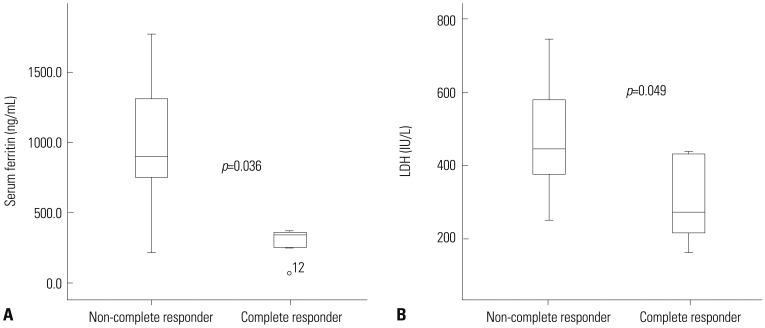
To assess clinical and laboratory factors affecting the treatment responses, the patients were divided into two groups according to their response to decitabine treatment. Six patients were considered responders, and 11 patients were considered non-responders. The differences in clinical characteristics between the groups are summarized in Table 1. One non-responder was not tested for baseline ferritin level and was excluded from the corresponding analysis. Univariate analysis showed that the responders had lower baseline serum ferritin levels than the non-responders [337.7 ng/mL (range, 71.4–362.0) vs. 933.3 ng/mL (range, 273.7–15000); p=0.036] (Fig. 2A). All of the responders and only three of the 10 non-responders had serum ferritin levels lower than 500 ng/mL (p=0.011). There was also a significant difference in BM iron-stain grade. The responders had significantly lower BM iron-stain grades than the non-responders (median 2.0 vs. 3.0; p=0.033). As expected, the BM blast percentage at diagnosis was significantly lower in the responders than in the non-responders (median 35.5% vs. 71.4%; p=0.027). In addition, the baseline serum lactate dehydrogenase (LDH) level was also significantly lower in the responders than in the non-responders (median 274.5 IU/L vs. 448.5 IU/L; p=0.049) (Fig. 2B). Other baseline factors, including age, gender, cytogenetic risk, performance status, peripheral blood blast count, WBC count, hemoglobin, platelets, serum creatinine, and C-reactive protein, were not significantly different between the responders and the non-responders. Unfortunately, it was not possible to identify any statistically significant predictive factors for the treatment response by multivariate analysis, because of the limited number of patients (data not shown).
Fig. 2. Boxplot of the baseline (A) serum ferritin and (B) LDH levels in patients according to the decitabine response (the responders and the non-responders). Serum ferritin and LDH levels at diagnosis were significantly lower in the responders than in the non-responders. LDH, lactic dehydrogenase.
Overall and progression-free survival
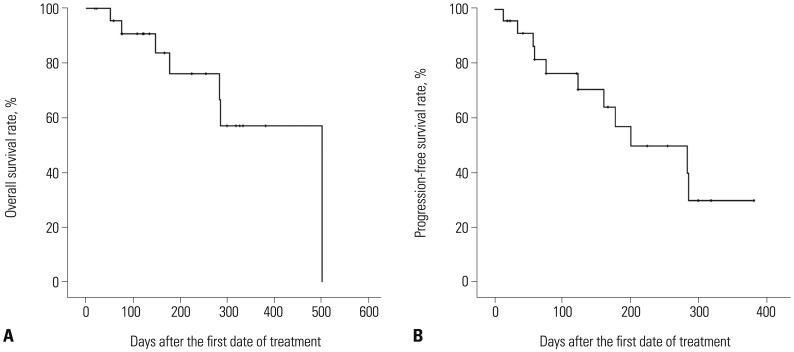
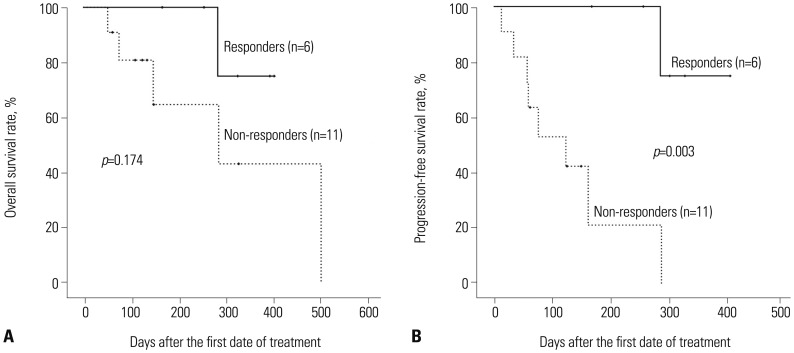
The median OS and PFS of all patients were 502 days and 201 days [95% confidence interval (CI), 50 to 351 days], respectively, from the starting date of the first decitabine treatment (Fig. 3). Estimation of the median OS and PFS among the responders was impossible, because five of the six patients (83.3%) were still alive without evidence of disease relapse at the end of the study period, with a median follow-up duration of 258 days (range, 139–351 days). On the other hand, the median OS and PFS among the non-responders were 286 days (95% CI, 89 to 483 days) and 59 days (95% CI, 33 to 85 days), respectively. Although the rate of OS was higher among the responders than among the non-responders, there was no statistically significant difference in OS between those groups (Fig. 4A). However, the rate of PFS among the responders was significantly higher than that among the non-responders (p=0.003) (Fig. 4B).
Fig. 3. (A) Overall (OS) and (B) progression-free survival (PFS) after decitabine treatment. The median OS and PFS of all patients were 502 days and 201 days (95% CI, 50 to 351 days), respectively, from the starting date of the first decitabine treatment. CI, confidence interval.
Fig. 4. (A) Overall (OS) and (B) progression-free survival (PFS) after decitabine treatment according to the decitabine response (the responders and the non-responders). The OS rate was higher (p=0.174), and the PFS rate was significantly higher (p=0.003) in the responders than in the non-responders.
Changes of BM and peripheral blood parameters according to treatment
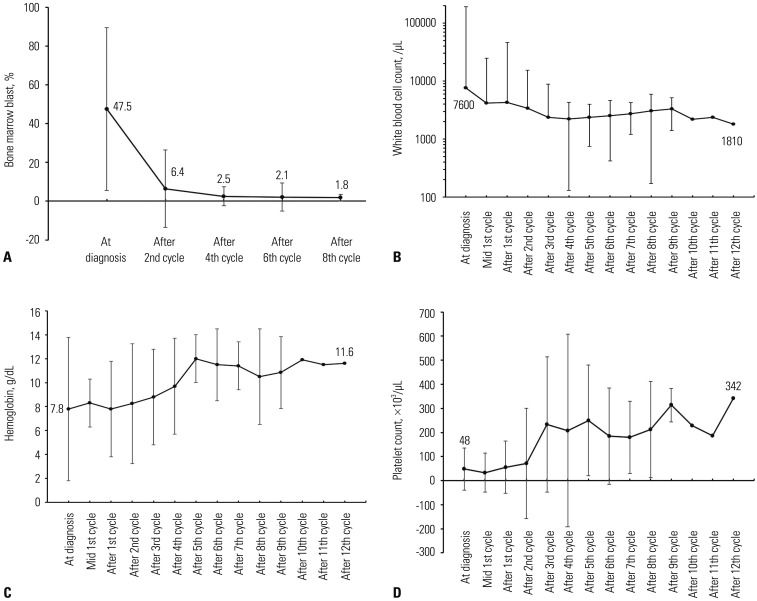
The BM blast percentage, WBC count, hemoglobin concentration, and platelet count at diagnosis and their numeric changes according to the treatment cycles in the patients who kept receiving decitabine treatment are shown in Fig. 5. As the decitabine treatment proceeded, the median BM blast percentage continuously decreased from 47.5% at diagnosis to 6.4% after the second cycle, 2.5% after the fourth cycle, 2.1% after the sixth cycle, and 1.8% after the eighth cycle. The median WBC count also decreased from 7600/µL at diagnosis to 2360/µL after the fourth cycle, and it was maintained thereafter until the end of the study. The median concentration of hemoglobin gradually increased from 7.8 g/dL at diagnosis to more than 10.0 g/dL after the fifth cycle. The median platelet count was also recovered from 48000/µL at diagnosis to more than 100000/µL after the third cycle.
Fig. 5. Changes in (A) bone marrow (BM) blast, (B) white blood cell count, (C) hemoglobin, and (D) platelet count during the decitabine treatment. Decitabine treatment consistently reduced the fraction of BM blast, and the hemogram was also continuously improved.
Toxicity
The most common non-hematologic treatment-related toxicities were febrile neutropenia and fatigue (Table 2). During the median four cycles of decitabine treatment, seven patients (29.2%) experienced febrile neutropenia of grade 3 or more, and three patients (12.5%) experienced fatigue of grade 2 or 3. One patient (4.2%) experienced grade 3 congestive heart failure, and one patient experienced grade 4 pulmonary thromboembolism. Three patients (12.5%) refused treatment after experiencing serious febrile neutropenia or fatigue, and two patients (8.3%) died from sepsis resulting from febrile neutropenia.
Table 2. Hematologic and Non-Hematologic Toxicity of Decitabine Treatment.
| Toxicity | No. of patients | % (per 24 patients) |
|---|---|---|
| Febrile neutropenia | ||
| Grade 3/grade 4 | 5/2 | 20.8/8.3 |
| Fatigue | ||
| Grade 2/grade 3 | 1/2 | 4.2/8.3 |
| Cardiovascular toxicity | ||
| Heart failure (grade 3) | 1 | 4.2 |
| Pulmonary thromboembolism (grade 4) | 1 | 4.2 |
DISCUSSION
In this study, which is the first report on a Korean population, we showed the efficacy of decitabine against AML in older adults with a tolerable toxicity profile. The median OS was 286 days, which might not be inferior to the results of other treatment modalities reported in previous studies showing a median OS ranging from 7.5 to 10 months after intensive chemotherapy15,16,17 and of 5.0 months after supportive care or low-dose cytarabine.12 During the decitabine therapy, the hemograms of the patients gradually improved. CR rates among elderly patients after intensive chemotherapy have been reported as less than 50%,15,16,17 and the CR and CRi rates of 35.3% in this study seem to be remarkable. Our data did not show a significant survival difference between patients according to the treatment response, which might be attributed to the small number of patients and the short period of the study. On the other hand, our data might suggest that even patients lacking a CR could have a substantial survival benefit with subsequent anti-leukemic therapy.
Finding the optimal treatment strategy for older adults with AML remains challenging for hematologists. Intensive chemotherapy based on cytarabine combined with an anthracycline, the so-called 7+3 regimen, has been a standard treatment for many years among younger patients with AML, with a CR rate of 70–80% and a cure rate of 40–50%.18 However, the remission rate after treatment with the 7+3 regimen among older individuals is around 50%, and approximately 85% of elderly patients achieving CR experience relapse within 2 to 3 years.18 Hence, less intensive chemotherapy might be an alternative approach to treat older patients with AML. In the phase I study of decitabine against AML,3 a 15 mg/m2 10-day dosing regimen was suggested as optimal and well tolerable. In the phase II study of decitabine against AML, with a schedule of 20 mg/m2 for 5 consecutive days in patients more than 60 years of age as the first-line treatment, the overall response rate was 25% and the median OS was 7.7 months.10 The primary toxicity was infection and cytopenia, and up to 47.3% of patients experienced at least one serious adverse event. In the phase III study,19 the baseline characteristics of older age, poor cytogenetics and performance status, BM blast >50%, and higher WBC and lower platelet counts were associated with shorter OS. In a subgroup analysis, even patients with higher blast counts (BM blasts ≥30%) could enjoy a survival benefit from decitabine treatment.20 Ten-day schedules of decitabine therapy have also been studied, and Bhatnagar, et al.21 reported outcomes with an overall response rate of 42% and a median OS of 9.0 months.
In the present study, 35.3% of older adult patients with AML achieved CR or CRi status after decitabine treatment with a dosing regimen of 20 mg/m2 for 5 days in 28-day cycles. In a univariate analysis, BM blasts, LDH, serum ferritin level, and BM iron were associated with CR after decitabine treatment (p=0.027, 0.049, 0.036, and 0.033, respectively). The significance of lower BM blast percentage at diagnosis to predicting the decitabine response is similar to the results of a previous study.21 Whereas BM blast and LDH are types of cancer cell factors affecting the treatment response to decitabine, iron status, such as serum ferritin or BM iron, might be a microenvironmental factor. Ferritin is a well-known indicator of the body's iron store and has various activities, such as that of an acute phase reactant, antioxidant, or growth factor for AML cell lines.22,23 Hyperferritinemia is a poor risk factor in patients with hematologic malignancies, probably because of the adverse impact of body iron accumulation in transfusion-dependent patients.24,25,26,27 However, hyperferritinemia might also affect prognosis through mechanisms other than iron accumulation, because several studies have demonstrated that even patients with hyperferritinemia at diagnosis without prior transfusion have poor prognosis.28,29 We could not perform a multivariate analysis because of the small study sample, so further study is necessary to elucidate the exact prognostic impact of baseline variables, such as serum ferritin, LDH, and BM blast percentage, and to identify patients that will likely benefit from decitabine treatment.
As decitabine must be incorporated into DNA to inhibit DNA methylation, it is necessary to give more applications of decitabine than that for conventional chemotherapy to conclude the activity of the drug against AML.5 In our study, continuous decitabine treatment led to one of the six patients (16.7%) who had PR status after two cycles of decitabine achieving CR after the fourth cycle. In fact, six patients (35.3%) achieved either CR or CRi after a median 3.0 cycles (range, 2–4) of decitabine treatment. Similarly, one study reported that up to nine cycles of decitabine treatment were required to achieve CR in elderly patients with AML.10 Taken together, these data suggest that the effectiveness of decitabine therapy should not be determined too early, and at least four cycles of decitabine treatment should be given to patients to obtain the best response, unless there is strong evidence of a lack of response, such as an increase in leukemic blast, despite decitabine therapy.
In summary, our study showed that decitabine treatment with a 5-day schedule is effective and well tolerated in Korean older adults with newly diagnosed AML. Therefore, decitabine could be recommended as a first-line treatment for such patients. A randomized prospective phase III trial comparing decitabine with standard 7+3 chemotherapy in patients with AML who are at least 60 years of age (NCT02172872) is currently recruiting participants and will further address the role of decitabine.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for (6-2010-0165).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Korea Central Cancer Registry NCC. Annual report of cancer statistics in Korea in 2012. Sejong: Ministry of Health and Welfare; 2014. [Google Scholar]
- 2.Sorm F, Veselý J. Effect of 5-aza-2'-deoxycytidine against leukemic and hemopoietic tissues in AKR mice. Neoplasma. 1968;15:339–343. [PubMed] [Google Scholar]
- 3.Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 4.Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2'-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruijsen M, Lübbert M, Wijermans P, Huls G. Clinical results of hypomethylating agents in AML treatment. J Clin Med. 2014;4:1–17. doi: 10.3390/jcm4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claus R, Pfeifer D, Almstedt M, Zucknick M, Hackanson B, Plass C, et al. Decitabine induces very early in vivo DNA methylation changes in blasts from patients with acute myeloid leukemia. Leuk Res. 2013;37:190–196. doi: 10.1016/j.leukres.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Klco JM, Spencer DH, Lamprecht TL, Sarkaria SM, Wylie T, Magrini V, et al. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood. 2013;121:1633–1643. doi: 10.1182/blood-2012-09-459313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fandy TE, Jiemjit A, Thakar M, Rhoden P, Suarez L, Gore SD. Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes. Clin Cancer Res. 2014;20:1249–1258. doi: 10.1158/1078-0432.CCR-13-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin DY, Park YS, Yang K, Kim GY, Kim WJ, Han MH, et al. Decitabine, a DNA methyltransferase inhibitor, induces apoptosis in human leukemia cells through intracellular reactive oxygen species generation. Int J Oncol. 2012;41:910–918. doi: 10.3892/ijo.2012.1546. [DOI] [PubMed] [Google Scholar]
- 10.Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 16.Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031) Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- 17.Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 18.Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 19.Mayer J, Arthur C, Delaunay J, Mazur G, Thomas XG, Wierzbowska A, et al. Multivariate and subgroup analyses of a randomized, multinational, phase 3 trial of decitabine vs treatment choice of supportive care or cytarabine in older patients with newly diagnosed acute myeloid leukemia and poor- or intermediate-risk cytogenetics. BMC Cancer. 2014;14:69. doi: 10.1186/1471-2407-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadia TM, Thomas XG, Dmoszynska A, Wierzbowska A, Minden M, Arthur C, et al. Decitabine improves outcomes in older patients with acute myeloid leukemia and higher blast counts. Am J Hematol. 2015;90:E139–E141. doi: 10.1002/ajh.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatnagar B, Duong VH, Gourdin TS, Tidwell ML, Chen C, Ning Y, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014;55:1533–1537. doi: 10.3109/10428194.2013.856425. [DOI] [PubMed] [Google Scholar]
- 22.Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta. 2013;1836:245–254. doi: 10.1016/j.bbcan.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Kikyo N, Hagiwara K, Yazaki Y, Okabe T. Growth stimulation of ferritin of human leukemia cells in vitro. J Cancer Res Clin Oncol. 1995;121:76–78. doi: 10.1007/BF01202216. [DOI] [PubMed] [Google Scholar]
- 24.Armand P, Sainvil MM, Kim HT, Rhodes J, Cutler C, Ho VT, et al. Does iron overload really matter in stem cell transplantation? Am J Hematol. 2012;87:569–572. doi: 10.1002/ajh.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazuaye GN, Buser A, Gerull S, Tichelli A, Stern M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2012;47:60–64. doi: 10.1038/bmt.2011.13. [DOI] [PubMed] [Google Scholar]
- 26.Mahindra A, Bolwell B, Sobecks R, Rybicki L, Pohlman B, Dean R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310–316. doi: 10.1111/j.1365-2141.2009.07774.x. [DOI] [PubMed] [Google Scholar]
- 27.Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Lebon D, Vergez F, Bertoli S, Harrivel V, De Botton S, Micol JB, et al. Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk Res. 2015;39:818–821. doi: 10.1016/j.leukres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi S, Kobune M, Iyama S, Sato T, Murase K, Kawano Y, et al. Prognostic significance of serum ferritin level at diagnosis in myelodysplastic syndrome. Int J Hematol. 2012;95:527–534. doi: 10.1007/s12185-012-1048-3. [DOI] [PubMed] [Google Scholar]