Abstract
Aim
To determine whether early clinical, laboratory and musculoskeletal ultrasound (MSUS) characteristics can be used as early detectors of juvenile idiopathic arthritis.
Patients and methods
Forty (40) patients with juvenile idiopathic arthritis (JIA) diagnosed according to the ILAR criteria [1] and 20 healthy control children. All patients were subjected to the following assessment at base line and at follow up after 6 months: Clinical evaluation, MSUS examination and laboratory evaluation.
Results
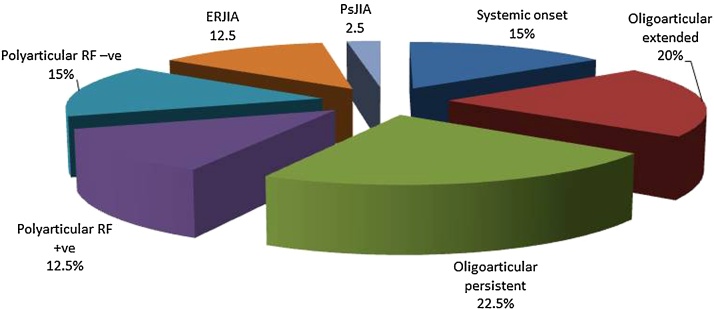
Of the 40 patients, 6 patients (15%) had systemic onset subtype, 8 (20%) oligoarticular extended, 9 (22.5%) oligoarticular persistent, 5 (12.5%) polyarticular rheumatoid factor (RF) +ve, 6 (15%) polyarticular RF −ve, 5 (12.5%) enthesitis related subtype and only one patient (2.5%) had psoriatic JIA. MSUS detected more synovitis than clinical examination (subclinical synovitis) both at base line and at follow up. MSUS is highly sensitive for early detection of joint involvement in JIA when compared to physical examination. Significant decrease in the mean cartilage thickness of the patients measured at follow up as compared to measures at base line.
Conclusion
MSUS is highly sensitive for early detection of joint involvement in JIA when compared to physical examination
1. Introduction
Juvenile Idiopathic Arthritis (JIA) is a chronic inflammatory disease that affects 1 of every 1,000 children worldwide (Ravalli and Martini, 2007). [2] Joint inflammation has a central role in the development of cartilage damage and bony erosion. Although a number of measures are applied in the evaluation of disease activity in, therapeutic decisions are primarily influenced by the presence of synovitis on clinical examination JIA (Ravalli et al., 1997) [3]. Currently evaluation of the disease status in children with JIA is based on clinical and laboratory measures. However, these measures have the limitation of not directly measuring inflammation at the primary site of pathology and may be subject to confounding influences. Imaging techniques, such as US and MRI, are capable of directly visualizing and objectively quantifying synovial inflammation and thus represent suitable tools to assess synovitis in children with JIA. These imaging modalities may also enable better and earlier detection of cartilage and bone changes than conventional radiography (Magni-Manzoni et al., 2012) [4].
The issue of subclinical synovitis may be particularly relevant in JIA. In the International League of Associations for Rheumatology classification, children with JIA are defined as having oligoarthritis or polyarthritis on the basis of the number of affected joints (≤4 or >4, respectively) (Giannini et al., 1992) [5]. Furthermore, the presence of active disease in a minimum of 5 joints is a prerequisite for patient inclusion in clinical trials of second line or biologic agents (Grassi, 2003), Lovell et al., 2008) [6], [7]. Therefore, the presence of subclinical disease in some joints may alter patient classification or affect the identification of patients requiring more aggressive treatment.
1.1. Aim: of the work
To determine whether early clinical, laboratory and musculoskeletal ultrasound (MSUS) characteristics can be used as early detectors of juvenile idiopathic arthritis.
2. Patients and methods
Forty patients with JIA and twenty subjects as a control group were included between Jun 2014 to April 2016, Twenty five (25) females and fifteen (15) males, their age ranged from 2 to 15 years with a mean of 8.8 ± 3.6 years and disease duration ranged from 6 weeks to three months. All patients were attending rheumatology and rehabilitation department at Minia university hospital. All patients were diagnosed according to the ILAR criteria (Petty et al., 1998) [1] if well fulfilled at the 1 st presentation or in the follow up after 6 months. They were included in our study after approval of the local ethical committee. An informed consent from each patient was taken before enrollment into the study.
2.1. Patient's selection
Inclusion criteria: Forty children aged <16 years presenting with any of the following manifestations with duration ranged from 6 weeks to three months: Arthritis (in at least one joint), arthralgia, skin rash, fever, psoriasis, sacroiliitis, enthesitis.
2.2. Exclusion criteria
Septic arthritis, haemarthrosis, arthritis caused by malignancy or trauma, connective tissue disorder such as systemic lupus erythematosus, mixed connective tissue disease or dermatomyositis. Eleven (11) children were excluded, 3 had juvenile lupus, 5 had viral infection and 3 had post traumatic arthritis.
2.3. Data collection
All patients were subjected to:
Initial assessment at base line and follow up assessment after 6 months, where the following assessments were done for all patients at base line and repeated at follow up after 6 months: Clinical evaluation, musculoskeletal ultrasonographic examination, laboratory evaluation.
The controls were subjected to: Musculoskeletal ultrasonographic examination and laboratory evaluation.
2.4. Locomotor system examination.
Each joint was examined by inspection (swelling, deformity, redness or scar), palpation (effusion, synovial thickening, hotness or tenderness), and movement (active and passive) with measurement of the active range of motion.
Articular index (AI) score was calculated according to modified Ritchie (AI) (Ritchie et al., 1968) [8].
Number of swollen joints was calculated.
Number of tender joints was calculated.
Number of joints with limited range of motion (LROM) was calculated.
Number of joints with clinical synovitis was calculated; a joint with clinical synovitis was defined as the presence of swelling or, if no swelling was present the presence of tenderness/pain on motion and LROM (Ruperto and Giannini, 1996;) [9] (Ravelli et al., 1997) [3].
Examination for enthesopathy (planter fascia, Achill's tendon, spinal process and iliac crest).
2.5. Musculoskeletal Ultrasonographic evaluation:
Ultrasonographic assessment was done for all patients at the 1st presentation and at follow up and for the control; the following data were recorded for the right and left metacarpophalangeal joints, proximal interphalangeal joints, wrists, knees and ankles:
Effusion.
Bone erosion.
Synovial membrane thickness.
Articular cartilage thickness.
2.5.1. Power Doppler signal (PDS)
US assessment was performed separately, immediately after clinical evaluation, by the same experienced radiologist and rheumatologist in musculoskeletal US (five years' experience in musculoskeletal US). The US examiner was blinded to clinical findings. Ultrasonographic examination was performed using Semins P300 Freq. up to 18MH linear transducer.
Joint effusion was defined as the presence of an abnormally anechoic space within the joint that was compressible. Bone erosion was defined as a cortical crater seen in two views, Synovial membrane thickness and articular cartilage thickness were measured in mm and compared to age and sex matched healthy control. Power Doppler signal was considered positive in the presence of vessel dots on PD images. All of the US findings were interpreted using both longitudinal and transverse planes. The number of joints with US effusion, erosions, increased synovial membrane thickness, positive PD signal and decreased articular cartilage thickness were computed.
A joint with US synovitis was defined as a joint in which any of the 5 US abnormalities was detectable. The US examination techniques as well as the definitions of US features were based on published guidelines and descriptions, particularly those provided by the outcome measures in rheumatology clinical trials
(Wakefield et al., 2005 [10]; Naredo et al., 2007 [11]; Naredo et al., 2008 [12]).
2.6. Laboratory evaluation:
The following laboratory investigations were done for patients (at base line and at follow up) and control:
Complete blood count (CBC) using automated cell counter.
Estimation of ESR by the Westergren method.
Rheumatoid factor by latex agglutination slide test for the qualitative and semi quantitative determination of RF in non diluted serum.
Liver and renal function tests as routine basic blood tests.
Antinuclear antibody titer and pattern (by immuno-fluorescent technique) (Aitcheson and Tan, 1982) [13].
Anti Cyclic Citrullinated Peptide antibody (Anti CCP), as follow (Schellekens et al., 2000) [14]: Kits used: Cyclic Citrullinated Peptide (CCP) IgG ELISA kit supplied from INOVA diagnostics, Inc.San Diego, CA USA 92131.
2.7. Statistical analysis
Data were coded, entered and analyzed by the Statistical Package for the Social Sciences (SPSS for windows version 16.0) (SPSS Inc., 2000). Two-tailed tests were used throughout, and statistical significance was set at the conventional level of less than 0.05.The range, means and standard deviation were calculated for interval and ordinary variables and frequencies and percentages for categorical variables (Bland, 1987) [15]. Comparisons were done by several procedures, depending on the type of variables: Student's t-test and chi-squared (c2) test. Pearson’s correlation coefficient was done (Bland, 1987) [15]. Sensitivity, specificity, positive and negative predictive values were calculated.
3. Results
Girls were 25 (62.5%) and boys were 15 (37.5%). The age of patients ranged from 2 to 15 years with a mean of 8.8 ± 3.6. Both patients and control were age and sex matched (p = 0.90 & p = 0.85).
The percentage of onset subtypes in JIA patients was represented on Fig. 1:
Fig. 1.
Represents the percentage of onset subtypes in JIA patients.
The disease duration was ranged from 6 to 12 weeks with a mean of 7.6 ± 2.0 weeks. Family history was positive in 5 (12.5%) of patients, 1 (2.5%) had oligoarticular subtype and 4 (10%) had enthesitis related arthritis.
The main clinical presentation was arthralgia in 4 (10%) of the 40 patients, arthritis in 28 patients (70%), fever in 3 patients (7.5%), skin rash in 2 patients (5%), and enthesitis in 3 patients (7.5%).
At base line, 5 patients (12.5%) were RF +ve and 35 (87.5%) were RF −ve, 14 patients (35%) were antinuclear antibodies (ANA) +ve {7 (50%) had homogenous ANA pattern, 4 (28.6%) speckled and 3 (21.4%) nucleolar pattern} and 26 (65%) were −ve, 5 patients (12.5%) were anti CCP antibody +ve and 35 (87.5%) were −ve, erythrocyte sedimentation rate (ESR) was elevated in 37 patients (92,5%), C-reactive protein (CRP) was positive in 32 patients (80%), anemia was present in 6 patients (15%), while thrombocytosis was present in 5 patients (12.5%).
3.1. Musculoskeletal Ultrasonographic findings
3.1.1. Frequency of clinical and US features in specific joints at base line: (Table 1, Table 2, Table 3)
Table 1.
Clinical data of patients at base line and at follow up.
| At base line | At follow up | P-value | ||
|---|---|---|---|---|
| Morning stiffness(min) | Range | 0.00–165.0 | 0.00–180 | 0.141 |
| Mean ± SD | 28.50 ± 70.24 | 68.12 ± 79.53 | ||
| Ritchie AI | Range | 1.00–19.00 | 2.00–25.00 | 0.000** |
| Mean ±SD | 6.65 ± 5.86 | 11.57 ± 7.29 | ||
| N. of swollen J. | Range | 0.00–5.00 | 0.00–7.00 | 0.000** |
| Mean ±SD | 1.77 ± 1.76 | 3.17 ± 2.07 | ||
| N. of tender J. | Range | 0.00–7.00 | 0.00–8.00 | 0.093 |
| Mean ±SD | 3.17 ± 0.55 | 3.45 ± 1.16 | ||
| N. of J. with clinical synovitis | Range | 0.00–6.00 | 0.00–7.00 | 0.000** |
| Mean ±SD | 1.95 ± 1.86 | 3.35 ± 2.20 | ||
| N. of J. with LROM | Range | 0.00–5.00 | 0.00–6.00 | 0.618 |
| Mean ±SD | 1.67 ± 0.82 | 1.75 ± 0.98 | ||
| Morning stiffness | Yes | 26(65%) | 19(47.5%) | 0.115 |
| No | 14(35%) | 21(52.5%) | ||
| Fever | Yes | 8(20%) | 5(12.5%) | 0.636 |
| No | 32(80%) | 35(37.5%) | ||
| Skin rash | Yes | 7(17.5%) | 5(12.5%) | 0.531 |
| No | 33(82.5%) | 35(37.5%) | ||
| Uveitis | Yes | 11(27.5%) | 8(20%) | 0.431 |
| No | 29(72.5%) | 32(80%) | ||
| Enthesitis | Yes | 10(25%) | 7(17.5%) | 0.412 |
| No | 30(75%) | 33(82.5%) |
Significant P-value < 0.01.
Table 2.
Ultrasonography determined synovitis in clinically symptomatic and asymptomatic joints.
| At base line |
At follow up |
|||
|---|---|---|---|---|
| Joints with clinical synovitis (n = 79) | Clinically asymptomatic joints (n = 321) | Joints with clinical synovitis (n = 138) | Clinically asymptomatic joints (n = 262) | |
| N. of joints with US synovitis | 79 (100%) | 44 (13.7%) | 138 (100%) | 45 (17.17%) |
Table 3.
Clinical and US examination in detection of synovitis in different JIA subtypes at follow up.
| JIA subtype | Patients with clinical synovitis (n = 30) |
Patients with US synovitis (n = 38) |
||
|---|---|---|---|---|
| Number | % | Number | % | |
| Systemic onset | 6 | 20 | 6 | 15.78 |
| Oligoarticular extended | 8 | 26.66 | 8 | 21.05 |
| Oligoarticular persistent | 5 | 16.66 | 9 | 23.68 |
| Polyarticular RF +ve | 3 | 10 | 5 | 13.15 |
| Polyarticular RF −ve | 4 | 13.33 | 6 | 15.78 |
| ERJIA | 3 | 10 | 3 | 7.89 |
| PsJIA | 1 | 3.33 | 1 | 2.63 |
In total, 400 joints {10 joints in every patient of the 40 patients RT and LT second proximal interphalangeal joint (PIP), RT and LT second metacarpophalangeal joint (MCP), RT and LT wrists, RT and LT knees, RT and LT ankles} were assessed both clinically and with US at base line.
3.1.1.1. Clinical findings
On clinical examination, 72 joints (18%) were swollen, 109 joints (27.25%) were tender, 26 joints (6.5%) had limited range of motion (LROM), and 79 joints (19.75%) had clinical synovitis.
Among the 79 joints with clinical synovitis, the most frequently affected were the LT MCP (15.18), followed by the LT wrist (13.92%) and RT wrist (13.92%), RT MCP (12.65%), RT knee (11.39%), RT PIP (7.59%) and both ankles (7.59% for each), and the less frequently affected were the LT PIP (5.06%) and LT knee joints (5.06%).
3.1.1.2. US findings
On US evaluation, 116 joints (29% of the total 400 joints) had joint effusion, 39 joints (9.75%) had erosions, 102 joints (25.5%) had synovial hypertrophy, 57 joints (14.25%) had +ve PDS, 94 (23.5%) joints had decreased cartilage thickness (Fig. 1).
A total of 123 joints (30.75%) had US synovitis (i.e., had 1 or more of the 5 US abnormalities). Ultrasonic synovitis was seen most frequently in the LT MCP (13.82%), followed by the RT MCP and RT wrist joints (13.00% for each), RT knee (12.19%), LT wrist (9.75%), LT ankle (8.94%), LT knee (8.13%), LT PIP and RT ankle (7.31%) and the less frequently affected was the RT PIP joint (6.50%).
3.1.2. Frequency of clinical and US features in specific joints at follow up: (Table 3)
The same 400 joints were assessed both clinically and with US at follow up.
3.1.2.1. Clinical findings
On clinical examination, 129 joints (32.25%) were swollen, 184 joints (46%) were tender, 32 joints (8%) had LROM, and 138 joints (34.5%) had clinical synovitis.
Among the 138 joints with clinical synovitis, the most frequently affected was the RT wrist (15.21)%), followed by the RT and LT MCP joints (12.31% for each), RT knee‘ (11.59%), LT PIP and LT wrist (9.42% for each), RT ankle (7.97%), LT ankle (8.69%), LT knee (7.24%) and the less frequently affected was the RT PIP (5.79%).
3.1.2.2. US findings
On US evaluation, 170 joints (42.5% of the total 400 joints) had joint effusion, 86 joints (21.5%) had erosions, 171 joints (42.75%) had synovial hypertrophy, 112 joints (28%) had +ve PDS, 163 (40.75%) joints had decreased cartilage thickness.
A total of 183 joints (45.75%) had US synovitis. Ultrasonic synovitis was seen most frequently in the RT wrist (13.11%), followed by the RT knee (12.56%), LT MCP and LT knee (10.92% for each), RT MCP (9.83%), LT wrist and LT ankle (9.28% for each), LT PIP and RT ankle (8.19% for each),and the less frequently affected was the RT PIP joint (7.65%).
Musculoskeletal ultrasonography led to classify 4 patients (had only arthralgia on clinical examination without any clinical synovitis) as oligoarticular persistent subtype, and also US led to classify 2 patients (who had oligoarthritis by clinical examination) as polyarticular RF +ve, and another 2 patients (who had oligoarthritis by clinical examination) as polyarticular RF −ve JIA.
3.2. Correlation between the number of joints with US synovitis and different clinical parameters (at follow up)
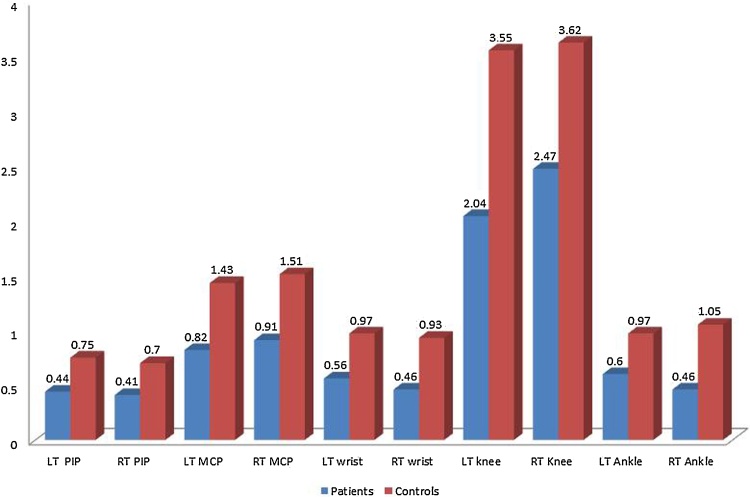
At follow up the number of joints with US synovitis had positive highly significant correlation with the number of swollen joints (p=0.000**), number of tender joints (p=0.000**), number of joints with clinical synovitis (p=0.000**), number of joints with LROM (p=0.006**), Ritchie AI (p=0.000**) and duration of morning stiffness (p=0.002**). The mean cartilage thickness measured in JIA patients at follow up was significantly lower in all examined joints (LT and RT PIPs, LT and RT MCPs, LT and RT wrists, LT and RT knee and LT and RT ankles) compared with the healthy control group Fig. 4, Fig. 5.
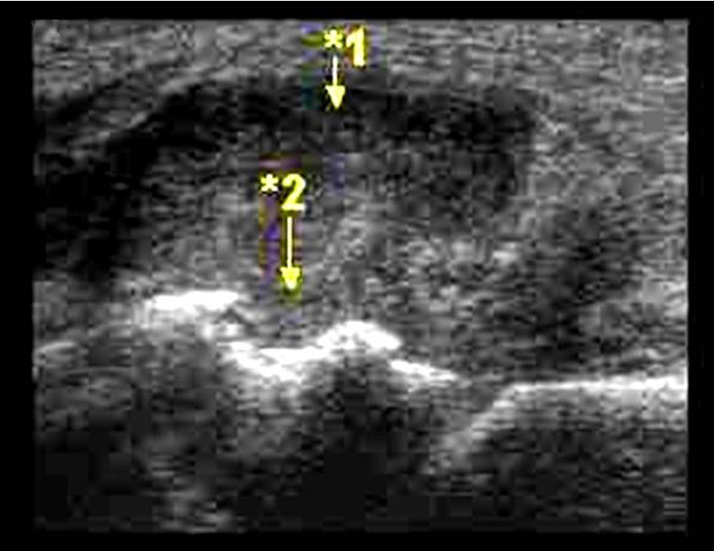
Fig. 4.
Ankle joint with synovial hypertrophy (1) and tibial erosions (2).
Fig. 5.
Represents comparison between joint cartilage thickness measured by MSUS in patients (at follow up) and control group (values are in mm).
3.3. Correlation by clinical examination and US evaluation for detection of synovitis
The sensitivity of clinical examination reached 34.5% compared to 45.7% for the MSUS with specificity of 100% for both. The +ve predictive value was 1 for each while the −ve predictive value was 0.43 for clinical examination compared to 0.47 for MSUS. (Table 4) Different degrees and joint involvements were represented in Figs. 2, 3, and 5.
Table 4.
The sensitivity, specificity, positive and negative predictive values of clinical examination and MSUS in detection of synovitis (calculated using the follow up values).
| Clinical examination | MSUS | |
|---|---|---|
| Sensitivity | 34.5 | 45.7 |
| Specificity | 100% | 100% |
| Positive predictive value | 1 | 1 |
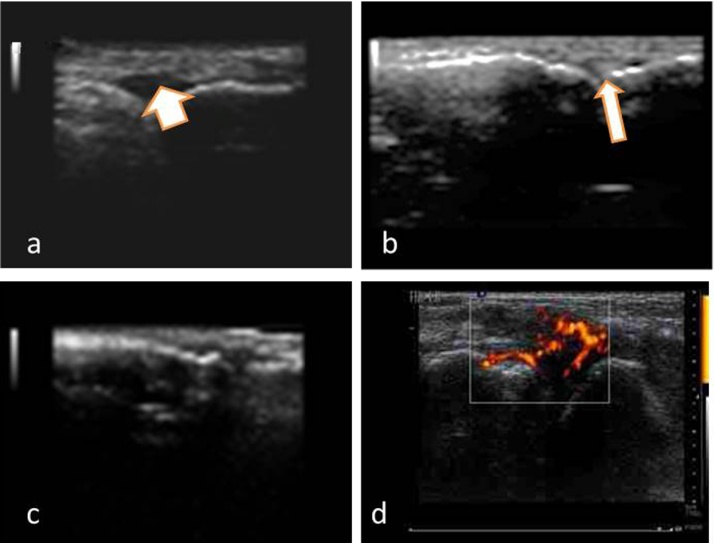
Fig. 2.
a- Effusion in the 2nd MCP joint (Thick short arrow). b- 2nd MCP effusion with erosion of the metacarpal head (arrow). c- Synovial hypertrophy in 2nd MCP joint with erosion of metacarpal head.
4. Discussion
In the past decade, there has been important progress in the management of JIA, which includes the shift towards early aggressive interventions, and the development of new therapeutic agents and combination treatment strategies (Hayward and Wallace, 2009 [16]). A reliable documentation of the advances in therapeutic effectiveness creates the need for sensitive methods that enable a precise monitoring of the course of the synovial inflammation process. Furthermore, it is desirable to identify patients with a high likelihood of developing erosive joint damage early, so as to institute appropriately aggressive therapy at an early stage of the disease. Of the diagnostic tools currently available, imaging studies are best suited for these purposes (Ruperto and Martini, 2011) [17].
Ultrasonography is ideally suited for multiple joint assessment. It has been suggested that its routine use allows a marked improvement of a clinician's capability to detect both early and hidden features of synovitis (Grassi, 2003) [6].
Silvia et al. (2009) [18], compared clinical and US examination of multiple joints in children with JIA where thirty two patients underwent clinical and US evaluation of 52 joints. They found that US detected more synovitis than clinical examination. Of the 1664 scanned joints, 104 (6.25%) had clinical synovitis and 167 (10.0%) had US synovitis. Furthermore, 86 (51.5%) of the 167 joints that had US documented synovitis were clinically normal (i.e., had subclinical synovitis). Subclinical synovitis was more common in small hand and wrist joints. Notably, discrepancies between pediatric rheumatologists in clinical examination of joints in children with JIA were found to be larger in small hand joints (Guzman et al., 1995) [19].
Detecting synovitis in clinically unaffected joints, US may increase the number of patients who are candidates to receive second line or biologic agents. Another important issue that may be affected by US application is the assessment of clinical remission. The absence of joints with active arthritis, defined on clinical grounds, is a fundamental component of criteria for inactive disease in JIA (Wallace et al., 2004) [20].
Our data support previous studies which indicate that US is able to detect subclinical disease (Magni et al., 2009 [21]; Filippou et al., 2011)[22].
Our study revealed that MSUS is highly sensitive for early detection of joint involvement in JIA when compared to physical examination.
This goes in concordance with the study by Ginger et al. (2011)[23], which aimed to determine sensitivity and specificity of the physical examination (PE) for identifying synovitis in the knee and ankle joints of children with JIA. Nineteen patients with JIA were referred for US. Both knees and ankles were examined using US. They found that there was agreement between US and PE in 75% of cases. PE was 64% sensitive and 86% specific for identifying active arthritis. physical examination was 100% specific if (1) the patient was positive for both PE criteria. When the PE was negative and the US was positive, 21.4% developed active disease on PE within 6 months. Limitations of this study were that neither the sonographer nor the interpreting radiologists were blinded to the patient’s diagnosis. Synovitis was not confirmed by another imaging technique, no US was performed at the time of the follow up. Moreover PE was performed by more than 1 clinician so that interobserver disagreement may have been present. While in the current study we performed US at the time of follow up, and PE was performed by only 1 clinician so that there is no interobserver disagreement.
The findings in our study have important implications for the classification of JIA. Currently, the number of joints affected over time is adopted as a criterion to classify patients in presumably homogeneous categories. Patients are defined as having oligoarthritis or polyarthritis if they have 4 or fewer or 5 or more joints involved, respectively, during the first 6 months of disease (Petty et al., 2004) [24].
We found that US led to classify 4 patients (had only arthralgia on clinical examination without any clinical synovitis) as oligoarticular persistent subtype, and also US led to classify 2 patients (who had oligoarthritis by clinical examination) as polyarticular RF +ve, and another 2 patients (who had oligoarthritis by clinical examination) as polyarticular RF −ve JIA.
Similarly, in the study of Silvia M et al. (2009)[18], US led them to classify 5 patients who were labeled as having oligoarthritis or were found to have no synovitis on clinical examination as having polyarthritis. In another study in JIA, 36% of clinically normal knees had evidence of effusion on US (McCarron et al., 2008) [25]. these findings suggest that US is more accurate than clinical assessment and may lead to reclassifying many patients with JIA. Furthermore, they add to the criticisms about the use of the number of affected joints as a classification parameter in JIA (Martini, 2003) [26].
The study of Silvia et al. (2009) [18], had some limitations which should be mentioned. The relationship between clinical and US findings was evaluated in a cross sectional assessment. Therefore, they could not investigate the predictive value of US in relation to the efficacy of therapeutic interventions or course of joint disease over time. A healthy control group, which would have strengthened the study, was not available. This is in contrast to our study in which we did a follow up US assessment; therefore, we could investigate the predictive value of US, and a healthy control group was examined to strengthen the study. However both studies did not validate the additional synovitis by other imaging techniques, such as MRI. Although this technique is limited by its inability to scan more than one joint and general anesthesia is required in younger children.
In our study the mean cartilage thickness measured in the JIA patients at follow up was significantly lower in all examined joints compared with the healthy control group.
Dan #XPS##x00D8;stergaard et al. (2013) [27], found that, Joint cartilage thickness was decreased in the knee, wrist, and second PIP joint in children with JIA compared with the healthy cohort (p < 0.001 for all). Whereas the cartilage thickness in the ankle and second MCP joint was not different from that of healthy controls. Interestingly, regardless of whether the knees, wrists, and second PIP joints were ever previously affected, they found significantly decreased cartilage thickness compared to similar joints from healthy controls.
A limitation of this study by Dan #XPS##x00D8;stergaard et al. (2013) [27], was that the US measurements were done by 1 observer who measured joint cartilage thickness in healthy children and 1 who did the measurements in the patients with JIA so that interobserver disagreement may have been present. However the investigators were blinded to clinical information about JIA subtype, disease activity, age, and sex. This limitation was not present in our study.
We found that, number of joints with US synovitis was significantly correlated with different clinical features. Silvia et al. (2009) [18], found that US findings were moderately correlated with clinical measures of joint swelling, but poorly correlated with those of joint tenderness/pain on motion and restricted motion.
We should emphasize that our results do not mean that US is an alternative to clinical examination. It should be regarded as a tool that complements conventional clinical examination.
US has several advantages over other imaging techniques for use in paediatric subjects, which include non-invasiveness, rapidity of performance, ease of repeatability, high patient acceptability and lack of exposure to ionizing radiation. In addition, it does not require sedation for scanning in younger children. US allows precise and thorough visualization of inflammatory and destructive joint abnormalities, including synovial hyperplasia, joint effusion, cartilage damage, bone erosion, tenosynovitis and enthesopathy (Filippucci et al., 2007 [28]).
Finally, the significance of new results when compared to recent studies can be as follow: Our results included children’s very early onset of disease and re-evaluate them after 6 months (short term evaluation). It is useful to use US for reclassification of the types of juvenile arthritis early which is important for disease prognosis. Assessment included 5 different joints rather other studies and clinical parameters were assessed and correlated with US findings.
5. Conclusions
MSUS is highly sensitive for early detection of joint involvement in JIA when compared to physical examination. Subclinical synovitis as detected by US is common in children with JIA. This finding may have important implications for patient classification. Abnormal US findings are significantly correlated with different clinical features
We recommend the use of MSUS for early detection of synovitis in JIA, however MSUS training focused on the assessment of pediatric patients and standardization of US assessment of joints in children might be very important in implementing the use of this technique in clinical practice and research. Studies on larger groups of children are needed to confirm the validation and variability of US in children. Moreover, longitudinal assessments are required to determine the true significance of subclinical disease as determined by MSUS in JIA.
Conflict of interest
We have no conflict of interest to declare.
Funding
No disclosure of funding received for this work from any organization.
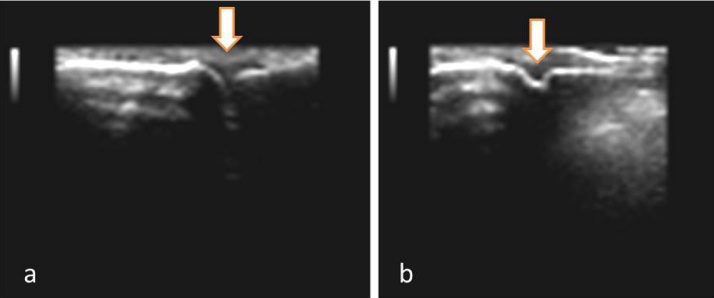
Fig. 3.
a- Synovial hypertrophy in the 2nd PIP joint. b- 2nd PIP joint with effusion.
References
- 1.Petty R.E., Southwood T.R., Baum J. Revision of the proposed classification criteria for juvenile idiopathic arthritis. J Rheumatol. 1998;25:1991–1994. [PubMed] [Google Scholar]
- 2.Ravelli A., Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli A., Viola S., Ruperto N. Correlation between conventional disease activity measures in juvenile chronic arthritis. Ann Rheum Dis. 1997;56:197–200. doi: 10.1136/ard.56.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magni-Manzoni S., Malattia C., Lanni S. Advances and challenges in imaging in juvenile idiopathic arthritis. Nat Rev Rheumatol. 2012;8:329–336. doi: 10.1038/nrrheum.2012.30. [DOI] [PubMed] [Google Scholar]
- 5.Giannini E.H., Brewer E.J., Kuzmina N. The Pediatric Rheumatology Collaborative Study Group and the Cooperative Children's Study Group Methotrexate in resistant juvenile rheumatoid arthritis: results of the USA-USSR double-blind, placebo- controlled trial. N Engl J Med. 1992;326:1043–1049. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 6.Grassi W. Clinical evaluation versus ultrasonography: who is the winner? J Rheumatol. 2003;30:908–909. [PubMed] [Google Scholar]
- 7.Lovell D.J., Ruperto N., Goodman S. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–820. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie D., Boyle J., Mc Innes J. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Quart J Med. 1968;37:393. [PubMed] [Google Scholar]
- 9.Ruperto N., Giannini E.H. Redundancy of conventional articular response variables used in juvenile chronic arthritis clinical trials. Ann Rheum Dis. 1996;55:73–75. doi: 10.1136/ard.55.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakefield R.J., Balint P.V., Szkudlarek M. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 11.Naredo E., Collado P., Cruz A. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–124. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 12.Naredo E., Rodriguez M., Campos C. For the Ultrasound Group of the Spanish Society of Rheumatology Validity, reproducibility, and responsiveness of a twelve-joint simplified power Doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2008;59:515–522. doi: 10.1002/art.23529. [DOI] [PubMed] [Google Scholar]
- 13.Aitcheson T., Tan M. Antinuclear antibodies. In: Panayi G.S., editor. vol. 6. Churchill Livingestone; Edinburgh, London, Melboune and New York: 1982. p. 87. (JIA: Scientific basis of Rheumatology). [Google Scholar]
- 14.Schellekens A., Visser H., De Jong A. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Bland M. Chrchill Livingstone; 1987. An introduction to medical statistics. [Google Scholar]
- 16.Hayward K., Wallace C.A. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res. 2009;11:216. doi: 10.1186/ar2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruperto N., Martini A. Current medical treatments for juvenile idiopathic arthritis. Front. Pharmacol. 2011;2:60. doi: 10.3389/fphar.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvia M., Oscar E., Angelo R. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Pediatric Rheumatology. 2009;61(11):1497–1504. doi: 10.1002/art.24823. [DOI] [PubMed] [Google Scholar]
- 19.Guzman J., Burgos-Vargas R., Duarte-Salazar C. Reliability of the articular examination in children with juvenile rheumatoid arthritis: interobserver agreement and sources of disagreement. J Rheumatol. 1995;22:2331–2336. [PubMed] [Google Scholar]
- 20.Wallace C.A., Ruperto N., Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–2294. [PubMed] [Google Scholar]
- 21.Magni S.M., Epis O., Ravelli A. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:1497–1504. doi: 10.1002/art.24823. [DOI] [PubMed] [Google Scholar]
- 22.Filippou G., Cantarini L., Bertoldi I. Ultrasonography vs: clinical examination in children with suspected arthritis. Does it make sense to use polyarticular Ultrasonographic screening? Clin Exp Rheumatol. 2011;29:345–350. [PubMed] [Google Scholar]
- 23.Ginger L., Janow S., Vikash P. Detection of Active Disease in Juvenile Idiopathic Arthritis: Sensitivity and Specificity of the Physical Examination vs Ultrasound. J Rheumatol. 2011;38:2671–2674. doi: 10.3899/jrheum.110360. [DOI] [PubMed] [Google Scholar]
- 24.Petty R.E., Southwood T.R., Manners P. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 25.McCarron M., Wray M., Pascoli L. Knee disease in juvenile idiopathic arthritis (JIA): correlation between clinical and ultrasonographic findings. Pediatric Rheumatology. 2008;6:95. [Google Scholar]
- 26.Martini A. Is the number of joints involved or the presence of psoriasis still useful tools to identify homogeneous disease entities in juvenile idiopathic arthritis? J Rheumatol. 2003;30:1900–1903. [PubMed] [Google Scholar]
- 27.Dan stergaard P., Anne H.S., Carsten H. Decreased Cartilage Thickness in Juvenile Idiopathic Arthritis Assessed by Ultrasonography. J Rheumatol. 2013;40:1596–1603. doi: 10.3899/jrheum.121077. [DOI] [PubMed] [Google Scholar]
- 28.Filippucci E., Iagnocco A., Meenagh G. Ultrasound imaging for the rheumatologist VII Ultrasound imaging in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:5–10. [PubMed] [Google Scholar]