Abstract
Background
Human papillomavirus (HPV) is a well-established oncogenic virus of cervical, anogenital, and oropharyngeal cancer. Various subtypes of HPV have been detected in 0% to 60% of breast cancers. The roles of HPV in the carcinogenesis of breast cancer remain controversial. This study was performed to determine the prevalence of HPV-positive breast cancer in Korean patients and to evaluate the possibility of carcinogenic effect of HPV on breast.
Methods
Meta-analysis was performed in 22 case-control studies for HPV infection in breast cancer. A total of 123 breast cancers, nine intraductal papillomas and 13 nipple tissues of patients with proven cervical HPV infection were tested by real-time polymerase chain reaction to detect 28 subtypes of HPV. Breast cancers were composed of 106 formalin-fixed and paraffin embedded (FFPE) breast cancer samples and 17 touch imprint cytology samples of breast cancers.
Results
The overall odds ratio between breast cancer and HPV infection was 5.43 (95% confidence interval, 3.24 to 9.12) with I<sup>2</sup> = 34.5% in meta-analysis of published studies with case-control setting and it was statistically significant. HPV was detected in 22 cases of breast cancers (17.9%) and two cases of intaductal papillomas (22.2%). However, these cases had weak positivity.
Conclusions
These results failed to serve as significant evidence to support the relationship between HPV and breast cancer. Further study with larger epidemiologic population is merited to determine the relationship between HPV and breast cancer.
Keywords: Breast neoplasms, Human papillomavirus, Real-time polymerase chain reaction, Meta-analysis
Human papillomavirus (HPV) is a known oncogenic virus associated with uterine cervical cancer, anogenital cancer, and oropharyngeal cancer. It has been proven that HPV proteins E6 and E7 can bind to p53 and retinoblastoma protein in epithelial cells and interact with growth-regulating system in uterine cervix, anus, and oropharynx [1,2]. Almost all uterine cervical cancers and anal cancers are associated with HPV infection [1]. HPV-mediated oropharyngeal squamous carcinomas have different disease entity from conventional oropharyngeal squamous carcinomas associated with chemical mutagens [3]. However, the role of HPV in mammary carcinogenesis still remains controversial because various risk factors such as genetic predisposition, diet, hormonal status, life style, and their interactions are involved complexly in the pathogenesis of breast cancer [4]. HPV infection has been reported in 4.4% to 60% of breast cancers [5-29]. Various subtypes of HPV including HPV-11, HPV-16, HPV-18, HPV-33, HPV-58, HPV-59, HPV-73, and HPV-82 are candidate subtypes of HPV associated with breast cancers. On the contrary, some studies could not detect HPV infection in breast cancers [30-33]. Furthermore, HPV infections have been detected not only in breast cancers, but also in various benign breast lesions such as fibroadenoma and intraductal papilloma. To the best of our knowledge, meta-analysis has not been performed yet to evaluate the relationship between HPV infection and breast cancer in a case-control setting. Therefore, the objective of this study was to perform meta-analysis between HPV infection and breast cancer to determine the prevalence of HPV-positivity in breast cancer, to determine whether any specific HPV subtypes are associated with breast cancer, and to evaluate the possibility of sexual transmission of HPV from genitals to breast.
MATERIALS AND METHODS
Meta-analysis
A systematic literature search was conducted in PubMed (January 1, 1992, to September 30, 2015) with the following keywords: “breast neoplasm” and “human papillomavirus.” All potentially relevant studies were reviewed. Studies with case-control setting were selected. Analyses were performed using R ver. 3.2.2 (2015-08-14) statistical software.
Tumor samples
The study was performed with 123 breast cancers and nine intraductal papillomas. These patients received surgeries at Korea University Guro Hospital from January 2007 to January 2015. The study protocol was approved by the Institutional Review Board of Guro Hospital. The 123 breast cancer samples were composed of 106 formalin-fixed and paraffin embedded (FFPE) tissues and 17 touch imprint cytology samples. The patients who received mastectomy and nipple resection were preferentially selected to obtain FFPE nipple tissues. The touch imprint cytology samples were also used to compare FFPE tissues and cytology samples. The nine intraductal papillomas samples were composed of nine FFPE tissues. To define the infection route by detecting HPV in nipple, 13 FFPE nipple tissues of breast cancer patients who had been confirmed with HPV infection in uterine cervix were included in this study. All materials were obtained from the tissue bank of Korea University Guro Hospital. Medical records and pathological reports of patients and histological features of breast cancer enrolled in this study were reviewed.
DNA isolation
Two or three 10-μm sections were taken from FFPE tissues. Deparaffinization of these sections was sufficiently done by xylene and ethanol treatment for 5 minutes alternately for three times. DNA was extracted using QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instruction. The concentration of extracted DNA was measured on Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). The quality of DNA was determined by evaluating A260/A280 ratio. DNA isolation from touch imprint sample from fresh breast cancer was also performed with QIAamp DNA Mini Kit according to the manufacturer’s instruction.
Real-time polymerase chain reaction
Extracted DNA samples were subject to real-time polymerase chain reaction (PCR) with gene specific primers provided with Anyplex II HPV 28 Detection System (Seegene, Seoul, Korea) using CFX96 Real-Time PCR (Bio-Rad, Hercules, CA, USA). Melting curves were analyzed using the exclusive analysis program provided with the Anyplex II HPV 28 Detection System. This system is able to detect 28 subtypes of HPV, including all subtypes reported in the literatures, such as HPV-11, HPV-16, HPV-18, HPV-33, HPV-58, HPV-59, HPV-73, and HPV-82. Because the Anyplex II HPV 28 Detection System was originally designed for cytological samples swabbed in uterine cervix, verification of Anyplex II HPV 28 Detection System with FFPE tissue of uterine cervical squamous cell carcinoma was performed.
RESULTS
Meta-analysis
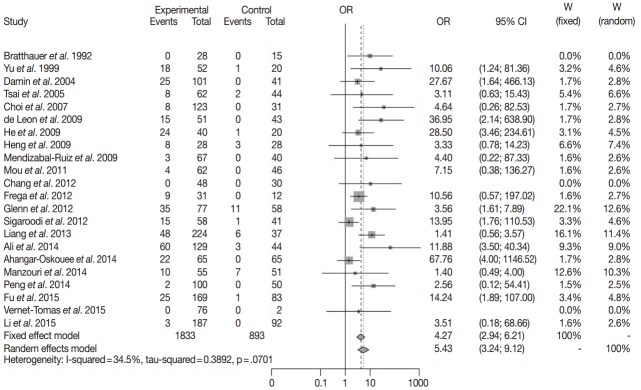
Twenty-two case-control studies for HPV infection in breast cancer were enrolled in meta-analysis with random effect model. HPV infection was detected in 342 of 1,833 breast cancers and in 36 of 857 benign breast lesions (Table 1). The overall odds ratio between breast cancer and HPV infection was 5.43 (95% confidence interval, 3.24 to 9.12) with I2=34.5% (Fig. 1). This result was statistically significant.
Table 1.
The published studies of HPV and breast cancer in case-control setting
| Study | Country | Case (n = 1,833) |
Control (n = 893) |
||
|---|---|---|---|---|---|
| HPV (+) | HPV (–) | HPV (+) | HPV (–) | ||
| Bratthauer et al. [34] (1992) | USA | 0 | 28 | 0 | 15 |
| Yu et al. [5] (1999) | China, Japan | 18 | 34 | 1 | 19 |
| Damin et al. [7] (2004) | Brazil | 25 | 76 | 0 | 41 |
| Tsai et al. [8] (2005) | Taiwan | 8 | 54 | 2 | 42 |
| Choi et al. [9] (2007) | Korea | 8 | 115 | 0 | 31 |
| de Leon et al. [11] (2009) | Mexico | 15 | 36 | 0 | 43 |
| He et al. [12] (2009) | China | 24 | 16 | 1 | 19 |
| Heng et al. [13] (2009) | Australia | 8 | 20 | 3 | 25 |
| Mendizabal-Ruiz et al. [14] (2009) | Mexico | 3 | 64 | 0 | 40 |
| Mou et al. [15] (2011) | China | 4 | 58 | 0 | 46 |
| Chang et al. [35] (2012) | China | 0 | 48 | 0 | 30 |
| Frega et al. [16] (2012) | Italy | 9 | 22 | 0 | 12 |
| Glenn et al. [17] (2012) | Australia | 35 | 42 | 11 | 47 |
| Sigaroodi et al. [18] (2012) | Iran | 15 | 43 | 1 | 40 |
| Liang et al. [19] (2013) | China | 48 | 176 | 6 | 31 |
| Ali et al. [23] (2014) | Iraq | 60 | 69 | 3 | 41 |
| Ahangar-Oskouee et al. [24] (2014) | Iran | 22 | 43 | 0 | 65 |
| Manzouri et al. [25] (2014) | Iran | 10 | 45 | 7 | 44 |
| Peng et al. [26] (2014) | China | 2 | 98 | 0 | 50 |
| Fu et al. [36] (2015) | China | 25 | 144 | 1 | 82 |
| Vernet-Tomas et al. [33] (2015) | Spain | 0 | 76 | 0 | 2 |
| Li et al. [28] (2015) | China | 3 | 184 | 0 | 92 |
| Total | 342 | 1491 | 36 | 857 | |
HPV, human papillomavirus.
Fig. 1.
The forest plot for relationship between breast cancer and human papillomavirus infection in case-control setting [5,7-9,11-19,23-26,28,33-36]. OR, odds ratio; CI, confidence interval.
Clinicopathologic data
The median age of 123 breast cancer patients was 51.6 years (range, 23 to 79 years). The 123 breast cancers included 103 invasive carcinomas of no special type, five invasive lobular carcinomas, one microinvasive carcinoma, three ductal carcinomas in situ, and 11 carcinomas of other specific subtypes. Other clinicopathological characteristics of the 123 cases of breast cancer are summarized in Table 2.
Table 2.
Clinicopathological characteristics of 123 cases of breast cancer
| Characteristic | Criteria | No. |
|---|---|---|
| Age | 34–50 | 67 |
| 51–66 | 56 | |
| Median (range) | 51.6 (23–79) | |
| Operation type | Mastectomy | 108 |
| Conserving operation | 15 | |
| Histologic grade | Grade 1 | 27 |
| Grade 2 | 41 | |
| Grade 3 | 52 | |
| Hormonal receptor | ER positive | 69 |
| PR positive | 62 | |
| HER2 positive | 52 | |
| Triple negative | 25 | |
| Lymph node metastasis | Positive | 54 |
| Negative | 69 | |
| HPV detection in cervix | Positive | 54 |
| Negative | 69 | |
| Histologic type | Invasive carcinoma of no special type | 103 |
| Invasive lobular carcinoma | 5 | |
| Microinvasive carcinoma | 1 | |
| Ductal carcinoma in situ | 3 | |
| Metaplastic carcinoma | 2 | |
| Carcinoma with medullary feature | 2 | |
| Apocrine carcinoma | 2 | |
| Carcinoma with neuroendocrine feature | 1 | |
| Adenoid cystic carcinoma | 1 | |
| Mucinous carcinoma | 1 | |
| Micropapillary carcinoma | 1 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HPV, human papillomavirus.
Of the 13 cases whose FFPE nipple tissues were tested for HPV, one case was positive for HPV-16 infection in uterine cervix and 12 cases were positive for HPV infection in uterine cervix by Hybrid Capture 2 (Qiagen, Gaithersburg, MD, USA) (Table 3). Of these 13 cases, five had low-grade squamous intraepithelial lesions, one had high-grade squamous intraepithelial lesion, and one had endocervical type adenocarcinoma in uterine cervix.
Table 3.
Clinicopathologic data of breast cancer patients confirmed HPV infection in uterine cervix
| Case No. | Age of breast surgery (yr) | Histology of breast | Age of HPV detection at uterine cervix (yr) | Histology of uterine cervix | Method |
|---|---|---|---|---|---|
| 15 | 50 | Invasive carcinoma of NST | 54 | NI | HC2 |
| 16 | 44 | Metaplastic carcinoma | 44 | LSIL | HC2 |
| 17 | 43 | Invasive carcinoma of NST | 44 | LSIL | HC2 |
| 18 | 48 | Invasive carcinoma of NST | 48 | NI | HC2 |
| 19 | 50 | Invasive carcinoma of NST | 50 | NI | HC2 |
| 20 | 46 | Invasive carcinoma of NST | 46 | LSIL | HC2 |
| 21 | 43 | Invasive carcinoma of NST | 41 | NI | HC2 |
| 22 | 49 | Invasive carcinoma of NST | 49 | NI | HC2 |
| 23 | 44 | Invasive carcinoma of NST | 44 | LSIL | HC2 |
| 24 | 63 | Invasive carcinoma of NST | 60 | Adenocarcinoma, endocervical type | HC2 |
| 25 | 50 | Invasive carcinoma of NST | 50 | NI | Medical record (HPV-16) |
| 38 | 56 | Invasive carcinoma of NST | 47 | LSIL | HC2 |
| 56 | 57 | Invasive carcinoma of NST | 46 | HSIL | HC2 |
HPV, human papillomavirus; NST, no special type; NI, in the biopsy, uterine cervical lesion was not identified; HC2, Hybrid Capture 2 (QIAGEN, Gaithersburg, MD, USA) in cytology sample; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
Availability of Anyplex II HPV 28 Detection System in FFPE tissue
HPV-16 was detected in the FFPE tissue of uterine cervical squamous cell carcinoma using Anyplex II HPV 28 Detection System, in concordance with the result of cytology sample using Anyplex II HPV 28 Detection System, proving that Anyplex II HPV 28 Detection System worked properly for FFPE tissue as for the cytology specimen. Therefore, Anyplex II HPV 28 Detection System can be used to detect HPV infection in FFPE tissue.
Real-time PCR
Proper DNA samples were isolated from 106 FFPE breast cancers, 17 touch imprint cytology samples of breast cancer, 13 FFPE nipple tissues, and nine FFPE intraductal papillomas. Amplification of dissociation curve of HPV subtypes 6, 16, 33, 39, 40, 51, 53, 58, and 61 was detected in real-time PCR of 22 FFPE breast cancers (17.9%) (Fig. 2). The 22 cases included 19 cases of invasive carcinoma of no special type, one case of adenoid cystic carcinoma, one case of metaplastic carcinoma, and one case of apocrine carcinoma (Table 4). Histological features of HPV infection were not identified by slide review of these 22 cases. In two FFPE tissues of intraductal papilloma, amplification of dissociation curve of HPV-33 and HPV-53 was detected in real-time PCR (22.2% of nine intraductal papilloma FFPE tissues). However, because HPV-positive cases showed weak amplification of the dissociation curve, these results were considered as weak positivity for HPV. HPV was not detected in 17 touch imprint cytology samples of breast cancer or 13 FFPE tissues of nipple.
Fig. 2.
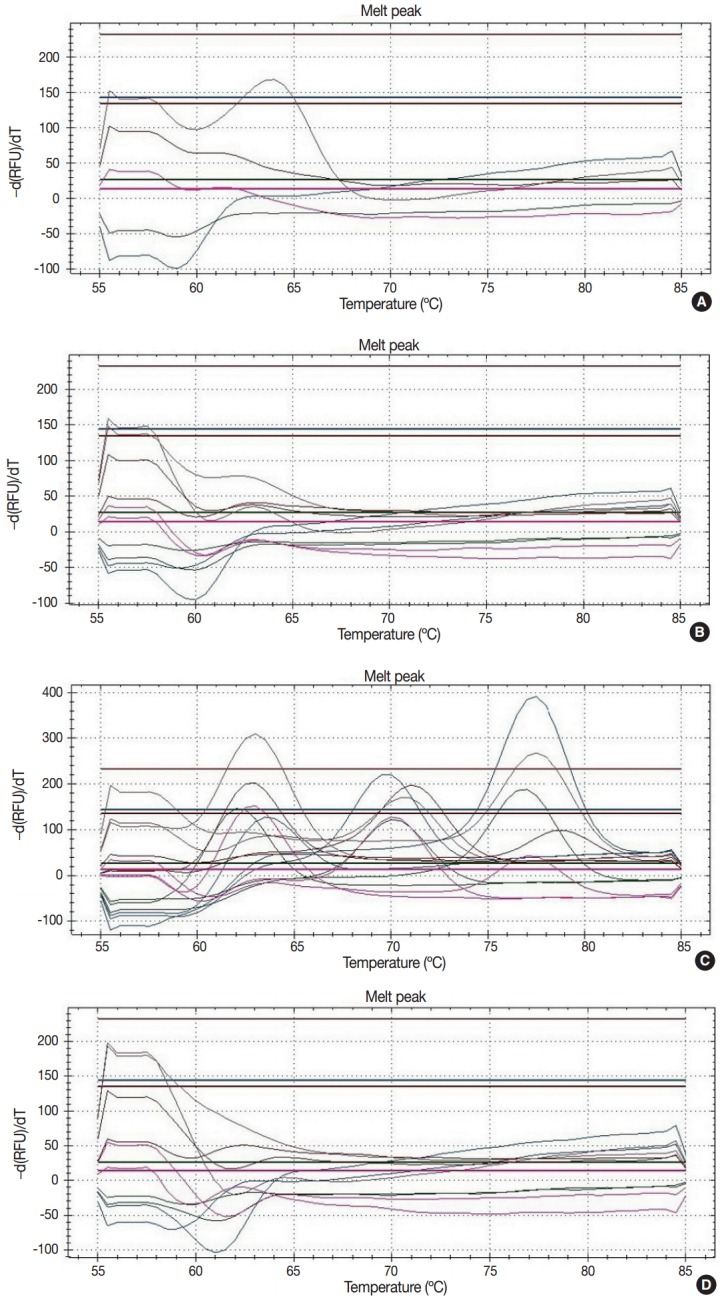
The representative results of dissociation curve in real-time polymerase chain reaction. (A) Weak positivity for human papillomavirus (HPV) 33. The dissociation curve of two cases shows the low melting peak. (B) Negative case. The dissociation curve of almost cases do not show the melting peaks. (C) Positive control. The dissociation curve of positive control shows 28 melting peaks about 28 subtypes of HPV. (D) Negative control.
Table 4.
Clinicopathologic data and HPV subtype of HPV-positive breast cancer
| Case No. | Age (yr) | Sample | Histologic type | HPV subtype |
|---|---|---|---|---|
| 1 | 50 | FFPE | Invasive carcinoma of NST | 33 |
| 3 | 46 | FFPE | Invasive carcinoma of NST | 33 |
| 8 | 55 | FFPE | Adenoid cystic carcinoma | 40, 51, 61 |
| 9 | 63 | FFPE | Metaplastic carcinoma | 40, 51, 53, 61 |
| 17 | 23 | FFPE | Invasive carcinoma of NST | 40, 51, 53, 61 |
| 20 | 32 | FFPE | Invasive carcinoma of NST | 51, 53 |
| 30 | 47 | FFPE | Invasive carcinoma of NST | 40, 51, 58 |
| 39 | 44 | FFPE | Invasive carcinoma of NST | 51 |
| 40 | 40 | FFPE | Invasive carcinoma of NST | 33, 51, 53 |
| 41 | 49 | FFPE | Invasive carcinoma of NST | 51, 53 |
| 49 | 55 | FFPE | Invasive carcinoma of NST | 51 |
| 50 | 38 | FFPE | Invasive carcinoma of NST | 51 |
| 63 | 70 | FFPE | Invasive carcinoma of NST | 6, 51, 58 |
| 73 | 42 | FFPE | Invasive carcinoma of NST | 6, 40, 53 |
| 74 | 58 | FFPE | Invasive carcinoma of NST | 6, 51 |
| 75 | 54 | FFPE | Invasive carcinoma of NST | 51 |
| 81 | 65 | FFPE | Invasive carcinoma of NST | 6, 40 |
| 93 | 35 | FFPE | Invasive carcinoma of NST | 53 |
| 99 | 54 | FFPE | Invasive carcinoma of NST | 6 |
| 109 | 50 | FFPE | Invasive carcinoma of NST | 51 |
| 120 | 63 | FFPE | Apocrine carcinoma | 16 |
| 122 | 50 | FFPE | Invasive carcinoma of NST | 39 |
HPV, human papillomavirus; FFPE, formalin-fixed and paraffin embedded tissue; NST, no special type.
DISCUSSION
Genetic and environmental factors such as mutation in BRCA1/2, ethnicity, hormonal effect, diet, and ionizing radiation are known to be involved in the carcinogenesis of breast cancer. However, two-thirds of patients with breast cancer have no association with these risk factors [37]. To elucidate viral carcinogenesis in mammary cancer development, many studies have focused on oncogenic virus. According to multistep carcinogenesis model of breast cancer, TP53 is considered as one predisposing gene. Because HPV E6 and E7 oncoproteins are able to inactivate TP53 gene, HPV can play a role in this multistep mammary carcinogenesis. On the other hand, Ohba et al. [38] have suggested that HPV infection induces overexpression of APOBEC3B associated with the early stage of carcinogenesis in breast cancer.
Recently, many studies have been performed to prove the relationship between breast cancer and HPV infection. These studies have revealed that approximately 19.8% of HPV infections are in breast cancer (range, 0% to 60%). The causes of no detection of HPV infection include geographic factors due to race and prevalence of HPV infection, selection bias due to difference in prevalence, and too low viral load to be technically detectable. At present, most studies performed in China and Middle East have showed high prevalence of positivity for HPV in breast cancer.
Meta-analysis from case-control setting revealed that the prevalence of HPV infection in breast cancer was higher than that in benign breast lesion. The I2 was appropriate for random effect model. The overall odds ratio between breast cancer and HPV infection was statistically significant. However, there are some limitations of meta-analysis. First, publication bias might exist due to low prevalence of HPV infection in breast cancers. Many studies that failed to detect HPV in breast cancer might not have been reported in the literature. Moreover, because 22 studies were performed with different methods that could only detect limited HPV subtypes, the prevalence of HPV infection in breast cancer might have been underestimated. Therefore, the quality of data from the literature might be questionable.
We found that variable HPV subtypes were detected in 22 of 123 Korean breast cancers by real-time PCR. Specific HPV subtypes including HPV-39, HPV-40, HPV-53, and HPV-61 have not been mentioned in the literature. HPV-51 was the most frequently found subtype. It was detected in 14 of 22 HPV-positive breast cancers. HPV-16 and HPV-18 were the most frequently found subtypes in cervical cancer and oropharyngeal cancer. However, they were not common HPV subtypes in breast cancer. They were not detected in the two intraductal papillomas either. If HPV infection is involved in carcinogenesis of breast, specific HPV subtypes hardly related in cervical cancer and oropharyngeal cancer can have a role as carcinogen in breast. Interestingly, this result showed that the prevalence of HPV infection in benign breast lesions (22.2%) was higher than that in breast cancers (17.9%), although meta-analysis supported correlation between breast cancer and HPV. However, because only nine intraductal papillomas were tested, this result might have been compromised.
It is important to note that most cases showed weak positivity for HPV. Fragmentation of extracted DNA in FFPE tissues could be one of the reasons responsible for this result. However, even when the reduction of positivity by fragmentation was considered, positivity for HPV in FFPE breast cancer samples was too weak. Khan et al. [39] have also found HPV DNA in 26 of 124 Japanese breast cancer patients by PCR for FFPE breast cancer samples. Because the viral load in breast cancers was very low compared to viral load examined in uterine cervical carcinoma, they concluded that HPV was not involved in the development of breast cancers in Japanese. Due to the weak positivity of HPV in this study, we also concluded that this result was not a significant evidence to support carcinogenesis of HPV in breast.
If HPV is involved in the development of breast cancer, some differences between HPV-positive and HPV-negative breast cancers should be present. Heng et al. [13] have found putative koilocytes as the proof of HPV infection in HPV-positive breast cancers. However, Khan et al. [39] reviewed all HPV-positive breast cancer and failed to find koilocytes. In this study, we also failed to find koilocytes after slide review of the 123 breast cancers and nine intraductal papillamas. Kan et al. [40] have reported that HPV-positive breast cancers are not correlated with grade, patient survival, hormonal receptor status, HER-2 expression, or p53 overexpression. The characteristics of HPV-positive breast cancer are still controversial.
Since HPV is known as a sexually transmitted virus, HPV may be transmitted to the breast tissue through nipple by sexual behavior. We didn’t detect HPV infection in 13 nipple FFPE tissues and FFPE breast cancers of patients who had HPV infection in uterine cervix (Table 3) and failed prove that nipple was the infection route. However, Glenn et al. [17] have suggested that HPV can be detected in the epithelial cells extracted from human milk. In addition, de Villiers et al. [41] have detected HPV infection in nipple tissues. Some studies have tried to prove the coexistence of HPV infection in both cervical lesion and breast cancer. Hennig et al. [6] have proved that HPV-16 positive breast cancer is corresponding to HPV-16 positive high-grade cervical intraepithelial neoplasia in 19 of 41 cases. On the contrary, Lv et al. [42] failed to find coexistence of HPV in breast or cervical tissues of 12 cases. In our study, there was no case of HPV coexistence in cervical lesion or breast cancer. Furthermore, although most cervical and oropharyngeal cancers are provoked by HPV-16 and HPV-18, and HPV-16 was found in one breast cancer only. Common infected HPV subtypes of breast cancer might be different from those of cervical and oropharyngeal cancers. It is not convincing that the cervical intraepithelial neoplasia is one of the risk factors of breast cancer.
Statistical significance of correlation between breast cancer and HPV was found in meta-analysis using published studies of case-control setting. In addition, we detected 22 HPV-positive breast cancers in 123 Korean patients and two HPV-positive intraductal papillomas in nine Korean patients without finding histological characteristics of HPV infection in breast cancers. Because all HPV-positive breast cancers showed weak positivity, a correlation between HPV and breast cancer was not confirmed in this study. Further study with larger epidemiologic population is necessary to elucidate the role of HPV in mammary carcinogenesis.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Cobos C, Figueroa JA, Mirandola L, et al. The role of human papilloma virus (HPV) infection in non-anogenital cancer and the promise of immunotherapy: a review. Int Rev Immunol. 2014;33:383–401. doi: 10.3109/08830185.2014.911857. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Chang P, Wang L, et al. The role of human papillomavirus infection in breast cancer. Med Oncol. 2012;29:48–55. doi: 10.1007/s12032-010-9812-9. [DOI] [PubMed] [Google Scholar]
- 3.Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology (Williston Park) 2015;29:616–26. [PubMed] [Google Scholar]
- 4.Alibek K, Kakpenova A, Mussabekova A, Sypabekova M, Karatayeva N. Role of viruses in the development of breast cancer. Infect Agent Cancer. 2013;8:32. doi: 10.1186/1750-9378-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Morimoto T, Sasa M, et al. HPV33 DNA in premalignant and malignant breast lesions in Chinese and Japanese populations. Anticancer Res. 1999;19:5057–61. [PubMed] [Google Scholar]
- 6.Hennig EM, Suo Z, Thoresen S, Holm R, Kvinnsland S, Nesland JM. Human papillomavirus 16 in breast cancer of women treated for high grade cervical intraepithelial neoplasia (CIN III) Breast Cancer Res Treat. 1999;53:121–35. doi: 10.1023/a:1006162609420. [DOI] [PubMed] [Google Scholar]
- 7.Damin AP, Karam R, Zettler CG, Caleffi M, Alexandre CO. Evidence for an association of human papillomavirus and breast carcinomas. Breast Cancer Res Treat. 2004;84:131–7. doi: 10.1023/B:BREA.0000018411.89667.0d. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JH, Tsai CH, Cheng MH, Lin SJ, Xu FL, Yang CC. Association of viral factors with non-familial breast cancer in Taiwan by comparison with non-cancerous, fibroadenoma, and thyroid tumor tissues. J Med Virol. 2005;75:276–81. doi: 10.1002/jmv.20267. [DOI] [PubMed] [Google Scholar]
- 9.Choi YL, Cho EY, Kim JH, et al. Detection of human papillomavirus DNA by DNA chip in breast carcinomas of Korean women. Tumour Biol. 2007;28:327–32. doi: 10.1159/000124238. [DOI] [PubMed] [Google Scholar]
- 10.Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer. 2008;99:404–7. doi: 10.1038/sj.bjc.6604503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leon DC, Montiel DP, Nemcova J, et al. Human papillomavirus (HPV) in breast tumors: prevalence in a group of Mexican patients. BMC Cancer. 2009;9:26. doi: 10.1186/1471-2407-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Q, Zhang SQ, Chu YL, Jia XL, Wang XL. The correlations between HPV16 infection and expressions of c-erbB-2 and bcl-2 in breast carcinoma. Mol Biol Rep. 2009;36:807–12. doi: 10.1007/s11033-008-9249-9. [DOI] [PubMed] [Google Scholar]
- 13.Heng B, Glenn WK, Ye Y, et al. Human papilloma virus is associated with breast cancer. Br J Cancer. 2009;101:1345–50. doi: 10.1038/sj.bjc.6605282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendizabal-Ruiz AP, Morales JA, Ramirez-Jirano LJ, Padilla-Rosas M, Moran-Moguel MC, Montoya-Fuentes H. Low frequency of human papillomavirus DNA in breast cancer tissue. Breast Cancer Res Treat. 2009;114:189–94. doi: 10.1007/s10549-008-9989-1. [DOI] [PubMed] [Google Scholar]
- 15.Mou X, Chen L, Liu F, et al. Low prevalence of human papillomavirus (HPV) in Chinese patients with breast cancer. J Int Med Res. 2011;39:1636–44. doi: 10.1177/147323001103900506. [DOI] [PubMed] [Google Scholar]
- 16.Frega A, Lorenzon L, Bononi M, et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. Eur J Gynaecol Oncol. 2012;33:164–7. [PubMed] [Google Scholar]
- 17.Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One. 2012;7:e48788. doi: 10.1371/journal.pone.0048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigaroodi A, Nadji SA, Naghshvar F, Nategh R, Emami H, Velayati AA. Human papillomavirus is associated with breast cancer in the north part of Iran. ScientificWorldJournal. 2012;2012:837191. doi: 10.1100/2012/837191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W, Wang J, Wang C, et al. Detection of high-risk human papillomaviruses in fresh breast cancer samples using the hybrid capture 2 assay. J Med Virol. 2013;85:2087–92. doi: 10.1002/jmv.23703. [DOI] [PubMed] [Google Scholar]
- 20.Pereira Suarez AL, Lorenzetti MA, Gonzalez Lucano R, et al. Presence of human papilloma virus in a series of breast carcinoma from Argentina. PLoS One. 2013;8:e61613. doi: 10.1371/journal.pone.0061613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera-Goepfert R, Vela-Chavez T, Carrillo-Garcia A, et al. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer. 2013;13:445. doi: 10.1186/1471-2407-13-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis IM, Al-Ayadhy B, Al-Awadhi S, Kapila K, Al-Mulla F. Prevalence and correlation of human papilloma virus and its types with prognostic markers in patients with invasive ductal carcinoma of the breast in kuwait. Sultan Qaboos Univ Med J. 2013;13:527–33. doi: 10.12816/0003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali SH, Al-Alwan NA, Al-Alwany SH. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr Health J. 2014;20:372–7. [PubMed] [Google Scholar]
- 24.Ahangar-Oskouee M, Shahmahmoodi S, Jalilvand S, et al. No detection of ‘high-risk’ human papillomaviruses in a group of Iranian women with breast cancer. Asian Pac J Cancer Prev. 2014;15:4061–5. doi: 10.7314/apjcp.2014.15.9.4061. [DOI] [PubMed] [Google Scholar]
- 25.Manzouri L, Salehi R, Shariatpanahi S, Rezaie P. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv Biomed Res. 2014;3:75. doi: 10.4103/2277-9175.125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J, Wang T, Zhu H, et al. Multiplex PCR/mass spectrometry screening of biological carcinogenic agents in human mammary tumors. J Clin Virol. 2014;61:255–9. doi: 10.1016/j.jcv.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Piana AF, Sotgiu G, Muroni MR, Cossu-Rocca P, Castiglia P, De Miglio MR. HPV infection and triple-negative breast cancers: an Italian case-control study. Virol J. 2014;11:190. doi: 10.1186/s12985-014-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Ding J, Zhai K. Detection of human papillomavirus DNA in patients with breast tumor in China. PLoS One. 2015;10:e0136050. doi: 10.1371/journal.pone.0136050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes A, Bianchi G, Feltri AP, Perez M, Correnti M. Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience. 2015;9:548. doi: 10.3332/ecancer.2015.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindel K, Forster A, Altermatt HJ, Greiner R, Gruber G. Breast cancer and human papillomavirus (HPV) infection: no evidence of a viral etiology in a group of Swiss women. Breast. 2007;16:172–7. doi: 10.1016/j.breast.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Hedau S, Kumar U, Hussain S, et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC Cancer. 2011;11:27. doi: 10.1186/1471-2407-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong A, Leung CP, Shin VY, Ng EK. No evidence of human papillomavirus in patients with breast cancer in Hong Kong, Southern China. ISRN Virol. 2013;2013:546503. [Google Scholar]
- 33.Vernet-Tomas M, Mena M, Alemany L, et al. Human papillomavirus and breast cancer: no evidence of association in a Spanish set of cases. Anticancer Res. 2015;35:851–6. [PubMed] [Google Scholar]
- 34.Bratthauer GL, Tavassoli FA, O’Leary TJ. Etiology of breast carcinoma: no apparent role for papillomavirus types 6/11/16/18. Pathol Res Pract. 1992;188:384–6. doi: 10.1016/S0344-0338(11)81229-X. [DOI] [PubMed] [Google Scholar]
- 35.Chang P, Wang T, Yao Q, et al. Absence of human papillomavirus in patients with breast cancer in north-west China. Med Oncol. 2012;29:521–5. doi: 10.1007/s12032-011-9945-5. [DOI] [PubMed] [Google Scholar]
- 36.Fu L, Wang D, Shah W, Wang Y, Zhang G, He J. Association of human papillomavirus type 58 with breast cancer in Shaanxi province of China. J Med Virol. 2015;87:1034–40. doi: 10.1002/jmv.24142. [DOI] [PubMed] [Google Scholar]
- 37.Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med (Berl) 1997;75:429–39. doi: 10.1007/s001090050128. [DOI] [PubMed] [Google Scholar]
- 38.Ohba K, Ichiyama K, Yajima M, et al. In vivo and in vitro studies suggest a possible involvement of HPV infection in the early stage of breast carcinogenesis via APOBEC3B induction. PLoS One. 2014;9:e97787. doi: 10.1371/journal.pone.0097787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan NA, Castillo A, Koriyama C, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J Cancer. 2008;99:408–14. doi: 10.1038/sj.bjc.6604502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kan CY, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer. 2005;93:946–8. doi: 10.1038/sj.bjc.6602778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005;7:R1–11. doi: 10.1186/bcr940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv YR, Wang JL, Zhang K, et al. Human papilloma viruses (HPVs) no co-existence in breast cancer and cervical cells in the same patient. Chin J Physiol. 2014;57:105–6. doi: 10.4077/CJP.2014.BAC207. [DOI] [PubMed] [Google Scholar]