Abstract
Mevalonate kinase deficiency (MKD) is caused by mutations in a key enzyme of the mevalonate–cholesterol biosynthesis pathway, leading to recurrent autoinflammatory disease characterised by enhanced release of interleukin-1β (IL-1β). It is currently believed that the inflammatory phenotype of MKD is triggered by temperature-sensitive loss of mevalonate kinase activity and reduced biosynthesis of isoprenoid lipids required for the prenylation of small GTPase proteins. However, previous studies have not clearly shown any change in protein prenylation in patient cells under normal conditions. With lymphoblast cell lines from two compound heterozygous MKD patients, we used a highly sensitive in vitro prenylation assay, together with quantitative mass spectrometry, to reveal a subtle accumulation of unprenylated Rab GTPases in cells cultured for 3 days or more at 40 °C compared with 37 °C. This included a 200% increase in unprenylated Rab7A, Rab14 and Rab1A. Inhibition of sterol regulatory element-binding protein (SREBP) activation by fatostatin led to more pronounced accumulation of unprenylated Rab proteins in MKD cells but not parent cells, suggesting that cultured MKD cells may partially overcome the loss of isoprenoid lipids by SREBP-mediated upregulation of enzymes required for isoprenoid biosynthesis. Furthermore, while inhibition of Rho/Rac/Rap prenylation promoted the release of IL-1β, specific inhibition of Rab prenylation by NE10790 had no effect in human peripheral blood mononuclear cells or human THP-1 monocytic cells. These studies demonstrate for the first time that mutations in mevalonate kinase can lead to a mild, temperature-induced defect in the prenylation of small GTPases, but that loss of prenylated Rab GTPases is not the cause of enhanced IL-1β release in MKD.
INTRODUCTION
Mevalonate kinase deficiency (MKD) is an autosomal recessive, autoinflammatory disorder caused by bi-allelic mutations in the MVK gene that encodes a critical enzyme in cholesterol and isoprenoid lipid biosynthesis.1, 2 With the severe form of MKD (mevalonic aciduria; OMIM 251170), patients typically have undetectable levels of mevalonate kinase (MVK) activity and display a variety of neurological and developmental abnormalities.3 The less severe form (hyperimmunoglobulinemia D syndrome or HIDS, OMIM 260920) is characterised by 1–7% normal MVK activity1 and the occurrence of frequent febrile, inflammatory episodes.4 Most patients with MKD are compound heterozygous, commonly with V377I or H20N/P point mutations.4, 5
The inflammatory phenotype of MKD appears to be caused principally by loss of synthesis of isoprenoid lipids downstream of MVK,6 particularly geranylgeranyldiphosphate.7, 8, 9 Geranylgeranyldiphosphate is necessary for the post-translational prenylation of proteins of the Rho/Rac/Rap and Rab families of small GTPases. In vitro models of MKD involve treatment of cells with statins (HMG-CoA reductase inhibitor), nitrogen-containing bisphosphonates (inhibitors of farnesyl diphosphate synthase10) or specific inhibitors of geranylgeranyltransferase I such as GGTI-29811 to mimic the block in protein prenylation that is assumed to occur in cells deficient in MVK. These inhibitors predispose cells to increased inflammasome activity and enhanced caspase-1-mediated cleavage of pro-interleukin (IL)-1β,9, 12, 13, 14, 15, 16, 17, 18 a characteristic feature of MKD.
It has been proposed that mutations in MVK may lead to temperature-sensitive changes in folding or stability of the enzyme.5, 19 Hence, elevations in body temperature caused by exercise, stress or other triggers could lead to reduced MVK activity and decreased flux through the mevalonate pathway. Inhibition of Rho or Rac prenylation could lead to increased inflammasome activity via defects in autophagy.20, 21 However, numerous steps in autophagy are also regulated by Rab GTPases,22 which are prenylated by geranylgeranyltransferase II (GGTase II or Rab GGTase). Despite proposed mechanistic models of enhanced IL-1β release involving decreased Rho/Rac prenylation,21, 22 previous studies have failed to demonstrate any intrinsic defect in protein prenylation in cells derived from MKD patients in the absence of statin treatment.23 Furthermore, whether loss of Rab prenylation contributes to the inflammatory phenotype of MKD has not been examined. Here, we took a novel approach, utilising a highly sensitive in vitro prenylation assay,24 to demonstrate for the first time that the prenylation of Rab proteins is altered in cell lines derived from MKD patients following mild heat stress. However, using NE10790 (a specific inhibitor of geranylgeranyltransferase II25, 26, 27) we found that the loss of Rab prenylation is not sufficient to enhance the release of IL-1β following lipopolysaccharide (LPS) stimulation of human monocytes.
Results and Discussion
In this study we specifically sought to answer the long-standing question whether protein prenylation is compromised in MKD cells. We identified two children with MKD. A 3-year-old daughter of unrelated parents (MKD1) was found to be compound heterozygous for missense MVK mutation V377I (c.1129G>A) and c.1058_1060del deletion in exon 11 (a mutation that, to our knowledge, has not previously been reported). She presented with recurrent fever, spastic cerebral palsy of unknown cause, failure to thrive, and elevated IgD and IgA levels (500 mg l−1, 2.74 g l−1, respectively). An 8-year-old boy (MKD2), born to non-consanguineous parents, presented by 12 months of age with 3–4 weekly episodes of periodic fevers lasting 3–4 days in association with abdominal pain, lethargy and arthralgia. His IgA and IgD levels were elevated (9.65 and 1.68 g l−1, respectively). He was confirmed to have compound heterozygous missense mutations in MVK; V377I and H20N (c.58C>A). His parents were confirmed heterozygous carriers.
Epstein-Barr Virus-transformed lymphoblastoid cell lines (EBV-LCLs) were generated using peripheral blood samples from the two MKD patients (MKD1 and MKD2) and from one heterozygous (V377I) parent of each child (Prnt1 and Prnt2, respectively).
MKD patient cells show temperature-dependent accumulation of unprenylated Rab proteins
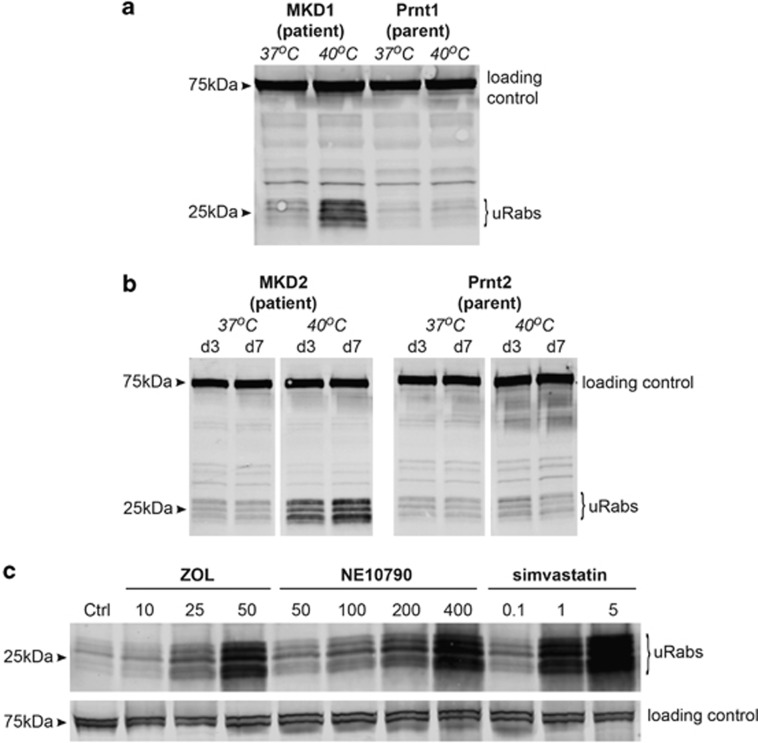
Previous studies have shown that fibroblasts from MKD patients, including those with V377I/H20P mutations, have an ~50% decrease in MVK activity after culturing for 24 h at 39 °C.19 We used a highly sensitive in vitro prenylation assay with GGTase II24 to determine whether mutations in MVK cause a temperature-dependent accumulation of unprenylated Rab GTPases in EBV-LCLs derived from MKD patients. Lysates from MKD1 and MKD2 cells (patients), and Prnt1 and Prnt2 cells (parents) grown under normal culture conditions (37 °C) showed very low levels of unprenylated Rab proteins (Figures 1a and b). However, culturing MKD1 patient cells at 40 °C caused an increase in the presence of unprenylated Rab proteins (a cluster of bands between 21–27 kDa) after 7 days (Figure 1a). Similarly, EBV-LCLs from MKD2 showed an increase in unprenylated Rab proteins when cultured at 40 °C for 3 days and this increased further after 7 days (Figure 1b).
Figure 1.
Increased temperature causes decreased Rab prenylation in MKD cell lines. (a) MKD1 (patient) cells, but not Prnt1 (parent) cells, have an accumulation of 21–27 kDa unprenylated Rab GTPases (uRabs) when cultured at 40 °C compared with 37 °C for 7 days. (b) MKD2 (patient) cells, but not Prnt2 (parent) cells, have an accumulation of 21–27 kDa unprenylated Rab GTPases (uRabs) when cultured at 40 °C compared with 37 °C for 3 days (d3) or 7 days (d7). (c) Culture of a control lymphoblast cell line at 37 °C for 48 h with 10–50 μM zoledronic acid (ZOL), 50–400 μM NE10790 or 0.1–5 μM simvastatin causes a concentration-dependent increase in unprenylated Rab proteins. The doublet (or broad singlet) of 75 kDa endogenous biotinylated protein was used as a loading control in a–c. The data shown are representative of three independent experiments.
Culturing EBV-LCLs from patients or parents at 40 °C did not affect cell viability (>90% in all cell lines based on trypan blue exclusion) or cell proliferation (based on 5-(and 6)-carboxyfluorescein succinimidyl ester (CFSE) dilution measured by flow cytometry; Supplementary Figure S1). Furthermore, as protein prenylation is a post-translational modification that occurs immediately after synthesis of newly transcribed protein, the accumulation of unprenylated Rab proteins observed in patient EBV-LCLs at 40 °C was not due to a general decrease in cell proliferation, protein synthesis or cell viability. Accumulation of unprenylated Rabs in patient EBV-LCLs was not observed at earlier time points and we could not detect any increase in unprenylated Rap1A (prenylated by GGTase I) by western blotting (data not shown), consistent with the far greater sensitivity of the in vitro Rab prenylation assay.24 To our knowledge, this is the first demonstration that protein prenylation is indeed reduced in cells derived from MKD patients under physiologically relevant conditions that mimic a febrile state.
The accumulation of unprenylated Rab proteins in MKD1 and MKD2 EBV-LCLs cultured for 7 days at 40 °C was comparable to the effect of treating normal EBV-LCLs with 25 μM zoledronic acid, 100 μM NE10790 or 0.1–1 μM simvastatin for 48 h at 37 °C (Figure 1c). This concentration of statin is considerably less than the amount frequently used to inhibit the mevalonate pathway in cell culture models of MKD (10–20 μM), raising the important question whether the effects of such high concentrations of statin in cell culture models are truly representative of changes in prenylation in MKD cells.
Preventing SREBP activation enhances the accumulation of unprenylated Rab proteins in MKD cells
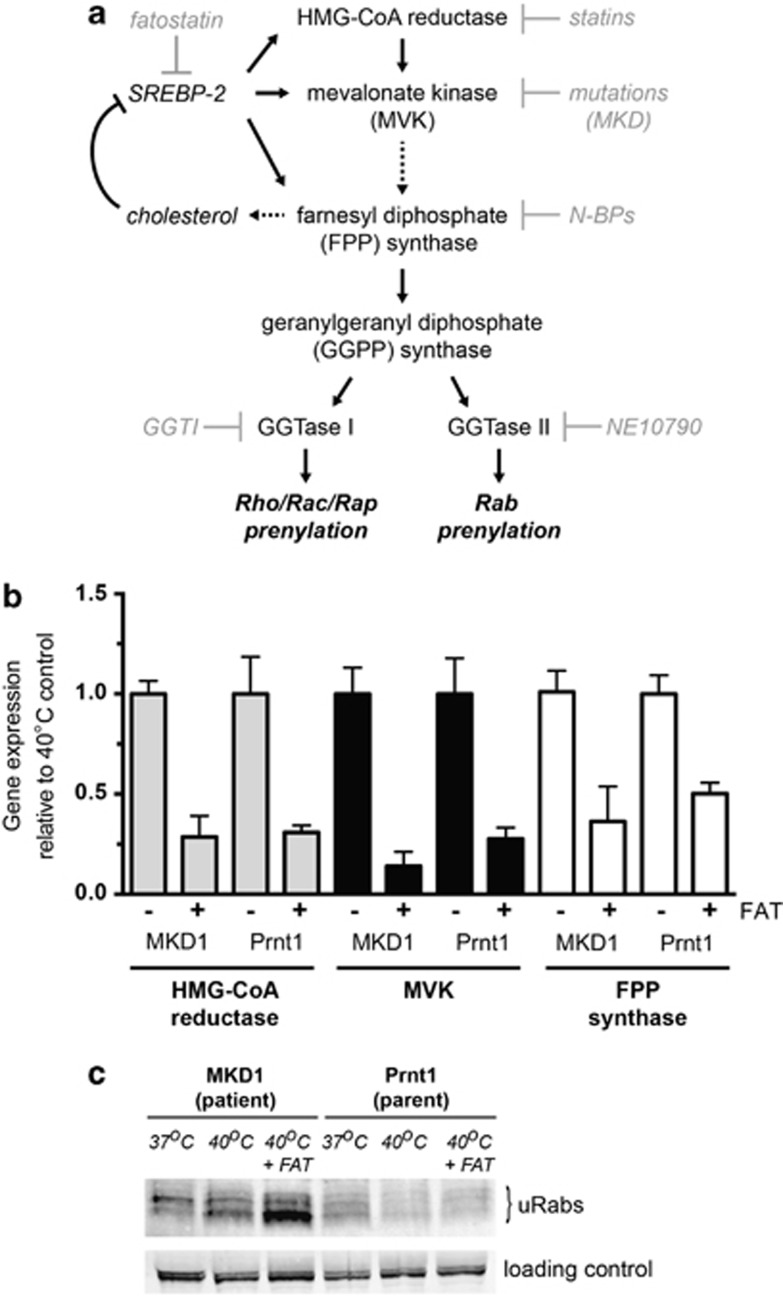
Previous studies have suggested that MKD cells are capable of SREBP transcription factor-mediated upregulation of HMG-CoA reductase and MVK19 and hence normal compensatory changes in the mevalonate pathway in response to lack of cholesterol.5, 6, 28 This suggests that any lack of protein prenylation in MKD cells would be at least partly overcome by upregulation of enzyme expression (Figure 2a). To examine whether lack of protein prenylation was more pronounced when SREBP-mediated transcriptional upregulation was inhibited, we cultured MKD1 and Prnt1 cells at 40 °C for 3 days in the presence and absence of 10 μM fatostatin, an inhibitor of SREBP cleavage and activation.29 Consistent with an earlier study,29 and in accordance with diminished SREBP-2 transcriptional activity, the expression of the SREBP-2-responsive genes HMG-CoA reductase, MVK and FPP synthase was substantially reduced in the presence of fatostatin in MKD1 and Prnt1 cells (Figure 2b). Although viability remained >96% in both cell lines throughout the experiment, 10 μM fatostatin treatment enhanced the accumulation of unprenylated Rab proteins only in MKD1 but not Prnt1 cells (Figure 2c). Most likely, after fatostatin treatment, the remaining wild-type MVK would be sufficient to maintain adequate flux though the mevalonate pathway and thus maintain normal protein prenylation in Prntl1 cells. However, in MKD1 cells treated with fatostatin, the decreased enzymatic activity of the mutated MVK at 40 °C, together with the inability to replace the defective enzyme pool, results in an even greater reduction in isoprenoid lipids and a more severe loss of protein prenylation.
Figure 2.
Fatostatin (FAT) decreases SREBP-dependent gene expression and enhances the inhibition of protein prenylation at 40 °C in MKD cells. (a) Schematic diagram of the flux through the mevalonate pathway leading to sterol biosynthesis and protein prenylation. Mutations resulting in reduced MVK activity, as well as pharmacological inhibitors such as statins and nitrogen-containing bisphosphonates (N-BPs), block the production of isoprenoid precursors essential for protein prenylation. The specific inhibitors GGTI-298 and NE10790, respectively, inhibit prenylation of Rho/Rac/Rap GTPases and Rab GTPases. FAT interferes with the upregulation of genes encoding mevalonate pathway enzymes by blocking SREBP-2 activation. (b) TaqMan gene expression analysis of three SREBP-regulated genes in patient (MKD1) and parent (Prnt1) EBV-LCLs shows substantial reduction upon FAT treatment. Cells were cultured with dimethyl sulfoxide vehicle (−) or 10 μM FAT (+) at 40 °C for 2 days before harvesting for RNA isolation and real time PCR. dd-CT values are the mean of three separate experiments and expressed relative to the vehicle control (± s.e.m.). (c) FAT increased the accumulation of unprenylated Rab proteins (uRabs) that occurs in MKD1 (patient) cells after culture for 3 days at 40 °C, but has no effect in Prnt1 (parent) cells. Endogenous 75 kDa biotinylated protein was used as a loading control. The data shown are representative of three independent experiments.
These data suggest that EBV-LCLs derived from MKD patients (at least those with partial loss-of-function mutations) can somewhat overcome the effect of MVK mutations on protein prenylation via mechanisms that include SREBP-mediated upregulation of enzymes of the mevalonate pathway.
Quantitative SILAC analysis reveals mild accumulation of unprenylated Rab7, Rab14 and Rab1 in MKD cells at 40 °C
To identify and quantify the unprenylated Rab proteins that accumulated in MKD cells at 40 °C versus 37 °C, we enriched the in vitro-prenylated (biotinylated) Rab proteins from lysates prepared from stable isotope labelling with amino acids in cell culture (SILAC)-labelled MKD2 EBV-LCLs cultured either at 37 or 40 °C for 7 days. Enriched, biotinylated Rab proteins were then identified by mass spectrometry24 and the relative abundance of each was calculated as the fold change between cells cultured at 37 or 40 °C. Ten in vitro-prenylated Rab proteins were identified with a false discovery rate <1% (Table 1). After culture at 40 °C compared with 37 °C, there was an ~200% increase in unprenylated Rab7A, Rab14 and Rab1A, an ~100% increase in unprenylated Rab11A/B, Rab5B and Rab21, and an ~40% increase in Rab6A/B/39 A, Rab1B/C and Rab2 A/B. Although the fold increase in the unprenylated form of these Rab proteins appears fairly small, the cumulative effect of a small increase in each of these unprenylated proteins, and probably other, less abundant Rab GTPases that could not be identified by mass spectrometry, is still evident in the corresponding protein blots of MKD2 cells in Figure 1b.
Table 1. Quantitative SILAC analysis reveals increased accumulation of unprenylated Rab proteins in MKD2 cells cultured at 40 °C compared with 37 °C.
| Name | Relative abundance (%) | Ratio 40 °C/37 °C (H/L) | Ratio 40 °C/37 °C (L/H) |
|---|---|---|---|
| Rab11A/11B | 8.7 | 2.1 | 1.8 |
| Rab6A/6B/39A | 23.4 | 1.6 | 1.4 |
| Rab1A | 0.8 | ND | 2.9 |
| Rab1B/1C | 12.6 | 1.6 | 1.5 |
| Rab5C | 4.6 | 1.4 | 0.8 |
| Rab7A | 10.0 | 3.0 | 2.7 |
| Rab2A/2B | 5.6 | 1.4 | 1.4 |
| Rab5B | 19.8 | 1.7 | 1.7 |
| Rab14 | 1.0 | 2.7 | ND |
| Rab21 | 13.5 | 2.4 | 1.3 |
Abbreviations: LC/MS, liquid chromatography–mass spectrometry; MKD2, mevalonate kinase deficiency; SILAC, stable isotope labelling with amino acids in cell culture.
Cells labelled in ‘heavy' (H) or ‘light' (L) medium were cultured for 7 days at 40 °C or 37 °C, respectively. As an inverse labelling control, cells labelled in ‘heavy' (H) or ‘light' (L) medium were cultured for 7 days at 37 °C or 40 °C, respectively. Equal amounts of protein from cell lysates were mixed prior to in vitro prenylation, then biotinylated proteins were enriched with streptavidin beads and analysed by LC/MS. The amount of individual Rab proteins was expressed as a ratio of levels in cells at 40 or 37 °C (either H/L or L/H). For the 10 Rab proteins identified, the average relative abundance was calculated from the analysis of whole-cell lysates of H- or L-labelled cells cultured at 37 °C. Rab1A and Rab14 could not be detected (ND) in one of the pair of H- or L-labelled samples.
The increases in unprenylated Rab proteins in MKD2 cells that we identified by SILAC are considerably lower than those observed after treatment of macrophages with very low (nanomolar) concentrations of nitrogen-containing bisphosphonates.24 This again raises the question whether treatment of cells in culture with high (micromolar) concentrations of nitrogen-containing bisphosphonatess or statins truly reflects the more subtle changes in prenylation that occur in cultured MKD cells.
Inhibition of Rab prenylation does not enhance IL-1β release by THP-1 monocytes or human PBMC
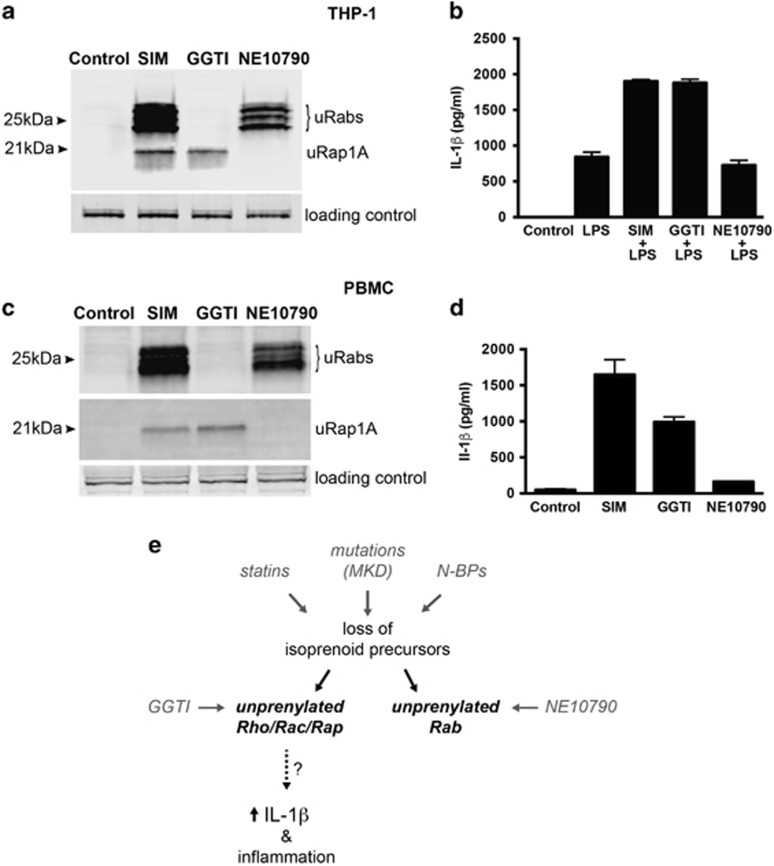
Human EBV-LCLs do not release IL-1β. Therefore, to determine whether lack of prenylation of Rab GTPases or Rho/Rac/Rap-family GTPases leads to enhanced IL-1β release, human THP-1 monocytic cells were treated with simvastatin (prevents prenylation of all small GTPases), GGTI-298 (prevents prenylation of Rho/Rac/Rap-family GTPases) or NE10790 (prevents prenylation of Rab GTPases) (Figure 2a). THP-1 cells were pretreated for 24 h with concentrations of each inhibitor that had similar effects on Rab or Rap1A prenylation: 5 μM simvastatin, 10 μM GGTI-298 or 2 mM NE10790 (Figure 3a), before stimulation with 200 ng ml−1 LPS for a further 6 h. These concentrations of inhibitors did not affect cell viability (which was consistently about 80% based on trypan blue exclusion) after 24 h treatment. Although exposure to simvastatin or GGTI-298 significantly enhanced the release of IL-1β caused by LPS stimulation, NE10790 had no effect (Figure 3b). IL-18 release (which, like IL-1β, is inflammasome-mediated) also increased with LPS stimulation, and, similar to IL-1β, LPS-mediated production of IL-18 was doubled by pretreating cells with simvastatin or GGTI-298, but not NE10790 (Supplementary Figure S2). Tumour necrosis factorα (TNFα) was not detected in the absence of LPS. Treatment with LPS alone for 6 h caused considerable production of TNFα but neither simvastatin, GGTI-2978 nor NE10790 showed any additional stimulatory effect on TNFα secretion (Supplementary Figure S2). This is consistent with a recent study demonstrating increased IL-1β, but not TNFα, production from MKD versus healthy control peripheral blood mononuclear cells (PBMC) following TLR4 ligation by LPS.30 In cultures of PBMC from healthy controls, we found that treatment with 5 μM simvastatin or 10 μM GGTI-298 alone for 24 h caused the release of IL-1β even in the absence of LPS. However, similar to THP-1 cells, NE1079 had no effect despite comparable inhibition of Rab prenylation with simvastatin (Figures 3c and d).
Figure 3.
Inhibition of Rab prenylation by NE10790 does not enhance IL-1β release from THP-1 monocytes. Unprenylated Rab GTPases (uRabs) or unprenylated Rap1A (uRap1A) were detected in THP-1 lysates (a) or human PBMC lysates (c) after treatment for 24 h with 5 μM simvastatin (SIM), 10 μM GGTI-298 or 2 mM NE10790. Endogenous 75 kDa biotinylated protein was used as a loading control. The data shown are representative of three independent experiments. (b) Pretreatment of THP-1 cells for 24 h with 5 μM SIM or 10 μM GGTI-298 enhanced the release of mature IL-1β after stimulation with 200 ng ml−1 LPS for 6 h, whereas pretreatment with 2 mM NE10790 had no effect. (d) Treatment of human PBMC for 24 h with 5 μM SIM or 10 μM GGTI-298 caused the release of mature IL-1β, whereas 2 mM NE10790 had no effect. ELISA values in b, d are the mean ± s.d. of triplicate samples and are representative of at least two independent experiments. (e) Disruption of the mevalonate pathway, by pharmacological inhibition or mutations in MVK, results in reduced synthesis of isoprenoid lipid precursors essential for protein prenylation. However, it is the loss of prenylation of GTPases modified by GGTase I (e.g., Rho, Rac and Rap), and not the loss of prenylated Rab GTPases (prenylated by GGTase II), that causes excessive IL-1-β production.
In conclusion, our studies demonstrate for the first time that raised temperature causes a mild inhibition of protein prenylation and accumulation of unprenylated Rab GTPases in EBV-LCLs from MKD patients. However, loss of Rab prenylation alone does not lead to enhanced IL-1β release from monocytes following LPS treatment. In contrast, inhibition of prenylation of Rho/Rac/Rap GTPases is sufficient to predispose monocytes to excessive IL-1β release (Figure 3e); the exact mechanisms involved remain to be definitively proven and validated in MKD cells. Importantly, decreased MVK activity in cultured EBV-LCLs from MKD patients appears to be partially counteracted by increased activity of endogenous SREBP, therefore cultured MKD cell lines may not be a good model to study the functional consequences of human MVK mutations.
Methods
Cells and reagents
With Human Research Ethics Committee (HREC) approval, informed consent was obtained prior to obtaining fresh blood samples from the patients and parents. Isolated PBMC were transformed with EBV using standard approaches. EBV-LCLs, THP-1 cells (mycoplasma-free, obtained from ATCC, Manassas, VA, USA) or human PBMC isolated from buffy coat preparations of healthy blood donors were cultured at 37 or 40 °C in RPMI with 10% foetal calf serum. Fatostatin (Tocris, Avonmouth, Bristol, UK), zoledronic acid (a gift from Dr Jonathan Green) and NE10790 (a gift from Dr Hal Ebetino) were dissolved as concentrated stocks in phosphate-buffered saline. GGTI-298 (Sigma, St Louis, MO, USA) was dissolved in dimethyl sulfoxide. Reagents for the prenylation assay were kindly provided by Professor Kirill Alexandrov and Dr Zakir Tnimov (University of Queensland).
Quantitative PCR analysis
MKD1 and Prnt1 cells (5 × 105 cells per ml) were cultured at 40 °C and treated for 2 days with 10 μM fatostatin or dimethyl sulfoxide vehicle before collection for RNA extraction (ISOLATE II RNA Mini Kit, Bioline) and cDNA synthesis (Tetro cDNA Synthesis Kit, Bioline, Alexandria, NSW, Australia) according to the manufacturer's instructions. RT-PCR was performed on a ABI Quantstudio7 (Scoresby, VIC, Australia) with specific Taqman primers for HMG-CoA reductase, MVK, FPP and beta-2-microglobulin (B2M) (Thermo Fisher Scientific Scoresby, VIC, Australia, Hs00168352_m1, Hs00176077_m1, Hs01560316_g1 and Hs99999907 respectively). Comparative values of expression (dd-CT) were calculated using beta-2-microglobulin as a housekeeping gene, and presented as relative to the vehicle control.
In vitro prenylation assay
Cells were washed twice with phosphate-buffered saline and lysed by sonication in prenylation buffer (50 mM HEPES, pH 7.2, 50 mM NaCl, 2 mM MgCl2, 100 μM GDP, 1 × Roche complete EDTA-free protease inhibitor cocktail, Basel, Switzerland). Protein was quantified using a BCA assay (Pierce, Scoresby, VIC, Australia). Dithiothreitol (DTT) was added to 50 μg cell lysate to a final concentration of 2 mM, with final concentrations of 2 μM Rab GGTase, 2 μM recombinant Danio rerio Rab escort protein-1, 0.5 μM Biotin-geranyl diphosphate (B-GPP) in a total volume of ~50 μl and the reactions incubated for 5 h at room temperature. In vitro-prenylated (that is, biotinylated) Rab proteins were detected on polyvinylidene difluoride blots using streptavidin-680RD (LiCOR, Lincoln, NE, USA).24 We also consistently detected a narrow doublet (often appearing as a broad singlet) of endogenous biotinylated 75 kDa proteins, which were used as a sample loading control. Blots were also analysed for unprenylated Rap1A using goat anti-Rap1A (sc-1482).24
SILAC and mass spectrometry
For quantitative Mass Spectrometry analysis, MKD2 cells were labelled using SILAC for at least 10 cell doublings, as previously described.24 Cells (106) were seeded into T25 flasks with 8 ml of SILAC medium (Sigma) in duplicate. ‘Light'-labelled cells were cultured at 37 °C for 7 days, whereas ‘heavy'-labelled cells were cultured at 40 °C for 7 days. The culture medium was supplemented once with 4 ml of fresh medium. Labelling conditions were reversed in a replicate experiment. Cells were washed three times with phosphate-buffered saline and lysed by sonication in prenylation buffer. Protein lysate (840 μg) was mixed in equal proportions from ‘light'- and ‘heavy'-labelled cells and the in vitro prenylation assay performed as described above. In vitro-prenylated proteins were isolated using streptavidin beads, then the bound proteins were analysed on an Orbitrap Velos Pro mass spectrometer (Thermo Scientific, Scoresby, VIC, Australia).24 For individual protein quantitation, normalised ratios were calculated from the MaxQuant data analysis software (Max Planck Institute of Biochemistry, Martinsried, Germany) for cells cultured at 37 and 40 °C.
IL-1β, IL-18 and TNF-α ELISA
THP-1 cells or human PBMC from healthy donors (2 × 105 cells in 200 μl in a 96-well plate) were treated with 5 μM simvastatin, 10 μM GGTI-298 or 2 mM NE10790 for 24 h. THP-1 cells were additionally stimulated with 200 ng ml−1 LPS for a further 6 h. Conditioned media were collected and analysed by ELISA for human TNF-α (Elisakit.com), IL-1β and IL-18 (Instant ELISA, eBioscience, San Diego, CA, USA). The cell pellets were harvested and analysed for unprenylated Rab proteins and Rap1A (as described above) to assess the effect of these drug concentrations on protein prenylation.
Acknowledgments
We gratefully acknowledge the patients and families involved in this study. We thank Emily Edwards and Danielle Priestley (Garvan Institute) for technical assistance, Kirill Alexandrov and Zakir Tnimov (University of Queensland) for providing reagents for the prenylation assay. We thank Dr Tri Phan for stimulating discussions and for comments on the manuscript. This work was supported in part by NHMRC project grant APP1079522 to MJR and by Mrs Janice Gibson and the Ernest Heine Family Foundation. The contents of this manuscript are solely the responsibility of the authors and do not reflect the views of the NHMRC.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 1999; 22: 175–177. [DOI] [PubMed] [Google Scholar]
- Drenth JP, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JG et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet 1999; 22: 178–181. [DOI] [PubMed] [Google Scholar]
- Simon A, Kremer HP, Wevers RA, Scheffer H, De Jong JG, Van Der Meer JW et al. Mevalonate kinase deficiency: evidence for a phenotypic continuum. Neurology 2004; 62: 994–997. [DOI] [PubMed] [Google Scholar]
- van der Burgh R, Ter Haar NM, Boes ML, Frenkel J. Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Clin Immunol 2012; 147: 197–206. [DOI] [PubMed] [Google Scholar]
- Mandey SH, Schneiders MS, Koster J, Waterham HR. Mutational spectrum and genotype-phenotype correlations in mevalonate kinase deficiency. Hum Mutat 2006; 27: 796–802. [DOI] [PubMed] [Google Scholar]
- Hoffmann GF, Wiesmann UN, Brendel S, Keller RK, Gibson KM. Regulatory adaptation of isoprenoid biosynthesis and the LDL receptor pathway in fibroblasts from patients with mevalonate kinase deficiency. Pediatr Res 1997; 41: 541–546. [DOI] [PubMed] [Google Scholar]
- Frenkel J, Rijkers GT, Mandey SH, Buurman SW, Houten SM, Wanders RJ et al. Lack of isoprenoid products raises ex vivo interleukin-1beta secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum 2002; 46: 2794–2803. [DOI] [PubMed] [Google Scholar]
- Schneiders MS, Houten SM, Turkenburg M, Wanders RJ, Waterham HR. Manipulation of isoprenoid biosynthesis as a possible therapeutic option in mevalonate kinase deficiency. Arthritis Rheum 2006; 54: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Marcuzzi A, Pontillo A, De Leo L, Tommasini A, Decorti G, Not T et al. Natural isoprenoids are able to reduce inflammation in a mouse model of mevalonate kinase deficiency. Pediatr Res 2008; 64: 177–182. [DOI] [PubMed] [Google Scholar]
- Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 2001; 296: 235–242. [PubMed] [Google Scholar]
- Coxon FP, Helfrich MH, van 't Hof RJ, Sebti SM, Ralston SH, Hamilton AD et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 2000; 15: 1467–1476. [DOI] [PubMed] [Google Scholar]
- Montero MT, Hernandez O, Suarez Y, Matilla J, Ferruelo AJ, Martinez-Botas J et al. Hydroxymethylglutaryl-coenzyme A reductase inhibition stimulates caspase-1 activity and Th1-cytokine release in peripheral blood mononuclear cells. Atherosclerosis 2000; 153: 303–313. [DOI] [PubMed] [Google Scholar]
- Kuijk LM, Beekman JM, Koster J, Waterham HR, Frenkel J, Coffer PJ. HMG-CoA reductase inhibition induces IL-1beta release through Rac1/PI3K/PKB-dependent caspase-1 activation. Blood 2008; 112: 3563–3573. [DOI] [PubMed] [Google Scholar]
- Kuijk LM, Mandey SH, Schellens I, Waterham HR, Rijkers GT, Coffer PJ et al. Statin synergizes with LPS to induce IL-1beta release by THP-1 cells through activation of caspase-1. Mol Immunol 2008; 45: 2158–2165. [DOI] [PubMed] [Google Scholar]
- Massonnet B, Normand S, Moschitz R, Delwail A, Favot L, Garcia M et al. Pharmacological inhibitors of the mevalonate pathway activate pro-IL-1 processing and IL-1 release by human monocytes. Eur Cytokine Netw 2009; 20: 112–120. [DOI] [PubMed] [Google Scholar]
- Norton JT, Hayashi T, Crain B, Cho JS, Miller LS, Corr M et al. Cutting edge: nitrogen bisphosphonate-induced inflammation is dependent upon mast cells and IL-1. J Immunol 2012; 188: 2977–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JT, Hayashi T, Crain B, Corr M, Carson DA. Role of IL-1 receptor-associated kinase-M (IRAK-M) in priming of immune and inflammatory responses by nitrogen bisphosphonates. Proc Natl Acad Sci 2011; 108: 11163–11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandey SH, Kuijk LM, Frenkel J, Waterham HR. A role for geranylgeranylation in interleukin-1beta secretion. Arthritis Rheum 2006; 54: 3690–3695. [DOI] [PubMed] [Google Scholar]
- Houten SM, Frenkel J, Rijkers GT, Wanders RJ, Kuis W, Waterham HR. Temperature dependence of mutant mevalonate kinase activity as a pathogenic factor in hyper-IgD and periodic fever syndrome. Hum Mol Genet 2002; 11: 3115–3124. [DOI] [PubMed] [Google Scholar]
- van der Burgh R, Nijhuis L, Pervolaraki K, Compeer EB, Jongeneel LH, van Gijn M et al. Defects in mitochondrial clearance predispose human monocytes to interleukin-1beta hypersecretion. J Biol Chem 2014; 289: 5000–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burgh R, Pervolaraki K, Turkenburg M, Waterham HR, Frenkel J, Boes M. Unprenylated RhoA contributes to IL-1beta hypersecretion in mevalonate kinase deficiency model through stimulation of Rac1 activity. J Biol Chem 2014; 289: 27757–27765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Diff 2014; 21: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Schneiders MS, Wanders RJ, Waterham HR. Regulation of isoprenoid/cholesterol biosynthesis in cells from mevalonate kinase-deficient patients. J Biol Chem 2003; 278: 5736–5743. [DOI] [PubMed] [Google Scholar]
- Ali N, Jurczyluk J, Shay G, Tnimov Z, Alexandrov K, Munoz MA et al. A highly sensitive prenylation assay reveals in vivo effects of bisphosphonate drug on the Rab prenylome of macrophages outside the skeleton. Small GTPases 2015; 6: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon FP, Helfrich MH, Larijani B, Muzylak M, Dunford JE, Marshall D et al. Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J Biol Chem 2001; 276: 48213–48222. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Ebetino FH, Mules EH, Seabra MC, McKenna CE, Rogers MJ. Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone 2005; 37: 349–358. [DOI] [PubMed] [Google Scholar]
- Baron RA, Tavare R, Figueiredo AC, Blazewska KM, Kashemirov BA, McKenna CE et al. Phosphonocarboxylates inhibit the second geranylgeranyl addition by Rab geranylgeranyl transferase. J Biol Chem 2009; 284: 6861–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Hoffmann G, Schwall A, Broock RL, Aramaki S, Sweetman L et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J Lipid Res 1990; 31: 515–521. [PubMed] [Google Scholar]
- Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol 2009; 16: 882–892. [DOI] [PubMed] [Google Scholar]
- Stoffels M, Jongekrijg J, Remijn T, Kok N, van der Meer JW, Simon A. TLR2/TLR4-dependent exaggerated cytokine production in hyperimmunoglobulinaemia D and periodic fever syndrome. Rheumatology 2015; 54: 363–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.