Abstract
Objective
To evaluate surgical outcome, complications, and patients satisfaction with the Tube® (Promedon, Cordoba, Argentina) malleable penile prosthesis in diabetic and non-diabetic patients with refractory erectile dysfunction (ED).
Patients and methods
The records of 128 eligible patients who received Tube malleable penile prostheses at our institute between September 2008 and October 2015 were reviewed.
Results
Of the 128 patients, who received Tube penile prostheses at our institute, 53 were diabetics and 75 were non-diabetics. Both groups of patients were comparable for mean age, education level, marital status, hospital stay, time to commencing sexual intercourse, and median follow-up. Complications included: inter-corporeal septal perforation (2.3%), glanular urethral injury (1.5%), acute urinary retention (3.9%), superficial wound infection (7%), penile discomfort (9.4%), and penile prostheses infection (5.5%). Moreover, 3.9% developed atrophy of the cavernosal tissue, 5.5% experienced bad cosmesis, 6.3% experienced ejaculatory disorders, and 2.3% developed bladder calculi. In all, 13 prostheses (9.4%) were removed, seven of them due to infection, three on the patients’ demand and three due to mechanical failure. The satisfaction rates with the prostheses were 77.3% and 79.4% in the diabetic and non-diabetic patients, respectively; with an overall satisfaction rate of 78.5%. There was no significant difference in the complication rate or prostheses infection between diabetic and non-diabetic patients.
Conclusion
Tube malleable penile prostheses are associated with low complication and high satisfaction rates. There was no significant difference in the complication rate or prostheses infection between diabetic and non-diabetic patients. A prospective comparative study with a large number of patients is recommended.
Abbreviations: cGMP, cyclic guanosine monophosphate; ED, erectile dysfunction; DM, diabetes mellitus; NO, nitric oxide; PDE5I, phosphodiesterase type 5 inhibitor; PGE1, prostaglandin E1; US, ultrasonography
Keywords: Erectile dysfunction, Malleable prostheses, Diabetic patients, Outcome
Introduction
The prevalence of erectile dysfunction (ED) increases significantly with age; with ∼50% of men aged 70 years having degree of ED [1]. The advent of phosphodiesterase type 5 inhibitors (PDE5Is) has revolutionised the treatment of ED and currently they are considered the first-line of treatment for ED [2]. Although, penile prostheses are the least selected and most invasive treatment option for ED, they offer a satisfactory and reliable method of treatment for restoring penile rigidity [3]. In 2000, the number of patients with penile implants in the USA was 17,540 increasing to 22,420 in 2009 [4]. The most common indication for penile implants is diabetes mellitus (DM), and ∼20% of patients with penile implants are diabetics [5]. The incidence of penile prosthesis infection ranges from 0.7% to 17.7%, and diabetic patients are more prone to infection than non-diabetics [6].
The most frequently used type penile prostheses (inflatable or malleable) is the inflatable type in the USA and other developed countries. However, inflatable types are costly, complex, and more liable to mechanical failure. Although, their reliability has improved in the last decade, their complications are still present [7]. The ease of use, simplicity of implantation, the rarity of mechanical failure, and cost-effectiveness make malleable penile prostheses more convenient for our patients [8]. The present study aimed to evaluate the surgical outcome, complications, and patients, satisfaction with Tube® malleable penile prosthesis (Promedon, Cordoba, Argentina) in diabetic and non-diabetic patients with refractory ED.
Patients and methods
The records of patients who received a Tube malleable penile prosthesis (Promedon) implantation at our institute between September 2008 and October 2015 were retrospectively reviewed. The strategy of our institute for penile prosthesis implantation is to offer this treatment to patients who have refractory ED unresponsive to a maximal dose of oral PDE5Is or intracavernosal injections of prostaglandin E1 (PGE1; alprostadil), with a normal examination and biochemical profile including a glycated haemoglobin A1cof <7% for diabetic patients. Therefore, all patients included in this study had refractory ED and controlled DM; patients with psychological instability or bad general health were excluded. All patients and their partners were fully informed that the prosthesis would solve the erection problem and have no effect on desire, orgasm, ejaculation or fertility. The data of 128 eligible patients, operated upon by the same experienced surgical team under a strict infection control protocol, were identified. Of these 128 patients, 53 were diabetics and 75 were non-diabetics. The patients’ preoperative characteristics are summarised in Table 1.
Table 1.
Preoperative demographics of 128 patients received 131 tube malleable penile prostheses.
| Variable | Non-diabetics | Diabetics | Total | P |
|---|---|---|---|---|
| Number of patients, n (%) | 75 (58.6) | 53 (41.4) | 128 (100) | |
| Mean (SD): | ||||
| Age, years | 43.8 (7.85) | 42.5 (7.73) | 0.441 | |
| Education, n (%) | ||||
| Not educated (illiterate) | 26 (20.3) | 17 (13.3) | 43 (33.6) | 0.079 |
| Educated | 49 (38.3) | 36 (28.1) | 85 (66.4) | 0.076 |
| Marital status, n (%) | ||||
| Married | 61 (47.65) | 42 (32.81) | 103 (80.5) | 0.077 |
| Divorced | 8 (6.25) | 5 (3.91) | 13 (10.2) | 0.078 |
| Widower | 4 (3.12) | 3 (2.34) | 7 (5.4) | 0.078 |
| Single | 2 (1.56) | 3 (2.34) | 5 (3.9) | 0.078 |
| Implantation type, n (%) | ||||
| Primary | 75 | 53 | 128 (100) | |
| Revision | 2 (2.66) | 1 (1.88) | 3 (2.34) | 0.078 |
Careful history taking (including sexual, medical, surgical, and drugs histories), physical examination (including genital, neurological and cardiovascular systems) and investigations [including hormonal profile, lipid profile, penile Doppler ultrasonography (US), optional cavernosography, and routine laboratory investigations] were performed preoperatively. Patients with an infection somewhere else in the body were treated first according to culture and sensitivity tests. In diabetic patients, the blood glucose level and glycated haemoglobin A1c were controlled with insulin to be <200 mg/dL and <7%, respectively. Moreover, strict antiseptic measures were taken including sterilisation and closure of the operation theatre the night before surgery, and a limited number of persons were allowed to attend the surgery. In addition, the surgical field was washed twice a day for 2 days preoperatively with povidone iodine and hospital admission was done on the morning of the operative day. Nevertheless, shaving of the genital area was performed immediately before surgery followed by povidone iodine and amikacin 500 mg/2 mL wash for at least 5 min. Perioperative ceftriaxone sodium 2 g i.v. once daily for 5 days followed by amoxicillin/clavulanate 1-g tablets twice daily for another 5 days were administered. Furthermore, clindamycin HCl hydrate 300 mg capsules/6 h was administered from the first operative day for 10 days.
Surgical technique
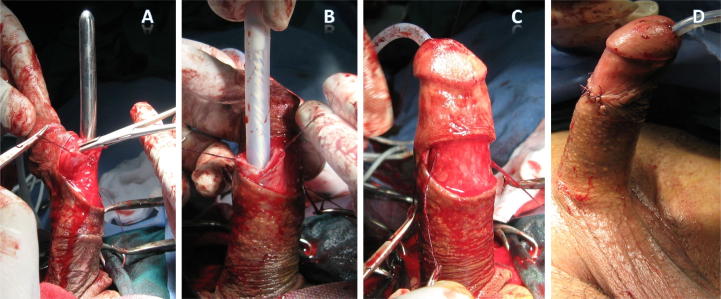
At the beginning of the procedure, a urethral catheter was inserted, in the operating room, and left for 24 h. Under spinal anaesthesia, a sub-coronal dorsal transverse incision was made followed by exposure of the corpus cavernosum lateral to the neurovascular bundles. Then, a longitudinal incision (∼15 mm long) was made through the tunica albuginea, followed by displacement of the cavernosal tissue from the tunica. A gradual expansion of the corpora into a suitable size was done using graduated Hegars dilators. Two cylinders of suitable size were implanted, followed by closure of the corpora with 3–0 polyglactin 910 (Vicryl®, Ethicon Endo-Surgery, Cincinnati, OH, USA) sutures and lastly, a compressive dressing was applied. Patients were discharged from the hospital on the second postoperative day and sexual intercourse was allowed after 4 weeks. All patients were followed up every month in the first year and twice yearly, thereafter. The steps of the surgical technique are summarised in Fig. 1.
Figure 1.
Surgical technique of malleable penile prostheses implantation; (A) sub-coronal incision, opening of the corpora cavernosum and expansion with Hegars dilators, (B) implantation of the prosthesis cylinder, (C) after implantation of both cylinders, (D) closure of the wound.
Results
A total of 128 eligible patients (53 diabetics and 75 non-diabetics) were included in this study (Table 1). All patients had refractory ED that did not respond to pharmacotherapy and received a total of 131 Tube malleable penile prostheses according to patients preferences and suitability of cost. Of the 131 implants, 128 were primary and three were revisions due to mechanical failure. Of the 53 diabetic patients, 22 had type I DM and 31 had type II DM. The aetiology of ED in the non-diabetic patients was venogenic in 27 cases diagnosed by penile Doppler US with an end diastolic velocity of >5 cm/s after intra-cavernosal injection of 60 mg papaverine or 15 μg PGE1 and venous leakage by dynamic infusion cavernosometry and cavernosography (done in some cases). ED was arteriogenic in 21 cases diagnosed by penile Doppler US with a peak systolic velocity of <25 cm/s after intra-cavernosal injection of 60 mg papaverine or 15 μg PGE1. Furthermore, post-spinal cord injury ED was diagnosed in seven patients with a history of spinal cord trauma and surgery followed by careful neurological examination with absent bulbo-cavernous reflex or a prolonged reflex latency of >45 ms. Also, post-priapism ED was diagnosed in five patient with a history of priapism followed by evacuation or shunt surgery and abnormal penile Doppler US findings after intra-cavernosal injection of 60 mg papaverine or 15 μg PGE1; peak systolic velocity of <25 cm/s or end diastolic velocity of >5 cm/s. Peyronie’s disease was diagnosed by penile examination and US showing cavernosal plaques and abnormal penile Doppler US findings, post-cystectomy in six patients with a history of radical pelvic surgery for bladder carcinoma and absent bulbo-cavernous reflex with neurological examination, and lastly a combination of two or more of these causes in six patients.
The diabetic and non-diabetic patients were comparable for age, level of education, marital status, hospital stay, time to commencing sexual intercourse, and mean follow-up period. The mean (SD; range) age was 42.5 (7.7; 29–55) years for the diabetic patients and 43.8 (7.8; 30–58) years for the non-diabetic patients. The hospital stay was 1 day in both groups, the mean (range) time to commencing sexual intercourse was 6 (4–8) weeks, and the median (range) follow-up was 42 (1–84) months and 43 (2–83) months in the diabetic and non-diabetic groups, respectively.
Intraoperative and early postoperative complications occurred in the form of inter-corporeal septal perforation in three patients (2.3%) due to the vigorous expansion and all of them were repaired intraoperatively; one patient (1.9%) was diabetic and two were non-diabetics (2.6%). Furthermore, a small glandular urethral injury occurred in two patients (1.5%) due to vigorous distal corporeal expansion and both cases, one diabetic (1.9%) and one non-diabetic (1.3%), were repaired intraoperatively with 3-0 polyglactin 910 and the urethral catheter was left in situ for 5 days. The inter-corporeal septal perforations and urethral injuries occurred in patients who had Type I DM for >10 years and post-priapism patients due to difficulty during corporeal expansion caused by the presence of cavernosal tissue fibrosis. In addition, five patients (3.9%) aged >55 years developed acute urinary retention immediately after catheter withdrawal, two (3.8%) were diabetics and three were non-diabetics (4%), and all cases were catheterised again for 5 days and commenced on tamsulosin hydrochloride 0.4 mg once daily. These five cases had an adenomatous prostate and acute urinary retention is related to BPH.
Moreover, nine patients (7%) had superficial wound infections, five of them (9.4%) were diabetics and four (5.3%) were non-diabetics, and all were treated with amoxicillin/clavulanate 1-g tablets twice daily and clindamycin HCl hydrate 300 mg capsules/6 h daily until healing of the wound within 15 days. In addition, 12 patients (9.4%) developed postoperative penile pain, numbness and hypothesia that resolved within a few months on NSAIDs and multivitamins. Of these 12 patients, seven (13%) were diabetics and five (6.6%) were non-diabetics. Penile prosthesis infections in this study occurred in seven patients (5.5%), four of them (7.5%) were diabetics and three were non-diabetics (4%), and all were treated by prosthesis removal (Table 2).
Table 2.
Perioperative complications of penile prosthesis implantation.
| Complication | Non-diabetics, n (%) | Diabetics, n (%) | Total, n (%) | P |
|---|---|---|---|---|
| Intraoperative | ||||
| Corporeal injury | 2 (2.6) | 1 (1.9) | 3 (2.3) | 0.111 |
| Urethral injury | 1 (1.3) | 1 (1.9) | 2 (1.5) | 0.5 |
| Early postoperative | ||||
| Urinary retention | 3 (4) | 2 (3.8) | 5 (3.9) | 0.374 |
| Superficial wound infection | 4 (5.3) | 5 (9.4) | 9 (7) | 0.347 |
| Penile pain and hypothesia | 5 (6.6) | 7 (13) | 12 (9.4) | 0.307 |
| Prosthesis infection | 3 (4) | 4 (7.5) | 7 (5.5) | 0.322 |
Long-term postoperative complications occurred in the form of penile cavernosal tissue pressure atrophy in five patients (3.9%), two of them (3.8%) were diabetics and three were non-diabetics (4%). Seven patients (5.5%) experienced bad cosmesis due to persistent erection, three of them (5.6%) were diabetics and four were non-diabetics (5.3%), and this complication may have occurred because of the patient’s misunderstanding of the nature by which the prosthesis functions or deficient preoperative explanation to the patients, thus inflatable prostheses were suitable for those patients. Ejaculatory disorders were reported by eight patients (6.3%), five were diabetics (9.4%) and three were non-diabetics (4%). In addition, three elderly patients (2.3%) aged >55 years developed bladder stones, which may have been related to their age and BPH complications, two were diabetics (3.8%) and one was non-diabetic (1.3%), and all of them were treated with cystolithotomy. A total of 13 prostheses (9.4%) were removed; seven prostheses (5.5%) were removed due to infection (four patients were diabetics and three non-diabetics), three prostheses (2.3%) were removed on the patients demand due to high expectations of the patient and psychological instability and lastly, three prostheses (2.3%) were removed due to mechanical failure caused by wire fracture within 13, 19, and 22 months of surgery, and these three patients were illiterate and they gave a history of frequent vigorous intercourse with an unusual sexual position (partner above the husband). In this study, three prostheses were removed on the patients demand and another three were removed due to mechanical failure, making the figure of prostheses removal high (Table 3).
Table 3.
Long-term complications of penile prosthesis implantation.
| Complication | Non-diabetics, n (%) | Diabetics, n (%) | Total, n (%) | P |
|---|---|---|---|---|
| Thin penis | 3 (4) | 2 (3.8) | 5 (3.9) | 0.374 |
| Bad cosmesis | 4 (5.3) | 3 (5.6) | 7 (5.5) | 0.685 |
| Ejaculatory disorders | 3 (4) | 5 (9.4) | 8 (6.3) | 0.274 |
| Bladder stone formation | 1 (1.3) | 2 (3.8) | 3 (2.3) | 0.257 |
| Prosthesis removal | ||||
| On patient’s demand | 2 (2.6) | 1 (1.9) | 3 (2.3) | 0.111 |
| Prosthesis infection | 3 (4) | 4 (7.5) | 7 (5.5) | 0.322 |
| Wire fracture | 1 (1.3) | 2 (3.8) | 3 (2.3) | 0.257 |
Lastly, 107 (83.6%) of the 128 patients included in the present study had regular follow-up, 44 of them were diabetics (41.1%) and 63 were non-diabetics (58.9%). In all, 21 patients were lost to follow-up (nine diabetics and 12 non-diabetics). Of the 21 patients who were lost to follow-up, 10 of them had their prostheses removed, due to infection in seven and on the patients demand in three cases. The patients and their partners were asked to complete a questionnaire of five domains including: desire, penile rigidity, orgasm, frequency of intercourse per week, and overall satisfaction. An educated nurse in the surgical team questioned the partners and reviewed their answers about satisfaction and dissatisfaction with their husbands’ prostheses. The satisfaction rates with penile prostheses were 77.3% and 79.4% in the diabetic and non-diabetic patients, respectively; with an overall satisfaction rate of 78.5%. The satisfaction rate in the study was less than reported in the literature because the wives of a number of patients who were illiterate reported dissatisfaction from frequent intercourse. The dissatisfaction was attributed to high patient’s expectation, persistent erection and flaccid penile glans, and partners’ dissatisfaction. In this study, penile prostheses were received by singles, widowers and divorced because they were preparing themselves for marriage.
Discussion
ED is the first manifestation of DM in 12–30% of cases and the prevalence of diabetic ED is 32–90% [9]. Moreover, diabetic men have a two- to fourfold higher risk of developing ED than the normal population [10]. Nitric oxide (NO), a product of the penile arterial endothelium, is the mediator of cavernosal tissue relaxation and penile erection through the production of cyclic guanosine monophosphate (cGMP) [11]. DM is associated with the production of superoxide radicals in the penile cavernosal tissues that impair the process of NO and cGMP synthesis through impairment of NO synthase and guanylyl cyclase, respectively. Furthermore, DM is associated with autonomic neuropathy that contributes to ED through reduced or absent parasympathetic activity required for cavernosal smooth muscle relaxation [12].
Pharmacotherapy is the first-line of treatment for ED, but in a case of failure, penile prosthesis (inflatable or malleable) implantation can be considered [13]. Although, malleable penile prostheses are easy to implant with a low risk of mechanical failure they are associated with a permanent penile erect state, a risk of chronic pain, difficult concealment, and erosion [14]. Patients with DM are more liable to infection than non-diabetics because of polymorphonuclear leucocyte dysfunction with subsequent impairment of the natural phagocytic and bactericidal activity. Moreover, diabetic-induced microangiopathy results in poor delivery of monocytes and polymorphonuclear leucocytes to the site of infection [15].
Penile prosthesis infection is a fearful and disastrous complication, occurring mostly in the first postoperative year and mandates immediate removal of the prosthesis in most situations, followed by revision surgery 3–6 months later, which is a challenging surgery due to the occurrence of corporeal fibrosis and associated with a high risk of infection [16]. Staphylococcus epidermidis is the most common organism found in penile prosthesis infections. This organism lives in the epidermis and surrounds itself with a protective biofilm, and patients infected with this organism might remain asymptomatic for a long period. Moreover, it was reported in the literature that S. epidermidis cultured from infected penile prostheses is highly sensitive to clindamycin antibiotics [17]. In the present series, the overall rate of penile prosthesis infection was only 5.5%, without a significant difference between the diabetic and non-diabetic patients. The lower infection rates in the present study could be explained by the strict infection-control protocol followed before and during surgery, and the use of clindamycin HCl hydrate 300 mg capsules/6 h. Cumming and Pryor [18] reported infection rates of 20% and 10% in diabetic and non-diabetic patients, respectively. Conversely, Minervini et al. [19] reported that a 10% infection rate in diabetic patients vs 21% in non-diabetics. Furthermore, Cakan et al. [16] reported a 10% infection rate in diabetics vs 15% in non-diabetics and Song et al. [20] reported penile prosthesis infection and erosion in one of nine diabetic patients with a malleable penile implant.
Recently, a systematic review has been conducted to study the relation between penile implant infection and the presence of DM in patients with organic ED. The authors concluded that the most recent and largest case series did not reveal any statistically significant increase in the risk of implant infection in diabetic patients. On the contrary, older studies showed an increased risk of implant infection in those patients and authors ascribed this discrepancy to smaller sample size and lack of robust statistical analysis in the older studies [21]. In the present study, inter-corporeal septal perforation occurred in 2.3% and small glanular urethral injury in 1.5% of patients due to difficult expansion caused by cavernosal tissue fibrosis and early experience with penile implant surgery. Whereas, Fathy et al. [8] reported urethral injury and corporeal perforation in 0% in their series and Song et al. [20] reported corporeal perforation in three of 224 patients in their series.
The overall incidence of mechanical failure in penile prosthesis implantation is reported to be 5% [22]. In the present study, a mechanical failure occurred in three cases (2.3%) at 13, 19, and 22 months after surgery, which were treated with revised implants following the diagnosis. Song et al. [20] reported in their series mechanical failure in four of 224 patients at a mean of 19 months after implantation. Furthermore, DM had no significant impact on the average duration of hospital stay or time to commencement of sexual intercourse in the present series. Cavernosal tissue fibrosis in diabetic patients has been reported to add to the difficulty of penile prosthesis implantation [23]. A similar result was found in the present series, where six diabetic patients with type I DM for >10 years and five post-priapism patients had penile cavernosal tissue fibrosis and difficulty was noted during the procedure. Eight patients (6.3%) had postoperative ejaculatory disorders, five of them were diabetic. Fathy et al. [8] reported a similar outcome of retarded ejaculation in 12 of 83 patients who underwent malleable penile prosthesis implantation for ED, seven of them were diabetic.
The satisfaction rate with Tube malleable penile prosthesis in the present series was not significantly different between the diabetic and non-diabetic patients, at 77.3% vs 79.4%, with an overall 78.5% satisfaction rate. This is comparable to satisfaction rates reported in other series [24]. The present study is a retrospective study that has the usual limitations and shortcomings including: the accuracy of written records or recall of individuals, some important data may not have been available, it is difficult to control bias and confounders, it may be impossible to access important information, and it is difficult to establish cause and effect. The present study is a retrospective one and has the drawbacks of a small and unequal number of patients, so a prospective comparative study with a large number of patients is recommended.
Conclusion
Tube malleable penile prostheses are associated with low complication and high satisfaction rates. There was no significant difference in the overall complication rate or prosthesis infection rate between diabetic and non-diabetic patients. A prospective comparative study with a large number of patients is recommended.
Conflict of interest
No conflict of interest to declare.
Source of funding
There was no funding or any disclosure to companies.
Acknowledgement
The author acknowledges his colleagues for their support and cooperation.
Andrology/Sexal Medicine
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Laumann E.O., West S., Glasser D., Carson C., Rosen R., Kang J.H. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the Male Attitudes Regarding Sexual Health survey. J Sex Med. 2007;4:57–65. doi: 10.1111/j.1743-6109.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 2.Jiann B.P., Yu C.C., Su C.C. Impact of introduction of sildenafil on other treatment modalities for erectile dysfunction: a study of nationwide and local hospital sales. Int J Impot Res. 2004;16:527–530. doi: 10.1038/sj.ijir.3901259. [DOI] [PubMed] [Google Scholar]
- 3.Mulcahy J.J., Austoni E., Barada J.H., Choi H.K., Hellstrom W.J.G., Krishnamurti S. The penile implant for erectile dysfunction. J Sex Med. 2004;1:98–109. doi: 10.1111/j.1743-6109.2004.10115.x. [DOI] [PubMed] [Google Scholar]
- 4.Montague D.K. Penile prosthesis implantation in the era of medical treatment for erectile dysfunction. Urol Clin North Am. 2011;38:217–225. doi: 10.1016/j.ucl.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Wilson S.K., Delk J.R., 2nd Inflatable penile implant infection: predisposing factors and treatment suggestions. J Urol. 1995;153:659–661. [PubMed] [Google Scholar]
- 6.Jarow J.P. Risk factors for penile prosthetic infection. J Urol. 1996;156:402–404. doi: 10.1097/00005392-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Carson C.C., Mulcahy J.J., Govier F.E. Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol. 2000;164:376–380. [PubMed] [Google Scholar]
- 8.Fathy A., Shamloul R., AbdelRahim A., Zeidan A., El-Dakhly R., Ghanem H. Experience with Tube® (Promedon) malleable penile implant. Urol Int. 2007;79:244–247. doi: 10.1159/000107957. [DOI] [PubMed] [Google Scholar]
- 9.Kamenov Z.A. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123:141–158. doi: 10.1055/s-0034-1394383. [DOI] [PubMed] [Google Scholar]
- 10.Lewis R.W. Epidemiology of erectile dysfunction. Urol Clin North Am. 2001;28:209–213. doi: 10.1016/s0094-0143(05)70132-4. [DOI] [PubMed] [Google Scholar]
- 11.Cellek S., Rodrigo J., Lobos E., Fernandez P., Serrano J., Moncada S. Selective nitrergic neurodegeneration in diabetes mellitus – a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999;128:1804–1812. doi: 10.1038/sj.bjp.0702981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegge N.C., Twomey A.M., Vaughton K., Gravenor M.B., Ramsey M.W., Price D.E. The role of endothelial dysfunction in the pathophysiology of erectile dysfunction in diabetes and in determining response to treatment. Diabet Med. 2006;23:873–878. doi: 10.1111/j.1464-5491.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans C. The use of penile prosthesis in the treatment of impotence. Br J Urol. 1998;81:591–598. doi: 10.1046/j.1464-410x.1998.00597.x. [DOI] [PubMed] [Google Scholar]
- 14.Montorsi F., Deho F., Salonia A., Briganti A., Bua L., Fantini G.V. Penile implants in the era of oral drug treatment for erectile dysfunction. BJU Int. 2004;94:745–751. doi: 10.1111/j.1464-410X.2004.05025.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson S.K., Carson C.C., Cleves M.A., Delk J.R. Quantifying risk of penile prosthesis infection with elevated glycosylated hemoglobin. J Urol. 1998;159:1537–1539. doi: 10.1097/00005392-199805000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Cakan M., Demirel F., Karabacak O., Yalçinkaya F., Altuğ U. Risk factors for penile prosthetic infection. Int Urol Nephrol. 2003;35:209–213. doi: 10.1023/b:urol.0000020300.23522.49. [DOI] [PubMed] [Google Scholar]
- 17.Teloken C., Souto J.C., Da Ros C., Thorel E., Souto C.A. Prosthetic penile infection: “rescue procedure” with rifamycin. J Urol. 1992;148:1905–1906. doi: 10.1016/s0022-5347(17)37067-2. [DOI] [PubMed] [Google Scholar]
- 18.Cumming J., Pryor J.P. Treatment of organic impotence. Br J Urol. 1991;67:640–643. doi: 10.1111/j.1464-410x.1991.tb15231.x. [DOI] [PubMed] [Google Scholar]
- 19.Minervini A., Ralph D.J., Pryor J.P. Outcome of penile prosthesis implantation for treating erectile dysfunction: experience with 504 procedures. BJU Int. 2006;97:129–133. doi: 10.1111/j.1464-410X.2005.05907.x. [DOI] [PubMed] [Google Scholar]
- 20.Song W.D., Yuan Y.M., Cui W.S., Wu A.K., Zhu Y.C., Liu J. Penile prosthesis implantation in Chinese patients with severe erectile dysfunction: 10-year experience. Asian J Androl. 2013;15:658–661. doi: 10.1038/aja.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christodoulidou M., Pearce I. Infection of penile prosthesis in patients with diabetes mellitus. Surg Infect (Larchmt) 2016;17:2–8. doi: 10.1089/sur.2015.164. [DOI] [PubMed] [Google Scholar]
- 22.Atienza Merino G. Penile prosthesis for the treatment of erectile dysfunction. Actas Urol Esp. 2006;30:159–169. doi: 10.1016/s0210-4806(06)73418-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Dai Y., Wang R. Treatment strategies for diabetic patients suffering from erectile dysfunction. Expert Opin Pharmacother. 2008;9:257–266. doi: 10.1517/14656566.9.2.257. [DOI] [PubMed] [Google Scholar]
- 24.Mulhall J.P., Ahmed A., Branch J., Parker M. Serial assessment of efficacy and satisfaction profiles following penile prosthesis surgery. J Urol. 2003;169:1429–1433. doi: 10.1097/01.ju.0000056047.74268.9c. [DOI] [PubMed] [Google Scholar]