Abstract
Transient receptor potential (TRP) channels are activated by environmental particulate materials. We hypothesized that polymorphic variants of transient receptor potential vanilloid-1 (TRPV1) would be uniquely responsive to insoluble coal fly ash compared with the prototypical soluble agonist capsaicin. Furthermore, these changes would manifest as differences in lung cell responses to these agonists and perhaps correlate with changes in asthma symptom control. The TRPV1-I315M and -T469I variants were more responsive to capsaicin and coal fly ash. The I585V variant was less responsive to coal fly ash particles due to reduced translation of protein and an apparent role for Ile-585 in activation by particles. In HEK-293 cells, I585V had an inhibitory effect on wild-type TRPV1 expression, activation, and internalization/agonist-induced desensitization. In normal human bronchial epithelial cells, IL-8 secretion in response to coal fly ash treatment was reduced for cells heterozygous for TRPV1-I585V. Finally, both the I315M and I585V variants were associated with worse asthma symptom control with the effects of I315M manifesting in mild asthma and those of the I585V variant manifesting in severe, steroid-insensitive individuals. This effect may be due in part to increased transient receptor potential ankyrin-1 (TRPA1) expression by lung epithelial cells expressing the TRPV1-I585V variant. These findings suggest that specific molecular interactions control TRPV1 activation by particles, differential activation, and desensitization of TRPV1 by particles and/or other agonists, and cellular changes in the expression of TRPA1 as a result of I585V expression could contribute to variations in asthma symptom control.
Keywords: asthma, inflammation, single nucleotide polymorphism (SNP), transient receptor potential channels (TRP channels), Western blot
Introduction
Suspended crustal and road materials, which often contain coal fly ash (CFA)2; combustion by-products from cigarettes and wood/biomass smoke; and emissions from motor vehicles, cooking, and power generation are common particulate materials (PM) in ambient air. However, the precise composition of air pollution varies widely by location, often promoting regionally specific effects on human health. In general, inhaled PM tend to cause airway irritation that can negatively impact respiratory conditions such as asthma (1–6).
The mechanisms by which different constituents of air pollution interact with and affect cells of the human airways and how these interactions affect respiratory physiology and human health are not fully understood. Transient receptor potential ankyrin-1 (TRPA1) and TRP vanilloid-1 (TRPV1) are activated by distinct environmental PM. TRPA1 is activated by electrophiles and oxidants in diesel exhaust, cigarette smoke, and wood smoke PM via covalent modification of intracellular cysteine and lysine residues as well as through cell surface interactions with insoluble PM such as CFA and diesel soot (7–11). TRPV1 is activated by insoluble PM including CFA, crystalline silica, diesel exhaust soot, residual oil fly ash, Mt. St. Helens volcanic ash, and urban particles collected in Salt Lake City, UT; St. Louis, MO; and Ottawa, Canada (12–15). CFA and other insoluble PM preferentially activate TRPV1 at the cell surface independent of the capsaicin-binding site (12, 16). C-fiber activation by TRPA1 and TRPV1 agonists reduces respiratory drive, induces cough, and triggers bronchoconstriction and neurogenic inflammation (17). Activation of TRPA1 (when expressed) and TRPV1 in non-neuronal cells including bronchial, small airway, and alveolar epithelial cells promotes proinflammatory cytokine/chemokine expression (IL-6, IL-8, and others) and cytotoxicity (12–16, 18–28). TRPA1 and TRPV1 are also involved in inflammation-induced airway hypersensitivity and play key roles in host defense responses (17, 29, 30).
Several studies have examined roles for TRPA1 and TRPV1 in asthma. TRPA1 has been shown to be integral in the development of asthma/allergic hypersensitivity in mice (31), and the R3C and R58T polymorphisms correlated with poorer asthma control in children (8).
The hypothesis of this study was that natural coding variants of TRPV1 would be uniquely responsive to traditional soluble (capsaicin) and atypical insoluble particle (CFA) agonists, which would manifest as differences in typical cellular responses to these agonists and perhaps an effect on asthma severity and symptom control among a cohort of asthmatic children in Utah. The hypothesis is based on results showing that some individuals with asthma, mainly cough variant asthma, exhibit greater sensitivity in capsaicin cough challenge tests (32–35). TRPV1 also has been shown to be expressed at higher levels in epithelial cells (36) and neurons in the bronchi of asthmatics with both mild and refractory (steroid-insensitive) asthma (37) as well as in neurons of individuals with chronic cough (38), in peripheral blood cells of children with asthma (39, 40), and in BALB/c but not C57Bl/6 mice challenged with IL-13 (41). Furthermore, the loss-of-function TRPV1 variant I585V (rs8065080) has been correlated with reduced cough and wheeze in a cohort of asthmatics from Barcelona and Sabadell, Spain (42, 43). Similarly, the T469I variant (rs224534) was correlated with small but non-significant increases in cough in non-asthmatics (43). Several intronic, 3′-, and 5′-untranslated region variants have also been associated with different asthma phenotypes (39, 40, 43). Further understanding of the effects of TRPV1 genotype on asthma could aid clinicians in understanding why certain individuals are more sensitive to various stimuli and potentially direct them toward more appropriate treatments.
In this study, we utilized a combination of in vitro tests of TRPV1 expression and function using both capsaicin and CFA as agonists to further explain unique mechanisms of TRPV1 activation by PM. We then assessed TRPV1-mediated desensitization, chemokine production, and TRP gene expression in primary human bronchial epithelial cells expressing either wild type or the TRPV1-I585V variant. Finally, we correlated genetic and clinical data from a cohort of children with asthma from Salt Lake City, UT (43) to assess whether TRPV1 variants with altered activity affected asthma.
Results
Functional Analysis of TRPV1 Variant Activation
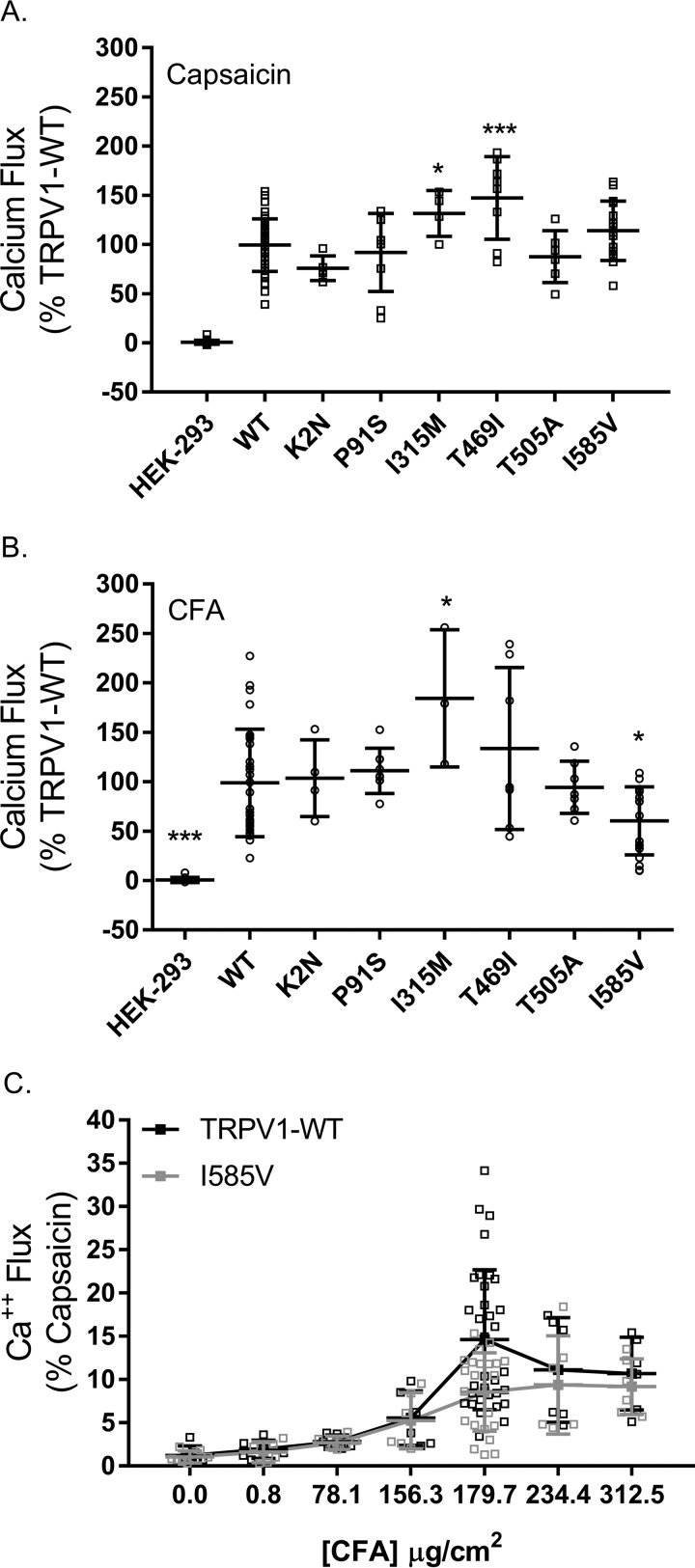
Activation of TRPV1 and six non-synonymous SNP variants by capsaicin and CFA particles was compared in transiently transfected HEK-293 cells using changes in intracellular calcium content as a measure of TRPV1 activity (Fig. 1). The SNP variants I315M, T469I, and I585V exhibited ∼32, 47, and 14% greater activity than wild-type TRPV1 using capsaicin as an agonist. The I315M and T469I variants were also ∼54 and 33% more responsive to CFA than wild-type TRPV1, whereas the I585V variant was ∼40% less responsive. Reduced sensitivity of the I585V variant was observed at multiple CFA concentrations (Fig. 1C).
FIGURE 1.
A and B, comparison of TRPV1 and TRPV1 variant activation by capsaicin (A) and CFA (B) is shown. C, dose response for activation of TRPV1-WT and TRPV1-I585V in transfected HEK-293 cells. Data are the mean ± S.D. (error bars) for n ≥ 3 independent assays. * represents p < 0.05, and *** represents p < 0.001 using one-way ANOVA and Dunnett's post hoc test for significant changes relative to wild-type TRPV1-transfected cells.
TRPV1 Variant Expression and Localization in HEK-293 Cells
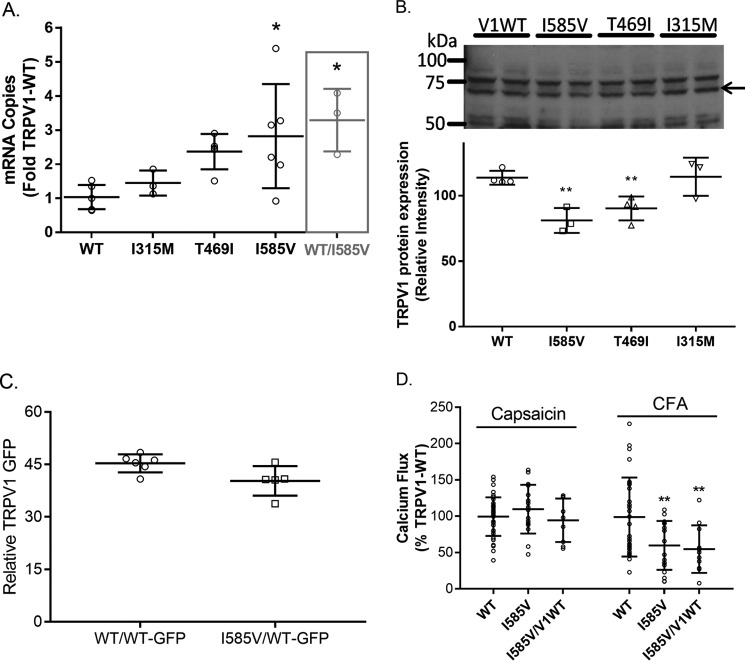
Results in Fig. 1 showing variations in TRPV1 activation among the I315M, T469I, and I585V variants could be explained by changes in the level of TRPV1 expression, unique subcellular localization of the variant proteins, or inherent changes in protein function. Fig. 2A shows that the expression of mRNA for the I315M, T469I, and I585V forms of TRPV1 were 1.4–3-fold greater than for wild-type TRPV1 in HEK-293 cells, similar to that reported by Xu et al. (44). Fig. 2B shows that there was ∼20–30% lower expression of TRPV1-I585V and -T469I protein compared with wild-type TRPV1 and I315M despite the increase in mRNA. No major changes in the proportion of calcium flux attributable to intracellular versus cell surface TRPV1 were observed between the wild-type and variant proteins; ∼90% of capsaicin-induced calcium flux for wild type, I315M, and T469I was inhibited using the cell-impermeable inhibitors EGTA and ruthenium red, and ∼82% of capsaicin-induced calcium flux was inhibited for I585V (data not shown).
FIGURE 2.
A, comparison of TRPV1 mRNA expression levels in HEK-293 cells transiently transfected with expression plasmids for wild-type TRPV1 (I585I), I315M, T469I, I585V, or a 1:1 mixture (same total plasmid amount as the individual plasmids) of wild-type TRPV1 and I585V expression plasmids (gray bar). Data represent the mean ± S.D. (error bars) of the ratio of TRPV1 mRNA to β2-microglobulin mRNA normalized to wild-type TRPV1. B, comparison of TRPV1 protein expression by Western blotting. Data represent the mean ± S.D. (error bars) relative to total protein determined by Amido Black staining of PVDF membranes. The arrow represents the TRPV1 protein. C, comparison of TRPV1 variant protein expression in HEK-293 cells co-transfected with TRPV1-WT/TRPV1-WT-GFP or TRPV1-I585V/TRPV1-WT-GFP by flow cytometry. D, comparison of TRPV1 activation (calcium flux) by capsaicin and coal fly ash in HEK-293 cells transfected with wild-type TRPV1, I585V, or a 1:1 mixture of expression plasmids. Data in C and D were analyzed for significant differences from the wild-type control group using one- or two-way ANOVA using Dunnett's post hoc test or Bonferroni's multiple comparisons test to assess changes relative to wild-type TRPV1. * represents p < 0.05, and ** represents p < 0.01.
TRPV1-WT/TRPV1-I585V Co-expression Studies
The possibility that the TRPV1-I585V variant altered the expression levels and/or functional properties of wild-type TRPV1 was also assessed. Transfection of HEK-293 cells with an equal quantity of plasmid DNA harboring either TRPV1-WT or TRPV1-I585V or co-transfecting cells with a 1:1 ratio of plasmid DNA for the TRPV1-WT and TRPV1-I585V demonstrated an increase in the overall expression of TRPV1 mRNA relative to cells transfected with wild-type TRPV1 alone (Fig. 2A, gray bar). As in Fig. 2B, TRPV1 protein expression in cells co-expressing TRPV1-WT/WT-green fluorescent protein (GFP) or TRPV1-I585V/WT-GFP was also slightly (∼10–15%) reduced as measured by flow cytometry (Fig. 2C). Finally, cells transfected with both wild-type TRPV1 and TRPV1-I585V were ∼40% less responsive to CFA as seen with TRPV1-I585V alone but not capsaicin (Fig. 2D).
Regulation of TRPV1 Internalization/Desensitization
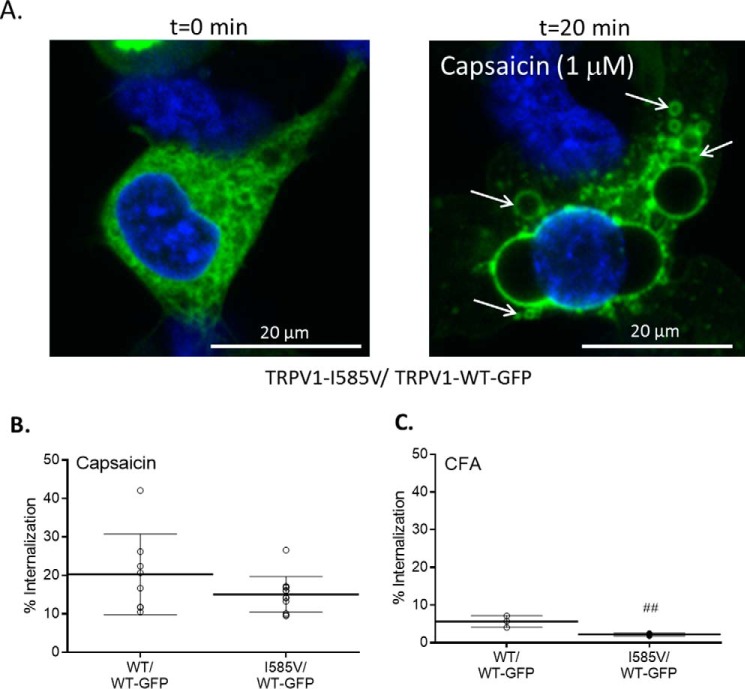
Agonist-induced and calcium-dependent desensitization/cellular internalization of TRPV1 (27) was also evaluated (Fig. 3). HEK-293 cells expressing TRPV1-WT-GFP and untagged TRPV1-WT exhibited increases in intracellular vesicles containing GFP within 20 min of capsaicin treatment (Fig. 3A; quantified in Fig. 3B). This response was substantially lesser in cells treated with CFA even at 2 h post-treatment (Fig. 3, B and C), consistent with CFA being a much weaker TRPV1 agonist than capsaicin. When cells expressing both TRPV1-WT-GFP and untagged TRPV1-I585V were treated with capsaicin, a ∼25% decrease in cellular internalization of TRPV1 was observed compared with TRPV1-WT-GFP/TRPV1-WT-transfected cells. With CFA, a ∼60% decrease in the internalization response was observed, consistent with I585V being preferentially less responsive to CFA.
FIGURE 3.
A, representative images demonstrating TRPV1-GFP internalization elicited by 1 μm capsaicin (arrows). B and C, quantification of TRPV1 internalization induced by capsaicin (1 μm) (B) and CFA (180 μg/cm2) (C). Internalization was quantified by counting any cell exhibiting a time-dependent increase in intracellular GFP-stained vesicles and normalizing to the total cell count (i.e. Hoechst 33342-stained nuclei). Data are represented as the percentage of cells showing TRPV1-WT-GFP internalization relative to the total number of cells in the field. Data represent the mean ± S.D. (error bars). # represents a difference relative to wild-type TRPV1 (p < 0.01).
IL-8 mRNA Induction and Secretion by Lung Cells Versus TRPV1 Genotype
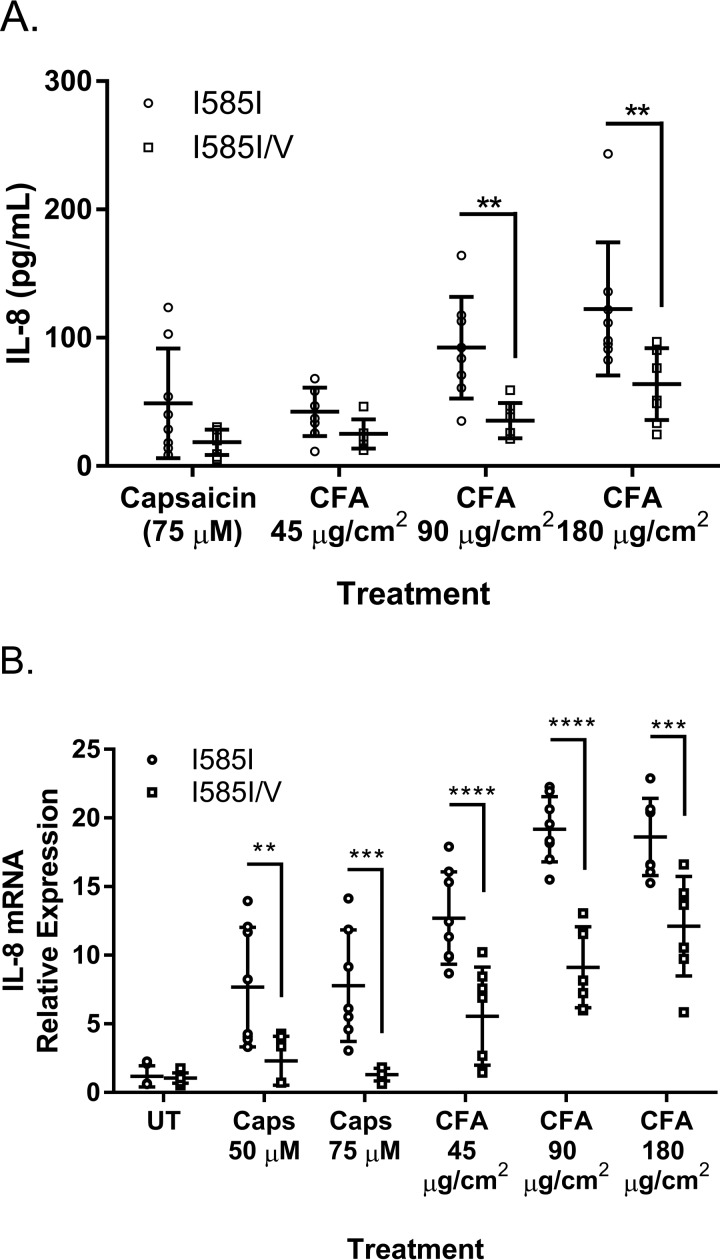
IL-8 is a common acute phase response protein secreted by lung epithelial cells treated with PM such as CFA. Primary normal human bronchial epithelial (NHBE) cells expressing either wild-type TRPV1 (I585I) or TRPV1-I585I/V (heterozygotes) exhibited dose-dependent increases in IL-8 accumulation in the culture medium following 24-h treatment with CFA. The response of the TRPV1-I585I/V cells was ∼30–60% lower depending upon the dose for both capsaicin and CFA (Fig. 4A). IL-8 mRNA expression mirrored the results for IL-8 protein (Fig. 4B).
FIGURE 4.
Comparison of IL-8 protein secretion by ELISA (A) and mRNA expression by quantitative PCR (B) by primary normal NHBE cells from four donor samples expressing either wild-type TRPV1 (I585I) or TRPV1-I585I/V (heterozygotes) following 24-h treatment with either capsaicin (Caps) (50 and 75 μm) or CFA (45, 90, and 180 μg/cm2). Data represent the mean ± S.D. (error bars) of six to eight biological replicates using two separate donor cell lines for wild-type TRPV1 (I585I) and TRPV1-I585I/V. ** indicates statistical significance (p < 0.01), *** represents p < 0.0005, and **** represents p < 0.0001 by two-way ANOVA with Bonferroni's multiple comparisons post hoc testing. UT, untreated.
TRP Channel Expression by Lung Cells Versus TRPV1 Genotype
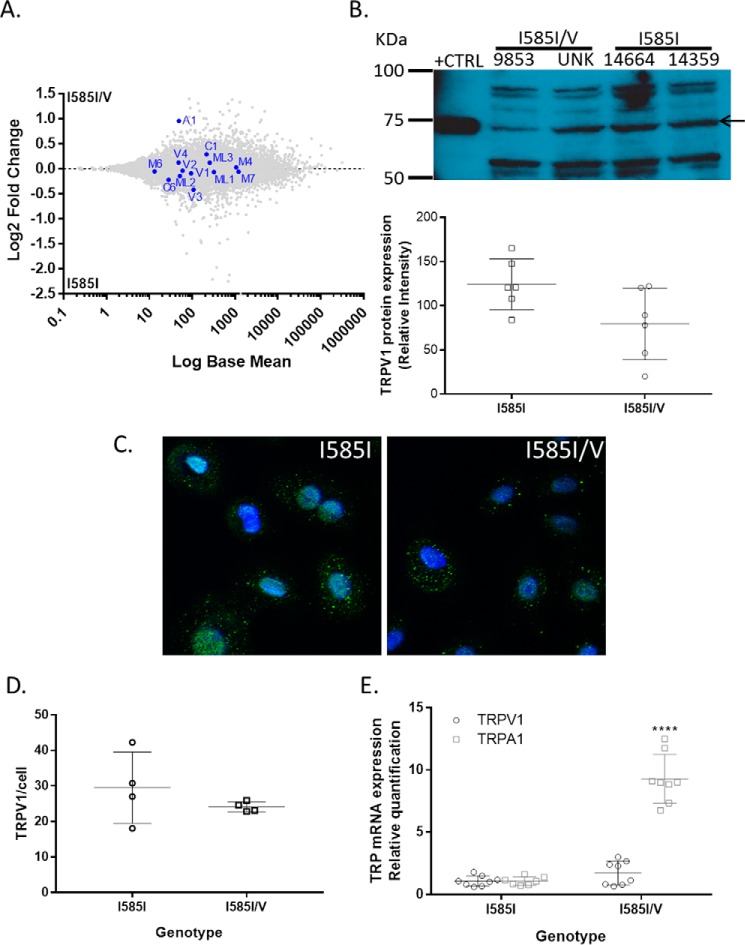
The expression levels of TRPV1 and other TRP channels in TRPV1-WT and TRPV1-I585I/V NHBE cells was also investigated via RNA sequencing analysis (Fig. 5A). The expression of mRNA for TRPV1 and many other TRP channels was not significantly altered as a result of the I585I/V genotype or agonist treatments. Immunocytochemistry and Western blotting analysis demonstrated a slightly (∼30%) lower, although not statistically significant, level of TRPV1 protein in I585I/V cells (Fig. 5, B–D), similar to but greater in magnitude than that observed in WT-GFP/I585V-co-transfected HEK-293 cells (Fig. 2C). However, cells expressing the TRPV1-I585I/V variant expressed significantly higher TRPA1 mRNA (∼10-fold wild type; Fig. 5, A and E).
FIGURE 5.
Comparison of TRP channel expression in primary NHBE cells expressing either wild-type TRPV1 (I585I; donors 9853 and unknown (UNK)) or I585I/V (donors 14664 and 14359). A, log ratio/mean average plot of RNA sequencing data comparing TRP channel gene expression as a function of TRPV1-I585 genotype. B–D, comparison of TRPV1 protein expression by Western blotting (B) and immunocytochemistry (C and D). E, confirmation of increased TRPA1 mRNA expression by quantitative PCR. Western blotting data represent the mean ± S.D. (error bars) relative to total protein. Blue (Hoechst 33342), nuclei; green (Alexa Fluor 488-conjugated antibody), TRPV1. Immunostaining was quantified in ImageJ by counting the number of green punctate foci per cell, representing TRPV1, and normalizing to the number of cells/nuclei, providing the number of TRPV1-positive foci/cell. Data were analyzed for significant differences from the I585I wild-type control group using a t test. **** represents p < 0.0001.
TRPV1 Genotype Analysis
The effect of I315M, T469I, and I585V SNPs on asthma control was evaluated in a cohort of children with asthma. General characteristics of the cohort are provided in Table 1. Genomic DNA samples were genotyped for I315M (n = 1179), T469I (n = 1226), and I585V (n = 1270) among other SNPs. The minor allelic and genotype frequencies for I315M, T469I, and I585V are shown in Table 2. The minor allele frequencies for I315M and T469I were comparable with those reported in the literature, whereas I585V was ∼5% more frequent in the overall cohort. However, all three SNPs were enriched 10–20% in the more severe electronic Asthma Tracker (eAT) population. For the I585V variant, there were also substantial differences in the percentages of patients with poorly controlled/severe asthma: for asthma control scores of 0–2, 41.6% of subjects were I585I/V versus 46.7% for subjects with scores of 3–12 and 64.7% for scores >13. Accordingly, the percentages of subjects with wild-type TRPV1 and I585V/V were lower among individuals with poor asthma control.
TABLE 1.
Population characteristics of children and young adults with asthma who underwent genotyping for TRPV1 polymorphisms
LTRA, leukotriene receptor antagonist; BMI, body mass index.
| Variable | Population value | eAT population value |
|---|---|---|
| Total subjects (n) | 1569 | 74 |
| Subjects utilizing a short acting bronchodilator (n (%)) | 1421 (90.6) | 73 (98.6) |
| Subjects treated with a long acting β-agonist (n (%)) | 12 (7.6) | 0 (0) |
| Subjects treated with an inhaled glucocorticoid (n (%)) | 958 (61.1) | 63 (85.1) |
| Subjects treated with other asthma drug (LTRA, anti-inflammatory, etc.) (n (%)) | 451 (28.7) | 24 (32.4) |
| Age, mean ± S.D. (years) | 8.84 ± 4.26 | 6.98 ± 3.31 |
| Sex (n (%)) | ||
| Male | 938 (59.9) | 43 (58.1) |
| Female | 627 (40.1) | 31 (41.9) |
| Race/ethnicity (n (%)) | ||
| American Indian/Alaskan Native | 15 (1.0) | 1 (1.4) |
| Asian | 13 (0.8) | 1 (1.4) |
| Black/African American | 57 (3.6) | 2 (2.7) |
| Native Hawaiian/Pacific Islander | 44 (2.8) | 3 (4.1) |
| White/Caucasian | 1101 (70.7) | 49 (67.1) |
| Unknown/not reported | 328 (21.1) | 17 (23.3) |
| Ethnicity (n (%)) | ||
| Hispanic | 308 (19.7) | 15 (20.5) |
| Non-Hispanic | 824 (52.9) | 25 (34.2) |
| Unknown, not reported | 427 (27.4) | 33 (45.2) |
| Weight, mean ± S.D. (kg) | 36.3 ± 21.9 | 27.1 ± 15.8 |
| Height, mean ± S.D. (cm) | 131.5 ± 25.2 | 120.8 ± 22.9 |
| BMI | 19.7 ± 9.1 | 20.6 ± 16 |
| Asthma control score, mean ± S.D. | 4.1 ± 3.3 | 4.9 ± 3.8a |
a Statistically different compared with the overall population (two-tailed p = 0.0432) using an unpaired t test.
TABLE 2.
The minor allele and genotype frequencies for I315M, T469I, and I585V variants in the study cohort
Global minor allele frequency (MAF) and sample size are as reported by the 1000 genomes project.
| TRPV1 SNP | Global MAF | Observed MAF | Wild-type frequency | Heterozygote frequency | Variant frequency |
|---|---|---|---|---|---|
| % | % | % | % | % | |
| I315M | 26.7 (n = 1335) | 25.3 | 56.3 | 36.7 | 7.0 |
| I315 M eAT | 37.0 | 37.3 | 50.8 | 11.9 | |
| T469I | 37.8 (n = 1893) | 37.4 | 40.1 | 45.0 | 14.8 |
| T469I eAT | 49.0 | 26.7 | 48.3 | 25.0 | |
| I585V | 31.8 (n = 1591) | 36.7 | 40.2 | 46.1 | 13.7 |
| I585V eAT | 52.0 | 18.6 | 59.3 | 22.0 |
Association of TRPV1 Variants with Asthma Phenotypes
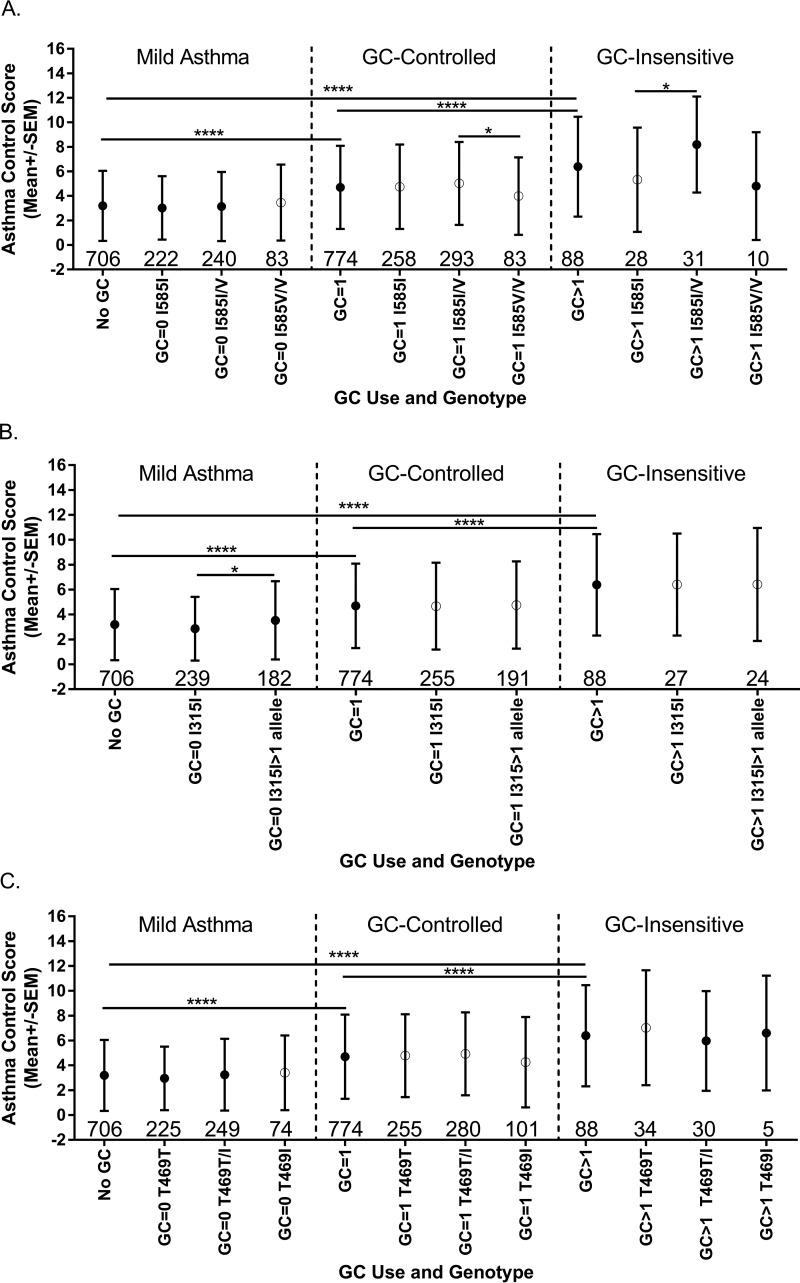
Asthma control scores and the asthma control score components were analyzed as a function of TRPV1 genotype. Odds ratio (OR) values for each SNP were plotted as a function of genotype and the aggregate asthma control score compared with a score of 0 (Fig. 6). Heterozygosity for I585V (Fig. 5A) or expression of >1 allele for TRPV1-I315M (Fig. 6B) was associated with poorer asthma symptom control, whereas T469I (Fig. 6C) had no apparent effect. Odds ratios for aggregate asthma control scores <2 versus >12 (excellent/good versus poor asthma control) were 2.23 (1.19–4.19; p = 0.0123) for ≥1 copy of I315M and 2.04 (1.06–3.95; p = 0.0339) for I585I/V. Symptom frequency (i.e. cough, wheeze, and chest tightness) was used as a more direct measure for TRPV1 due to its role in controlling the cough reflex (Fig. 7). The odds ratio for having a symptom frequency >0 was 1.25 (0.98–1.61; p = 0.0782) for ≥1 copy of I315M and 1.33 (1.03–1.72; p = 0.0287) and 1.08 (0.74–1.58; p = 0.6691) for I585I/V and I585V/V, respectively. For a symptom frequency score of 0 versus 3, the OR values were 2.42 (1.37–4.26; p = 0.0023) for ≥1 copy of I315M and 1.67 (0.96–2.92; p = 0.0709) and 1.16 (0.5–2.68; p = 0.7356) for I585I/V and I585V/V, respectively. These effects were typically more pronounced, albeit not statistically significant, among the eAT cohort (Fig. 7) and specifically among patients with more severe glucocorticoid (GC)-insensitive asthma (Fig. 8, A–C). Statistically significant increases in medical care frequency (overall and eAT population) were also observed for I315I/M. Statistically significant increases in activity interference (total and eAT population), nighttime awakenings (eAT population), and medical care frequency (eAT population), as determined using a questionnaire described elsewhere (8, 45), were also observed for I585I/V.
FIGURE 6.
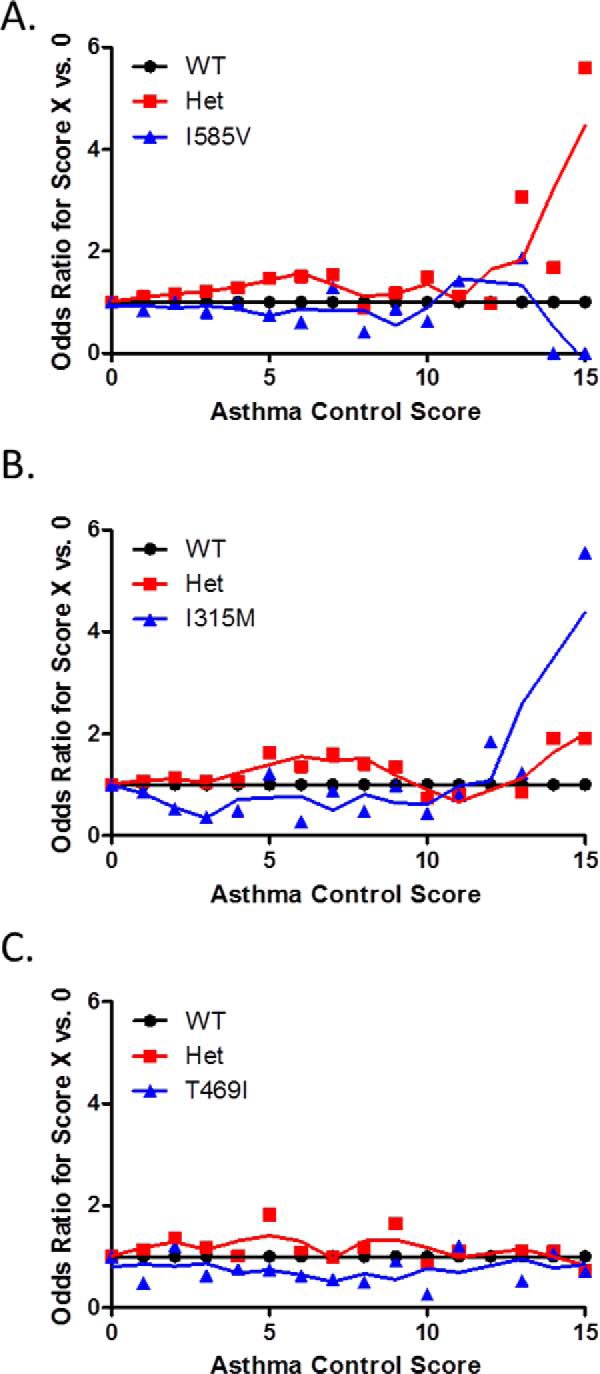
Simple OR values for subjects having an aggregate asthma control score of “X” versus 0, representing ideal asthma symptom control, as a function of TRPV1 genotype. A, I585V; B, I315M; C, T469I. OR calculations were performed using MedCalc. Het, heterozygote.
FIGURE 7.
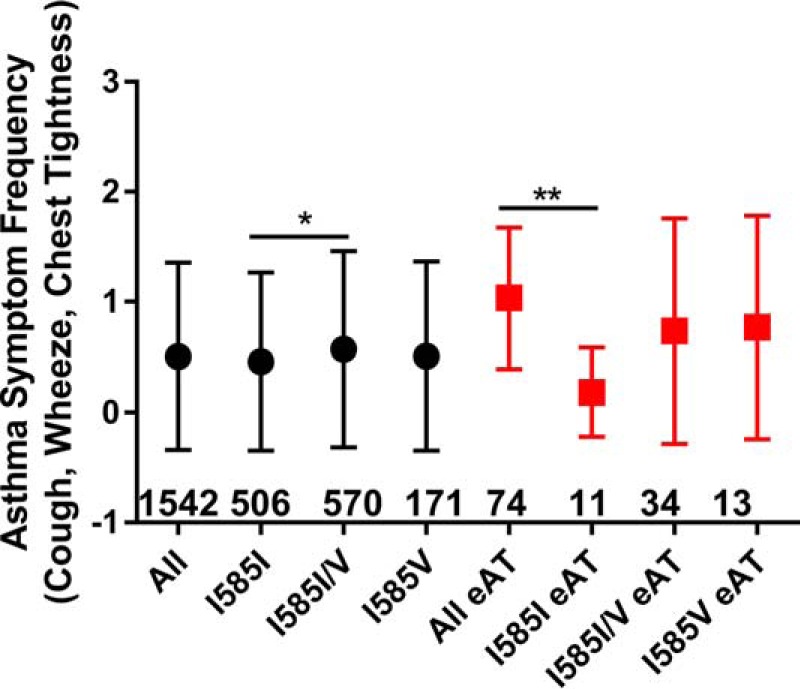
Comparison of asthma symptom frequency among subjects with wild-type TRPV1 versus I585V in a population of children with asthma (black circles) or subclassified by enrollment in the eAT tracking program (red squares) with the number of subjects in each group provided below or adjacent to the data point. Data are shown as the mean ± S.D. (error bars) and were analyzed for significant differences by one-way ANOVA using either Dunnett's or Bonferroni's multiple comparisons post hoc tests for changes relative to wild-type TRPV1. * represents p < 0.05, and ** represents p < 0.01. Subject numbers for each group are shown above the x axis within the figures.
FIGURE 8.
Comparison of aggregate asthma control scores among asthmatic subjects as a function of asthma (GC) controller medication use and TRPV1 genotype, I585V (A), I315M (B), or I585V (C). Mild asthma is defined as a lack of GC medication use (GC = 0). GC-controlled is based on the use of one GC medication (GC = 1), whereas GC-insensitive is defined by the use of >1 GC medication (GC > 1). Data are shown as the mean ± S.D. (error bars) and were analyzed for significant differences by one-way ANOVA using Bonferroni's multiple comparisons post hoc tests. * represents p < 0.05, and **** represents p < 0.0001. Subject numbers for each group are shown above the x axis within the figures.
Discussion
The molecular processes that influence how cells within the respiratory tract respond to inhaled PM are not fully understood. Evidence from this study further supports a role for TRPV1 in regulating cellular responses to particulate air pollutants and ultimately asthma.
Fig. 1, A and B, show that the TRPV1-I315M, -T469I, and -I585V variants were differentially activated by capsaicin and CFA. The I315M and T469I variants were more sensitive to capsaicin and CFA, whereas the I585V variant was less responsive to CFA PM; these trends persisted at multiple doses of CFA (Fig. 1C). Fig. 2A shows that in transiently transfected HEK-293 cells the level of mRNA expression for the T469I and I585V variants was higher than that of wild-type TRPV1 and I315M, but Fig. 2B shows that the expression of I585V and T469I protein was lower than either wild-type TRPV1 or I315M. Furthermore, co-expression of TRPV1-I585V with wild -type TRPV1 slightly decreased overall expression despite overexpression of TRPV1 mRNA (Fig. 2, A and C).
Thr-469 resides on extracellular loop 1, and based on the mRNA and protein expression data, the T469I variant appears to be inherently more responsive to TRPV1 agonists via a yet undetermined mechanism. This same conclusion can be made for I315M. Ile-315 resides within ankyrin domain 5, and ankyrin repeat domains in TRP channels are believed to play roles in TRP channel gating through interactions with cytoskeletal proteins and other regulatory molecules/processes (46). Intracellular interactions involving the ankyrin domains may be particularly relevant to mechanical/physical stimuli such as CFA. For example, we have shown previously that SNPs within and surrounding the N-terminal ankyrin repeat domains of TRPA1 variably affected activation by both soluble and insoluble agonists with the gain-of function SNPs R3C and R58T correlating with poorer asthma control (8). We speculate that I315M may also increase TRPV1 activity through unique intracellular interactions that can affect channel gating and ultimately asthma control (Figs. 6B and 8B).
The mechanistic basis for decreased responses of the I585V variant to CFA was also explored. Fig. 2, A and B, show that I585V protein was expressed at lower levels in transfected HEK cells compared with wild-type TRPV1 despite elevated mRNA expression. Furthermore, I585V suppressed (∼10%) wild-type TRPV1-GFP expression as measured by flow cytometry; similar effects were observed in NHBE cells heterozygous for the I585V variant (Fig. 5, B–D). As such, the functional differences between I585V and wild-type TRPV1 in Figs. 1, 3, and 4 could be explained by a reduction in TRPV1 protein expression. However, this conclusion is not fully supported by results showing that wild-type TRPV1 and I585V (and I585I/V in wild type/I585V-co-transfected cells) were comparably activated by a maximum stimulating concentration of capsaicin. Furthermore, the degree of TRPV1 protein reduction associated with expression of both wild-type TRPV1 and I585V or with I585V alone did not directly correlate with the degree of functional losses observed across multiple assays. Thus, I585V also appears to be inherently less sensitive to CFA, and the effect of the I585V substitution on TRPV1 activation by CFA may ultimately be multifaceted. First, Ile-585 resides on what cumulative results suggest is a mechanically sensitive helical component of the ion pore based on similar decreases in TRPV1 activation by CFA by mutation of C578 to alanine (12). Second, the helical segment containing both Cys-578 and Ile-585 resides between transmembrane (TM) segments 4 and 5 and has been suggested to engage in subunit assembly and allosteric modulation of channel gating (47). Specifically, this linker may form critical intrasubunit interactions with the TM5-loop-TM6 pore region, which is involved in functional coupling through weak trans-subunit interactions that regulate gating of the pore (48); this feature appears to be particularly important for TRPV1 activation by CFA. Third, Ile-585 substitution appears to decrease overall TRPV1 protein expression including perhaps that of wild-type TRPV1.
Treatment of human lung epithelial cells with TRPV1 antagonists has been shown to promote higher overall and greatly increased cell surface expression of TRPV1 in lung epithelial cells (49). This effect was associated with increased sensitivity of cells to agonists (calcium flux), exacerbated cytotoxicity, and increased IL-6 and IL-8 gene induction. Others have shown that TRPV1 agonists promote rapid internalization and lysosomal degradation of cell surface TRPV1 (27). This latter “desensitization” process was calcium-dependent, was inhibited by TRPV1 antagonists, and was not observed with a non-functional “pore-less” TRPV1 mutant. This latter process is believed to be a mechanism by which TRPV1 activity is “fine-tuned” and becomes desensitized to a broad array of stimuli. Based on these collective data and results herein, TRPV1 expression/function appears to be dynamically regulated by cells in response to changes in TRPV1 activity. Fig. 3, A and B, show in HEK-293 cells that TRPV1-WT-GFP undergoes rapid internalization into vesicles following stimulation with capsaicin. However, with CFA, significantly less TRPV1 internalization occurred even with 2 h of treatment (Fig. 3C) due presumably to the fact that CFA is a much weaker agonist of TRPV1. However, in both cases, co-transfection of TRPV1-WT-GFP with I585V suppressed TRPV1 internalization with a greater effect on CFA-induced internalization due presumably to its inhibitory effect on wild-type TRPV1 expression and decreased sensitivity to CFA. These data suggest that TRPV1 could exist in greater abundance at the surface of lung cells expressing the I585V variant. Accordingly, co-expression of the I585V variant could alter how cells typically respond to insoluble TRPV1 agonists such as PM.
Cytokine and chemokine gene induction by lung epithelial cells treated with TRPV1 agonists has been primarily associated with activation of TRPV1 at the cell surface, and this association was stronger for CFA than capsaicin, which can also induce IL-8 through endoplasmic reticulum stress (12, 20, 23). It was hypothesized that CFA-induced IL-8 secretion by bronchial epithelial cells would vary as a function of Ile-585 genotype. Fig. 4, A and B, show that IL-8 secretion and mRNA induction were lower among NHBE cells from donors heterozygous for TRPV1-I585I/V using both capsaicin and CFA as agonists. These data agree with findings that the I585V and heteromeric I585I/V channels are less responsive to CFA but did not show selectivity for CFA and did not support the hypothesis that I585V might promote cell surface TRPV1 enrichment or an overall increase in TRPV1 expression as an explanation for the effects of I585I/V on asthma. Rather these data support the finding that I585V may interfere with wild-type TRPV1 expression when co-expressed. Accordingly, confocal immunofluorescence staining and Western blotting analysis of TRPV1 in these same donor cells (Fig. 5, C and D) revealed that I585I/V cells expressed lower levels of TRPV1 compared with the wild type-expressing cells. These data collectively imply that a combination of inherent functional changes in the TRPV1-I585I/V protein and a decrease in the efficiency of TRPV1 translation and/or folding into an active proteins likely drive the change in IL-8 induction by the I585V protein.
The impact of TRPV1 variants on asthma control was also explored using samples obtained from children with asthma from the Salt Lake City, UT area. A prior study investigating the effect of TRPV1 SNPs on asthma symptom frequency (i.e. cough and wheeze) demonstrated that the I585V variant was ∼20–30% less responsive to temperature and capsaicin (42), similar to what we show for CFA. Furthermore, I585V expression was correlated with lower odds ratios for active wheezing (OR = 0.51, p = 0.01) and cough (OR = 0.57, p = 0.02) in Spanish children with asthma (42). Smit et al. (43) also reported that the I585V variant was associated with a decrease in cough in asthmatics (OR = 0.62, p = 0.03) but that this effect was not present in a pooled analysis of cough in a more heterogeneous cohort.
The cumulative biochemical results of this study provide molecular insights for how the I585V variant may reduce asthma symptom frequency. However, results from our cohort study (Figs. 6–8) correlated I315M and I585V expression with poorer asthma control. The findings on I315M were novel and perhaps intuitive because I315M was expressed comparably with wild-type TRPV1 and was more sensitive to both capsaicin and CFA, which could increase TRPV1 activity in the airways. In this regard, the lack of effect of T469I on asthma may suggest that lower levels of protein expression may counteract the apparent increase in sensitivity to agonists. However, the differences for I585V were puzzling. We could not directly compare many factors that could possibly explain the disparity on the I585I/V and I585V genotypes between this study and that of Cantero-Recasens et al. (42), but there were no differences in the minor/major allelic frequencies between studies, and thus genotype enrichment was not a factor.
We propose that regional differences in air pollutants could be involved. The composition of air pollution in Barcelona and Sabadell, Spain is consistently high and primarily composed of traffic-related PM (50, 51). Salt Lake City area PM tend to be a mixture of traffic- and geologically derived PM with the relative and absolute levels of different types of PM varying by season and weather. There are six active coal burning power plants in Utah including one just west of Salt Lake City. The CFA used in this study was collected from an active coal-burning power plant in Utah and shares mineral composition similar to regional geological dusts that dominate during dry months. However, winter high pollution events concentrate PM and are enriched in traffic-related and wood smoke PM, which are believed to have a greater impact on asthma.
We and others have found that traffic-related, wood smoke, and similar combustion-derived PM preferentially activate TRPA1 (8, 12). TRPA1 agonists, which would be consistently abundant in air pollution in Barcelona and Sabadell, Spain, may continually activate TRPA1/TRPV1-expressing C-fibers promoting cough, but eventual “pharmacological” cross-desensitization of both TRPA1 and TRPV1 would occur (13, 52). Thus, cross-desensitization of I585V, which is already less active to agonists such as CFA and similar PM, could manifest as a reduction in sensory neuron activity, cough, and asthma severity. Similarly, in cases where TRPA1 may be expressed by epithelial cells (e.g. people with the I585I/V genotype), exposure to TRPA1 agonists may also promote cross-desensitization, leading to an overall reduction in epithelial responses to PM and their contributions to asthma/lung homeostasis.
However, Salt Lake City area PM likely contain more direct acting TRPV1 agonists that can engage both C-fibers and epithelial cells via both TRPV1 and TRPA1 (if expressed) with the relative balance determined by the local environmental conditions at a given time. As such, the combination of increased expression of TRPA1 by epithelial cells expressing the TRPV1-I585I/V variant (Fig. 5, A and E), attenuated desensitization of I585I/V by agonists, and reduced internalization/desensitization of TRPA1 due to reduced TRPV1-I585V internalization (13, 52) could collectively exacerbate responses to environmental irritants and affect asthma. In particular, the expression of TRPA1 by epithelial cells may render these cells more sensitive to cyclical increases in combustion-derived PM in air and/or other similar irritants, promoting the expression of multiple epithelially derived proinflammatory cytokines, such as IL-8, via both TRPV1 (12) and TRPA1 (28). Interestingly, IL-8 has been reported to be more abundant among severe, steroid-resistant asthmatics with “neutrophilic asthma” endotype (14).
In summary, this study identified Ile-315, T469I, and Ile-585 as particle-sensitive components of TRPV1. Furthermore, the I585V variant affected several typical cellular responses to PM and capsaicin, the expression of TRPA1, and ultimately asthma control. Establishing more exact mechanistic links among local air pollution composition, TRPA1, TRPV1, TRPV1 variants, and asthma clearly requires more sophisticated studies for full understanding. Nonetheless, we suggest that assessing TRPV1 genetics and TRPA1 and -V1 channel expression may be useful in future particle toxicology studies as well as in identifying asthma subpopulations with particular sensitivity toward specific asthma triggers including local air pollutants. Such information could provide a more comprehensive understanding of a patient's asthma and consequently support more effective personalized interventions and therapies.
Materials and Methods
Reagents
Capsaicin, ruthenium red, EGTA, and all other chemicals were purchased from Sigma-Aldrich unless otherwise specified. CFA, also referred to as CFA1 in prior publications by our group (7, 12), is a size-fractionated (<30 μm) sample collected from a power plant in Castle Dale, Emery County, UT as described elsewhere (53).
Cell Culture
Cells were maintained in a humidified cell culture incubator at 37 °C with a 95% air, 5% CO2 atmosphere. HEK-293 cells (ATCC, Manassas, VA) were cultured in DMEM/F-12 containing 5% fetal bovine serum and 1× penicillin/streptomycin. NHBE cells (Lonza, Walkersville, MD) were cultured in bronchial epithelial growth medium for four to six passages as recommended by the manufacturer. NHBE cells from multiple donors were obtained and genotyped using TaqMan Genotyping Master Mix for TRPV1-I585V (rs8065080) (Life Technologies). Two separate donor cell sources for wild type and heterozygotes for TRPV1-I585V were identified and used. The donor identification numbers were 14359 and 14664 (wild-type TRPV1) and 9853 and unknown (TRPV1-I585I/V).
Cloning and Site-directed Mutagenesis
Human TRPV1 was cloned as described previously (21). Plasmids for the non-synonymous TRPV1 SNPs K2N (rs9894618), P91S (rs222749), I315M (rs222747), T469I (rs224534), and T505A (rs17633288) were generously provided by Dr. David M. Cohen of the Oregon Health Sciences University (44). Construction of the TRPV1-I585V (rs8065080) variant plasmid was achieved using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) (7, 12). Wild-type TRPV1 also was subcloned into the pEGFP-C2 (TRPV1-GFP) vector at the HindIII and XbaI sites for use in internalization/desensitization and flow cytometry experiments.
Quantification of TRPV1 Function (Calcium Assay)
HEK-293 cells were plated in 96-well plates precoated with 1% gelatin and grown to 80–90% confluence. Transient transfection of HEK-293 cells with TRPV1 plasmids was achieved using Lipofectamine 2000 (Life Technologies). Transfection efficiency, relative mRNA expression, and protein expression were compared with wild type for all variants. Cells were assayed for TRPV1 function (calcium flux) using the Fluo-4 Direct assay kit (Life Technologies). Treatment-induced changes in cellular fluorescence were quantified from fluorescence micrographs as described previously (7, 9, 12). Briefly, the average value for the change in fluorescence was corrected for background changes, normalized to the maximum attainable response elicited by ionomycin (10 μm), and represented as a percentage of TRPV1-WT or the positive control (capsaicin). All agonist treatment solutions were prepared in LHC-9 medium at 3× concentration and added to cells at room temperature. Particle suspensions were prepared and applied to cells using an area dose at 180 μg/cm2. It should be noted that the particle suspension concentration used (2.3 mg/ml; equivalent to 180 μg/cm2 if all particles settled out of solution) does not indicate the actual cellular dose of PM, which varies over the course of the 3-min assay as particles settle. The actual concentration of particles on cells during the course of the functional assays was substantially lower (maximum possible amount of 10–20 ng/cell assuming a cell area of ∼50–100 μm2 but typically <5–10 visible particles/cell), which plausibly approaches inhalation exposures.
Assessment of TRPV1 and IL-8 mRNA Expression
HEK-293 cells were cultured in 25-cm2 flasks, grown to 95% density, and transiently transfected with equal quantities (5.2 μg) of the TRPV1 plasmids using Lipofectamine 2000 (lipid to DNA ratio of 2:1) for 24 h. Cells were recovered in growth medium for 24 h. 48 h after transfection, cells were harvested. For PCR, cells were collected using trypsin, spun, and rinsed two times with cold PBS before RNA isolation. NHBEs cells from the four donor samples were plated into 12-well plates and treated with either vehicle; 50 or 75 μm capsaicin; or 45, 90, or 180 μg/cm2 CFA for 24 h. After treatment, the cell culture medium was removed, and cells were washed and frozen at −80 °C until RNA isolation. For both cell types, total RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) with on-column DNase digestion, and 2.5 μg of the total RNA was converted to cDNA using iScript (Bio-Rad). The resulting cDNA was diluted 1:20 for analysis by quantitative real time PCR using a Life Technologies QuantStudio 6 instrument and the TaqMan probe-based assays for human TRPV1 (assay Hs.00218912_m1) and IL-8 (assay Hs00174103_m1). Values for TRPV1 and IL-8 were normalized to β2-microglobulin (assay Hs.00984230_m1).
Assessment of TRPV1 Protein Expression
For Western blotting, protein was isolated from the cells using radioimmune precipitation assay lysis buffer (Pierce) supplemented with Halt protease inhibitor mixture (Thermo Fisher), sonicated (50% amplitude) 3 × 2 s, and clarified by centrifugation at 13,000 × g for 5 min. Protein concentration was measured using the BCA assay (Pierce). As a positive control, cell surface protein isolates from TRPV1-overexpressing BEAS-2B cells pretreated with the TRPV1 antagonist LJO-328, which we have previously shown to enrich TRPV1 at the cell surface (12, 49), were used. TRPV1 protein expression was assayed using a TRPV1 rabbit polyclonal antibody (ab3487, lot GR210703-16, Abcam (1:1000)) and a donkey anti-rabbit HRP secondary antibody (NA934VS, lot 9653375, GE Healthcare (1:10,000)) and normalized to total protein. Total protein was measured by poststaining the PVDF membrane with 0.1% Amido Black. Band densities were quantified using ImageJ software. Validation of the TRPV1 antibody consisted of reduced antibody staining in BEAS-2B versus TRPV1-overexpressing BEAS-2B cells, enrichment of TRPV1 immunoreactivity in cell surface protein isolates in the LJO-328-pretreated cells, and a lack of staining in naïve HEK-293 cells. By Western blotting, the TRPV1 band was ∼75 kDa.
Immunocytochemistry
NHBE cells were plated on flame- and alcohol-sterilized glass coverslips coated with Lechner and LaVeck LHC basal medium fortified with 30 μg/ml collagen, 10 μg/ml fibronectin, and 10 μg/ml bovine serum albumin. After 48 h, cells were fixed with 4% paraformaldehyde and treated with 0.1% Triton X-100. Nonspecific antibody binding was blocked using 10% goat serum (Thermo Fisher) for 1 h at room temperature, and subsequently cells were incubated with the TRPV1 rabbit polyclonal antibody (ab3487, lot GR210703-16, Abcam (1:200 in goat serum)) overnight followed by the Alexa Fluor 488-labeled goat anti-rabbit detection antibody for 1 h (ab150077, lot GR243699-1, Abcam (1:500)) at room temperature. Cells were postfixed with 4% paraformaldehyde, and the nucleus was stained with Hoechst 33342 (Molecular Probes). Cells were imaged by a 40× oil immersion lens on a Nikon A1R confocal microscope, imaging the depth of the cell across z-planes and keeping all imaging parameters (gain, offset, and laser) constant during imaging to allow for comparisons among test groups. TRPV1 staining appeared as small punctate foci. Images were flattened over the z-planes, and the number of punctate foci/cell was counted using ImageJ. Data are represented as the number of TRPV1-positive foci/cell.
Assessment of TRPV1 Internalization/Desensitization
The relative level and location of TRPV1 protein expressed in HEK-293 cells were studied using a C-terminal enhanced GFP-tagged TRPV1 construct (TRPV1-WT-GFP). Subcellular localization of TRPV1-WT-GFP co-expressed with TRPV1-WT or TRPV1-I585V was assessed using confocal microscopy. Cells were plated into gelatin-coated 8-well coverslip chamber slides and grown to confluence before transient transfection with TRPV1-WT/TRPV1-WT-GFP or TRPV1-I585V/TRPV1-WT-GFP. After 48 h, cells were stained with Hoechst 33342 (Molecular Probes) in LHC-9 medium, and cellular internalization of TRPV1-GFP in response to 1 μm capsaicin or 180 μg/cm2 CFA treatment was imaged over time. During experimentation, cells were maintained at 37 °C with 5% CO2 using an environmental chamber. Cell imaging was performed utilizing a 40× oil immersion lens on a Nikon A1R confocal microscope. Images were taken in one z-plane, utilizing perfect focus, every minute for up to 2 h. Images were processed using NIS elements acquisition software (Nikon). Internalization was quantified by visual inspection of de-identified time lapse images, counting any cell exhibiting a time-dependent increase in intracellular GFP-stained vesicles, with data represented as the percentage of the total number of cells counted (i.e. Hoechst 33342-stained nuclei).
Flow Cytometry
HEK-293 cells were seeded in 6-well plates, grown to 95% density, and transiently transfected as described above with the TRPV1 plasmids TRPV1-WT and TRPV1-WT-GFP or TRPV1-I585V and TRPV1-WT-GFP. 48 h after transfection, cells were trypsin-dissociated, pelleted, and washed with ice-cold PBS. The final pellet was resuspended in Opti-MEM I and incubated with propidium iodide for 15 min to determine live/dead cells for exclusion. Cells (≥20,000 events/sample) were analyzed on a Cell Lab Quanta flow cytometer (Beckman Coulter, Fullerton, Ca). The percentage of GFP-positive cells, indicative of TRPV1-positive live cells, was monitored with data expressed as the percentage of live cells expressing GFP.
Assessment of CFA-induced Chemokine Production
NHBE cells from four donors, two TRPV1-WT and two TRPV1-I585I/V, were treated with either vehicle; 50 or 75 μm capsaicin; or 45, 90, or 180 μg/cm2 CFA for 24 h. Medium was collected, clarified by centrifugation, and assayed for IL-8 using ELISA according to the manufacturer's recommendations (R&D Systems, Minneapolis, MN).
RNA Sequencing
RNA sequencing was performed by the High Throughput Genomics Core Facility at the Huntsman Cancer Institute, University of Utah. NHBE cells from the four donors (two TRPV1-WT and two TRPV1-I585I/V) treated with CFA and nonivamide were harvested, and total RNA was isolated as described above. RNA quality was assessed by RNA nanochip technology, and library construction was performed using the Illumina TruSeq Stranded mRNA Sample Preparation kit using established protocols. The sequencing libraries (18 pm) were then chemically denatured and applied to an Illumina TruSeq v3 single read flow cell using an Illumina cBot. Hybridized molecules were clonally amplified and annealed to sequencing primers with reagents from an Illumina TruSeq SR Cluster kit, v3-cBot-HS (GD-401-3001). Following transfer of the flow cell to an Illumina HiSeq instrument (HCS v2.0.12 and RTA v1.17.21.3), a 50-cycle single read sequence run was performed using TruSeq SBS v3 sequencing reagents (FC-401-3002). Data were processed by the University of Utah Bioinformatics core. The data presented in this publication have been deposited in NCBI's Gene Expression Omnibus (57) and are accessible through GEO Series accession number GSE85447(www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE85447).
Patient DNA Sample Collection and Purification
Saliva samples were obtained prospectively from 1569 children 2–17 years of age with a physician-confirmed diagnosis of asthma as part of a prior study. Briefly, subjects with a primary diagnosis of asthma were recruited from the emergency department and inpatient wards of Primary Children's Hospital in Salt Lake City, UT (45). Information about chronic medical conditions, concomitant medication use, and chief complaints were collected through a combination of structured interviews and medical chart abstraction. Data were de-identified before genotype analysis. Characteristics of the patient population studied are provided in Table 1. Oragene DNA kits (DNA Genotek Inc., Ottawa, Ontario, Canada) were used to collect 2 ml of saliva/patient. DNA was extracted as described previously (45).
Characterization of Asthma Phenotypes
National Institutes of Health Asthma Control Classification
Each patient's level of chronic asthma control was determined by a questionnaire based upon guidelines modified from National Heart Lung and Blood Institute Expert Panel Report 3 (54) as described previously (8, 16, 43). In this study, asthma control scores were analyzed as a numeric variable that could range from 0 (ideally controlled) to 15 (poorly controlled). Selected criteria scores (values = 0 (ideally controlled) to 3 (poorly controlled)) were also evaluated separately to isolate any differential effects on individual measures of asthma control.
Longitudinal Asthma Control Classification
A subset of the cohort (74 patients) consisting of individuals with more severe asthma (Table 1) and who actively participate in a real time asthma symptom self-monitoring program, referred to as the eAT (55, 56), were also evaluated separately. The eAT uses a weekly questionnaire to longitudinally classify patients as well, not well, or poorly controlled and collects medication compliance and exposure data for environmental triggers. Patients using the eAT system were enrolled during hospitalization for poor asthma control but soon achieved substantially improved and consistent control (55, 56), making this a unique subpopulation to assess the effects of TRPV1 variation on symptom control.
SNP Genotyping
SNP genotyping was performed using custom TaqMan Open Array cards (Life Technologies) at the University of Utah Genomics core facility. Prior to genotyping, 4 ng of purified genomic DNA was amplified using a custom TaqMan PreAmp Mastermix (Life Technologies). TaqMan probe-based SNP genotyping assays for Q85R (rs55916885), P91S (rs222749), R95G (rs56016548), N412D (rs61753456), I315M (rs222747), T401A (rs61751059), T469I (rs224534), T505A (rs17633288), I585V (rs8065080), and several other asthma-associated gene SNPs were contained on the array cards. TaqMan reactions were cycled as recommended by the manufacturer on a Life Technologies QuantStudio 12K instrument. Data clustering analysis was performed using TaqMan Genotyper software (Life Technologies). Only SNPs with minor allelic frequencies greater than 1% and with functional changes (Fig. 1) were further evaluated for an impact on asthma.
Statistical Analysis
Data are represented as the mean ± S.E. One-way or two-way ANOVA with post-testing at the 95% confidence interval was used to determine significance as specified in each figure legend. Analysis was performed using GraphPad 5.0 software (GraphPad Inc., San Diego, CA). OR calculations were performed using an online calculator in MedCalc, and logistic and ordinal logistic regression models were also used to compare asthma measures as a function of TRPV1 genotypes in R 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Author Contributions
C. E. D.-R. designed, performed, and analyzed the experiments shown in Figs. 3–5 and wrote the paper. E. G. R. and D. S. performed and analyzed the experiments shown in Figs. 1 and 2. Z. L. performed and analyzed the experiments shown in Figs. 2, 4, and 5. C. S. designed experiments, performed statistical analyses, and wrote the paper. C. S., B. L. S., B. F., F. N., D. A. U., and R. M. W. diagnosed, recruited, obtained consent, and collected patient DNA and asthma phenotype data in Figs. 6–8 and Tables 1 and 2. J. M. V. collected and characterized the coal fly ash material and participated in experimental design and writing the manuscript. C. A. R. obtained funding, conceived and coordinated the study, and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
Imaging was performed at the Fluorescence Microscopy Core Facility, a part of the Health Sciences Cores at the University of Utah. Microscopy equipment was purchased using National Center for Research Resources NCRR Shared Equipment Grant 1S10RR024761-01.
This work was supported by National Institutes of Health NIEHS Grant R01 ES017431 and Eunice Kennedy Shriver NICHD Grant R01 HD060559 and by the University of Utah Department of Pediatrics. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CFA
- coal fly ash
- PM
- particulate materials
- TRP
- transient receptor potential
- TRPV1
- transient receptor potential vanilloid-1
- TRPA1
- transient receptor potential ankyrin-1
- NHBE
- normal human bronchial epithelial
- OR
- odds ratio
- GC
- glucocorticoid
- TM
- transmembrane
- eAT
- electronic Asthma Tracker
- ANOVA
- analysis of variance.
References
- 1. Allen R. W., Mar T., Koenig J., Liu L. J., Gould T., Simpson C., and Larson T. (2008) Changes in lung function and airway inflammation among asthmatic children residing in a woodsmoke-impacted urban area. Inhal. Toxicol. 20, 423–433 [DOI] [PubMed] [Google Scholar]
- 2. Epton M. J., Dawson R. D., Brooks W. M., Kingham S., Aberkane T., Cavanagh J. A., Frampton C. M., Hewitt T., Cook J. M., McLeod S., McCartin F., Trought K., and Brown L. (2008) The effect of ambient air pollution on respiratory health of school children: a panel study. Environ. Health. 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iskandar A., Andersen Z. J., Bønnelykke K., Ellermann T., Andersen K. K., and Bisgaard H. (2012) Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax 67, 252–257 [DOI] [PubMed] [Google Scholar]
- 4. Jacquemin B., Sunyer J., Forsberg B., Aguilera I., Bouso L., Briggs D., de Marco R., García-Esteban R., Heinrich J., Jarvis D., Maldonado J. A., Payo F., Rage E., Vienneau D., and Künzli N. (2009) Association between modelled traffic-related air pollution and asthma score in the ECRHS. Eur. Respir. J. 34, 834–842 [DOI] [PubMed] [Google Scholar]
- 5. McConnell R., Islam T., Shankardass K., Jerrett M., Lurmann F., Gilliland F., Gauderman J., Avol E., Künzli N., Yao L., Peters J., and Berhane K. (2010) Childhood incident asthma and traffic-related air pollution at home and school. Environ. Health Perspect. 118, 1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Portnov B. A., Reiser B., Karkabi K., Cohen-Kastel O., and Dubnov J. (2012) High prevalence of childhood asthma in Northern Israel is linked to air pollution by particulate matter: evidence from GIS analysis and Bayesian model averaging. Int. J. Environ. Health Res. 22, 249–269 [DOI] [PubMed] [Google Scholar]
- 7. Deering-Rice C. E., Romero E. G., Shapiro D., Hughen R. W., Light A. R., Yost G. S., Veranth J. M., and Reilly C. A. (2011) Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem. Res. Toxicol. 24, 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deering-Rice C. E., Shapiro D., Romero E. G., Stockmann C., Bevans T. S., Phan Q. M., Stone B. L., Fassl B., Nkoy F., Uchida D. A., Ward R. M., Veranth J. M., and Reilly C. A. (2015) Activation of TRPA1 by insoluble particulate material and association with asthma. Am. J. Respir. Cell Mol. Biol. 53, 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shapiro D., Deering-Rice C. E., Romero E. G., Hughen R. W., Light A. R., Veranth J. M., and Reilly C. A. (2013) Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 26, 750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinman A., Chuang H.-H., Bautista D. M., and Julius D. (2006) TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., and Patapoutian A. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 [DOI] [PubMed] [Google Scholar]
- 12. Deering-Rice C. E., Johansen M. E., Roberts J. K., Thomas K. C., Romero E. G., Lee J., Yost G. S., Veranth J. M., and Reilly C. A. (2012) Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material. Mol. Pharmacol. 81, 411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akopian A. N., Ruparel N. B., Jeske N. A., and Hargreaves K. M. (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J. Physiol. 583, 175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes P. J. (2008) The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 118, 3546–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veronesi B., Wei G., Zeng J. Q., and Oortgiesen M. (2003) Electrostatic charge activates inflammatory vanilloid (VR1) receptors. Neurotoxicology 24, 463–473 [DOI] [PubMed] [Google Scholar]
- 16. Stockmann C., Reilly C. A., Fassl B., Gaedigk R., Nkoy F., Stone B., Roberts J. K., Uchida D. A., Leeder J. S., Sherwin C. M., Spigarelli M. G., Yost G. S., and Ward R. M. (2015) Effect of CYP3A5*3 on asthma control among children treated with inhaled beclomethasone. J. Allergy Clin. Immunol. 136, 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bessac B. F., Sivula M., von Hehn C. A., Escalera J., Cohn L., and Jordt S.-E. (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 118, 1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agopyan N., Li L., Yu S., and Simon S. A. (2003) Negatively charged 2- and 10-microm particles activate vanilloid receptors, increase cAMP, and induce cytokine release. Toxicol. Appl. Pharmacol. 186, 63–76 [DOI] [PubMed] [Google Scholar]
- 19. Oortgiesen M., Veronesi B., Eichenbaum G., Kiser P. F., and Simon S. A. (2000) Residual oil fly ash and charged polymers activate epithelial cells and nociceptive sensory neurons. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L683–L695 [DOI] [PubMed] [Google Scholar]
- 20. Reilly C. A., Johansen M. E., Lanza D. L., Lee J., Lim J. O., and Yost G. S. (2005) Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J. Biochem. Mol. Toxicol. 19, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reilly C. A., Taylor J. L., Lanza D. L., Carr B. A., Crouch D. J., and Yost G. S. (2003) Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol. Sci. 73, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas K. C., Roberts J. K., Deering-Rice C. E., Romero E. G., Dull R. O., Lee J., Yost G. S., and Reilly C. A. (2012) Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L111L119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas K. C., Sabnis A. S., Johansen M. E., Lanza D. L., Moos P. J., Yost G. S., and Reilly C. A. (2007) Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J. Pharmacol. Exp. Ther. 321, 830–838 [DOI] [PubMed] [Google Scholar]
- 24. Veranth J. M., Cutler N. S., Kaser E. G., Reilly C. A., and Yost G. S. (2008) Effects of cell type and culture media on Interleukin-6 secretion in response to environmental particles. Toxicol. in Vitro 22, 498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veranth J. M., Reilly C. A., Veranth M. M., Moss T. A., Langelier C. R., Lanza D. L., and Yost G. S. (2004) Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol. Sci. 82, 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veronesi B., Carter J. D., Devlin R. B., Simon S. A., and Oortgiesen M. (1999) Neuropeptides and capsaicin stimulate the release of inflammatory cytokines in a human bronchial epithelial cell line. Neuropeptides 33, 447–456 [DOI] [PubMed] [Google Scholar]
- 27. Sanz-Salvador L., Andrés-Borderia A., Ferrer-Montiel A., and Planells-Cases R. (2012) Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 287, 19462–19471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nassini R., Pedretti P., Moretto N., Fusi C., Carnini C., Facchinetti F., Viscomi A. R., Pisano A. R., Stokesberry S., Brunmark C., Svitacheva N., McGarvey L., Patacchini R., Damholt A. B., Geppetti P., et al. (2012) Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One 7, e42454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee L. Y., and Gu Q. (2009) Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr. Opin. Pharmacol. 9, 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meseguer V., Alpizar Y. A., Luis E., Tajada S., Denlinger B., Fajardo O., Manenschijn J.-A., Fernández-Peña C., Talavera A., Kichko T., Navia B., Sánchez A., Señarís R., Reeh P., Pérez-García M. T., et al. (2014) TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caceres A. I., Brackmann M., Elia M. D., Bessac B. F., del Camino D., D'Amours M., Witek J. S., Fanger C. M., Chong J. A., Hayward N. J., Homer R. J., Cohn L., Huang X., Moran M. M., and Jordt S.-E. (2009) A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U.S.A. 106, 9099–9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dicpinigaitis P. V. (2001) Capsaicin responsiveness in asthma and COPD. Thorax 56, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doherty M. J., Mister R., Pearson M. G., and Calverley P. M. (2000) Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55, 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujimura M., Kamio Y., Hashimoto T., and Matsuda T. (1998) Airway cough sensitivity to inhaled capsaicin and bronchial responsiveness to methacholine in asthmatic and bronchitic subjects. Respirology 3, 267–272 [DOI] [PubMed] [Google Scholar]
- 35. Nieto L., de Diego A., Perpiñá M., Compte L., Garrigues V., Martínez E., and Ponce J. (2003) Cough reflex testing with inhaled capsaicin in the study of chronic cough. Respir. Med. 97, 393–400 [DOI] [PubMed] [Google Scholar]
- 36. McGarvey L. P., Butler C. A., Stokesberry S., Polley L., McQuaid S., Abdullah H., Ashraf S., McGahon M. K., Curtis T. M., Arron J., Choy D., Warke T. J., Bradding P., Ennis M., Zholos A., et al. (2014) Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 133, 704.e4–712.e4 [DOI] [PubMed] [Google Scholar]
- 37. McGarvey L. P., Butler C. A., Stokesberry S., Polley L., McQuaid S., Abdullah H., Ashraf S., McGahon M. K., Curtis T. M., Arron J., Choy D., Warke T. J., Bradding P., Ennis M., Zholos A., Costello R. W., Heaney L. G. (2014) Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 133, 704–712 [DOI] [PubMed] [Google Scholar]
- 38. Groneberg D. A., Niimi A., Dinh Q. T., Cosio B., Hew M., Fischer A., and Chung K. F. (2004) Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am. J. Respir. Crit. Care Med. 170, 1276–1280 [DOI] [PubMed] [Google Scholar]
- 39. Chen C. L., Li H., Xing X. H., Guan H. S., Zhang J. H., and Zhao J. W. (2015) Effect of TRPV1 gene mutation on bronchial asthma in children before and after treatment. Allergy Asthma Proc. 36, e29–36 [DOI] [PubMed] [Google Scholar]
- 40. Ren Y. F., Li H., Xing X. H., Guan H. S., Zhang B. A., Chen C. L., and Zhang J. H. (2015) Preliminary study on pathogenesis of bronchial asthma in children. Pediatr. Res. 77, 506–510 [DOI] [PubMed] [Google Scholar]
- 41. Rehman R., Bhat Y. A., Panda L., and Mabalirajan U. (2013) TRPV1 inhibition attenuates IL-13 mediated asthma features in mice by reducing airway epithelial injury. Int. Immunopharmacol. 15, 597–605 [DOI] [PubMed] [Google Scholar]
- 42. Cantero-Recasens G., Gonzalez J. R., Fandos C., Duran-Tauleria E., Smit L. A., Kauffmann F., Antó J. M., and Valverde M. A. (2010) Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J. Biol. Chem. 285, 27532–27535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smit L. A., Kogevinas M., Antó J. M., Bouzigon E., González J. R., Le Moual N., Kromhout H., Carsin A. E., Pin I., Jarvis D., Vermeulen R., Janson C., Heinrich J., Gut I., Lathrop M., et al. (2012) Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir. Res. 13, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu H., Tian W., Fu Y., Oyama T. T., Anderson S., and Cohen D. M. (2007) Functional effects of nonsynonymous polymorphisms in the human TRPV1 gene. Am. J. Physiol. Renal Physiol. 293, F1865–F1876 [DOI] [PubMed] [Google Scholar]
- 45. Stockmann C., Fassl B., Gaedigk R., Nkoy F., Uchida D. A., Monson S., Reilly C. A., Leeder J. S., Yost G. S., and Ward R. M. (2013) Fluticasone propionate pharmacogenetics: CYP3A4*22 polymorphism and pediatric asthma control. J. Pediatr. 162, 1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sotomayor M., Corey D. P., and Schulten K. (2005) In search of the hair-cell gating spring: elastic properties of ankyrin and cadherin repeats. Structure 13, 669–682 [DOI] [PubMed] [Google Scholar]
- 47. Latorre R., Zaelzer C., and Brauchi S. (2009) Structure-functional intimacies of transient receptor potential channels. Q. Rev. Biophys. 42, 201–246 [DOI] [PubMed] [Google Scholar]
- 48. Liao M., Cao E., Julius D., and Cheng Y. (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johansen M. E., Reilly C. A., and Yost G. S. (2006) TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells. Toxicol. Sci. 89, 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aguilera I., Guxens M., Garcia-Esteban R., Corbella T., Nieuwenhuijsen M. J., Foradada C. M., and Sunyer J. (2009) Association between GIS-based exposure to urban air pollution during pregnancy and birth weight in the INMA Sabadell Cohort. Environ. Health Perspect. 117, 1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schembari A., Nieuwenhuijsen M. J., Salvador J., de Nazelle A., Cirach M., Dadvand P., Beelen R., Hoek G., Basagaña X., and Vrijheid M. (2014) Traffic-related air pollution and congenital anomalies in Barcelona. Environ. Health Perspect. 122, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Akopian A. N., Ruparel N. B., Patwardhan A., and Hargreaves K. M. (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 28, 1064–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith K. R., Veranth J. M., Kodavanti U. P., Aust A. E., and Pinkerton K. E. (2006) Acute pulmonary and systemic effects of inhaled coal fly ash in rats: comparison to ambient environmental particles. Toxicol. Sci. 93, 390–399 [DOI] [PubMed] [Google Scholar]
- 54. (2007) Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J. Allergy Clin. Immunol. 120, S94–S138 [DOI] [PubMed] [Google Scholar]
- 55. Nkoy F. L., Stone B. L., Fassl B. A., Koopmeiners K., Halbern S., Kim E. H., Poll J., Hales J. W., Lee D., and Maloney C. G. (2012) Development of a novel tool for engaging children and parents in asthma self-management. AMIA Annu. Symp. Proc. 2012, 663–672 [PMC free article] [PubMed] [Google Scholar]
- 56. Nkoy F. L., Stone B. L., Fassl B. A., Uchida D. A., Koopmeiners K., Halbern S., Kim E. H., Wilcox A., Ying J., Greene T. H., Mosen D. M., Schatz M. N., and Maloney C. G. (2013) Longitudinal validation of a tool for asthma self-monitoring. Pediatrics 132, e1554–e1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Edgar R., Domrachev M., and Lash A. E. (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]