Abstract
Proper positioning of neurons is fundamental for brain functions. However, little is known on how adult-born neurons generated in the hilar side of hippocampal dentate gyrus migrate into the granular cell layer. Because class 3 Semaphorins (Sema3) are involved in dendritic growth of these newborn neurons, we examined whether they are essential for cell positioning. We disrupted Sema3 signaling by silencing neuropilin 1 (NRP1) or 2 (NRP2), the main receptors for Sema3A and Sema3F, in neural progenitors of adult mouse dentate gyrus. Silencing of NRP2, but not NRP1, affected cell positioning of adult newborn neurons. Glycogen synthase kinase-3β (GSK3β) knockdown phenocopied this NRP2 silencing-mediated cell positioning defect, but did not affect dendritic growth. Furthermore, GSK3β is activated upon stimulation with Sema3F, and GSK3β overexpression rescued the cell positioning phenotypes seen in NRP2-deficient neurons. These results point to a new role for NRP2 in the positioning of neurons during adult hippocampal neurogenesis, acting via the GSK3β signaling pathway.
Keywords: cell migration, glycogen synthase kinase 3 (GSK-3), hippocampus, neurogenesis, neuron, adult-born neurons, cell positioning, neuropilin
Introduction
Precise cell positioning of migrating neurons during development is essential for the function of neurons, leading to proper wiring of neural circuitry. Although the functional implication of cell positioning in adult newborn neurons is still not well studied, migration of these newborn neurons has been reported. Newborn neurons in adult subventricular zone migrate a long distance through the rostral migratory stream to the olfactory bulb to become granule neurons and periglomerular neurons (1, 2). However, newborn neurons in dentate gyrus (DG)3 only migrate a short distance within the granule cell layer (GCL) (3–5). Although these studies suggested the positioning of adult newborn neurons within the GCL to be important for their connection into the local circuitry with preexisting neurons, the significance and mechanisms of this short distance migration of granule cells within GCL are still largely unknown.
Several proteins involved in regulating adult neurogenesis were implicated in controlling the positioning of newborn granule cells in adult brain. These proteins include GABA receptors (6–8), Disrupted-In-Schizophrenia 1 (DISC1) (3, 9), and NMDA receptor (10), which affect the positioning of newborn neurons within GCL or influence newborn neurons to migrate out into the molecular layer. Although these proteins have been implicated in affecting the positioning of newborn granule cells in adult brains, the upstream and downstream signaling pathways and how these proteins are involved remain to be clarified.
Various intracellular signaling cascades were identified to be essential for neurons to find their correct positions during embryonic and early postnatal development. These include signaling pathways stimulated by extracellular guidance cues such as Reelin, Netrin, and Semaphorin (11). Class 3 Semaphorins are well characterized in the developing nervous system (12). The presence of class 3 Semaphorins and their receptors in the adult hippocampus also implied their importance in adult neuronal development (12). However, the role of class 3 Semaphorins during adult neurogenesis in hippocampus is still largely unknown. We previously found that Sema3A and Sema3F mediate dendritic growth and branching of adult-born neurons (13). Therefore, we asked whether Class 3 Semaphorin signaling regulates cell positioning of newborn neurons in the adult DG. Using retroviral birth-dating and shRNA knockdown approaches, we found that NRP2 but not NRP1 has a specific influence on cell positioning of adult-born neurons in DG through GSK3β.
Results
NRP2 Is Essential for Positioning of Adult Newborn Neurons in GCL
We have previously shown that NRP1 and NRP2 regulate dendritic growth of adult-born neurons in the DG (13). To investigate whether these Semaphorin receptors regulate cell positioning of adult-born neurons in DG, we stereotaxically injected retroviruses encoding for control or NRP1- or NRP2-targeting short hairpin RNAs (shCTR, shNRP1 or shNRP2) into the hippocampus of 6-week-old mice and quantitatively assessed the cell positions of adult-born neurons in DG at 28 days post-infection (28 DPI).
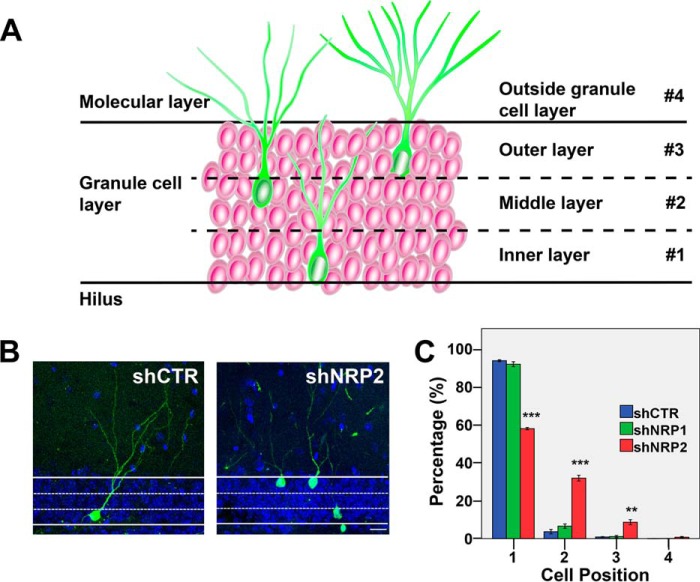
Cell position analysis was carried out to determine the migration pattern of newborn neurons in the adult DG (5). For this cell position analysis, DG was divided into three layers within the GCL (layers 1–3) and the molecular layer (layer 4) as described previously (5) (Fig. 1A). Neurons with the larger portion of their cell bodies positioned within a specific layer will be classified as residing within that particular layer. At 28 DPI, most of the control newborn neurons (shCTR) in the DG (∼90%) reside in the inner two-thirds of the GCL, (Fig. 1, B and C). Similar observations were also demonstrated in previous studies (3–5). However, knocking down NRP2 resulted in newborn neurons residing farther into the GCL (60, 30, and 10% in layers 1–3 of the GCL, respectively) (Fig. 1, B and C). On the other hand, knocking down NRP1 does not affect cell positioning (Fig. 1C). Hence, NRP2, but not NRP1, specifically influences cell positioning of adult-born neurons in the DG.
FIGURE 1.
Neuropilin-2 affects cell positioning of adult-born neurons in the dentate gyrus. A, diagram showing the granule cell layer (#1–3) and the molecular layer (#4) in adult dentate gyrus. B, representative images of shCTR or shNRP2-expressing newborn neurons in the DG at 28 DPI (scale bar, 20 μm). C, quantification of the positioning of adult-born neurons infected with retrovirus of control, shNRP1, or shNRP2. Colored bars represent the percentages of cells of each experimental group in layers 1, 2, and 3 of the granular layer and layer 4 (molecular layer). **, p < 0.01, ***, p < 0.001, as compared with shCTR, one-way ANOVA with Newman-Keuls' post hoc test.
Time Course of NRP2-mediated Neuronal Positioning
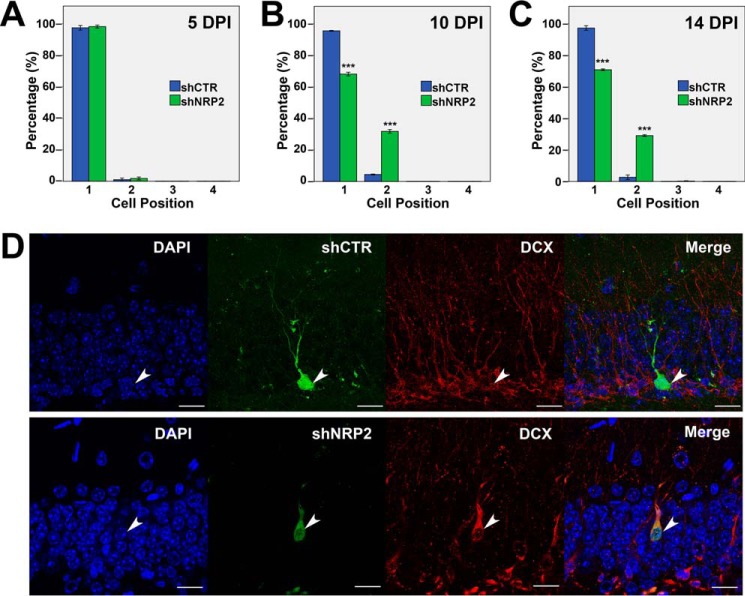
We observed the mispositioning of NRP2 KD neurons at 28 DPI (Fig. 1), but to investigate the temporal changes in neuron positioning during adult neurogenesis, we examined the cell positions of newborn neurons expressing shNRP2 or shCTR in vivo at earlier time points (5, 10, and 14 DPI). Time-course analysis indicated that at 5 DPI, all shCTR- and shNRP2-expressing neurons resided in the subgranular zone or inner granular layer (layer 1) (Fig. 2A). At 10 DPI, 70% of shNRP2-expressing cells are positioned in layer 1 of the GCL, whereas 30% are in layer 2 as compared with the 95 and 5%, respectively, seen in control cells (Fig. 2B). The cell positions of both shCTR-expressing and shNRP2-expressing neurons did not appear to change significantly from 10 DPI to 14 DPI (Fig. 2C). However, more cells were found farther into the GCL toward the molecular layer at 28 DPI (Fig. 1, B and C). Taken together, this time-course analysis showed that the cell positioning of newborn neurons in DG first occurred between 5 and 10 DPI and was likely to continue migrating slowly over time, at least until the last time point (28 DPI) that we examined.
FIGURE 2.
Time-course analysis of cell positioning of shNRP2-expressing newborn neurons. A–C, quantification of cell positioning of adult-born neurons infected with retrovirus at 5 (A), 10 (B), and 14 DPI (C). Colored bars represent the percentages of cells in the four layers as indicated. ***, p < 0.001, Student's t test. D, representative images showing expression of DCX in shCTR or shNRP2-expressing newborn neurons in DG at 14 DPI (scale bar, 20 μm).
To investigate whether the mispositioned neurons (located farther into the GCL) upon NRP2 knockdown are the results of enhanced neuronal maturation, we immunostained brain sections at 14 DPI with the immature neuronal marker, doublecortin (DCX) (Fig. 2D). Similar to shCTR-expressing neurons, shNRP2-expressing mispositioned neurons are still DCX-positive, (Fig. 2D). These results indicated that NRP2 knockdown-mediated cell positioning is not a consequence of enhanced maturation.
Stimulation of NRP2 by Sema3F Activates GSK3β
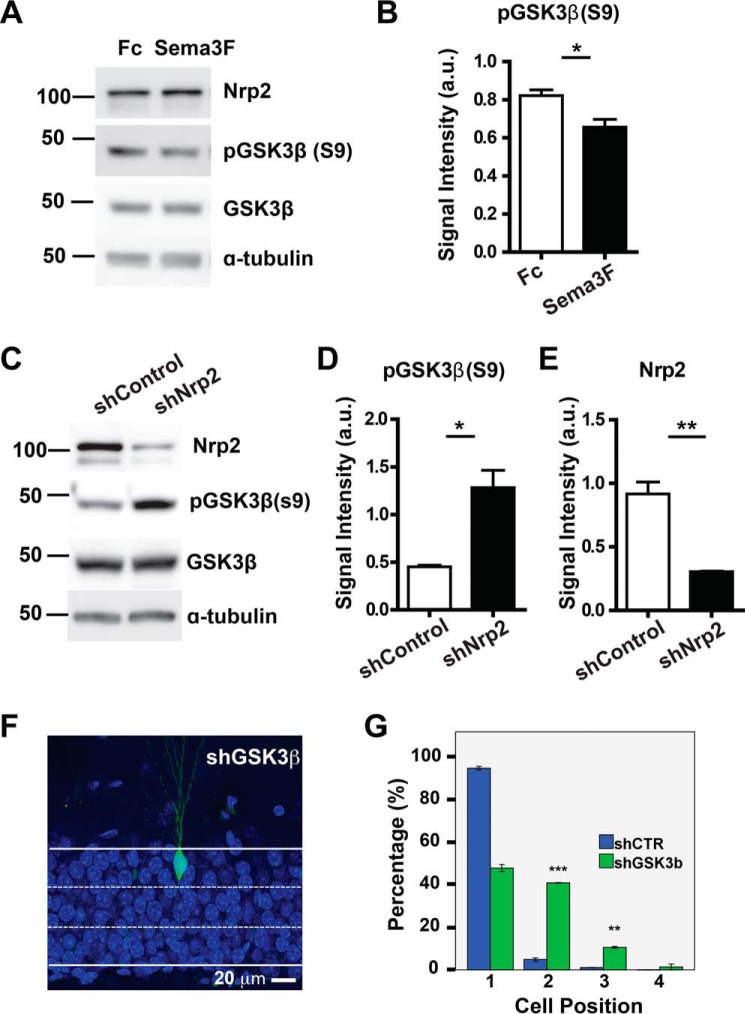
GSK3β regulates many key events during neuronal development (14) and is involved in synaptic plasticity and cognitive functions (15). GSK3β has also been shown to function downstream of Class 3 Semaphorin signaling (16–18) Therefore, we first examined whether GSK3β is activated by NRP2 upon Sema3F stimulation in primary hippocampal neurons. The activity of GSK3β is negatively regulated by phosphorylation at Ser-9 (19, 20). Hence, a decrease or increase, respectively, in phosphorylation at Ser-9 of GSK3β will indicate activation or inactivation of this protein. We found that Sema3F stimulation significantly decreases GSK3β Ser-9 phosphorylation by about 20% and hence activation of GSK3β (Fig. 3, A and B). Sema3F treatment of primary hippocampal neurons did not affect the level of NRP2. Next, to determine whether this activation of GSK3β is dependent on the presence of NRP2, serine phosphorylation of GSK3β was examined in NRP2 knockdown cells. Serine 9 phosphorylation of GSK3β was increased (by ∼2.8-fold) in NRP2 shRNA-expressing cells, confirming that GSK3β is the downstream molecule in Sema3F-NRP2 pathway (Fig. 3, C–E).
FIGURE 3.
Stimulation of NRP2 by Sema3F activates GSK3β and consequently affects cell positioning. A, representative Western blots showing serine phosphorylation of GSK3β (Ser-9) (pGSK3β (S9)) upon stimulation of NRP2 by Sema3F-Fc in primary hippocampal neurons, with anti-GSK3β, anti-NRP2, and anti-tubulin antibodies as loading controls. B, graph shows the quantification for signal intensity of Western blot bands for phosphorylated GSK3β. a.u., arbitrary units. C, representative Western blots show that serine phosphorylation of GSK3β is increased in NRP2 knockdown cells. Knockdown efficiency of shNRP2 was shown with anti-NRP2 antibody, while anti-GSK3β and anti-tubulin antibodies were used as loading controls. D and E, graphs show the quantification for signal intensity of Western blot bands for phosphorylated GSK3β and NRP2. F, representative image of shGSK3β-expressing newborn neurons in the DG at 28 DPI (scale bar, 20 μm). G, quantification of cell positioning of adult-born neurons expressing shCTR (blue bars) or shGSK3β (green bars). *, p < 0.05,**, p < 0.01, ***, p <0.001, t test.
Knockdown of GSK3β Affects Cell Positioning of Newborn Neurons
To determine whether the mispositioning of shNRP2-expressing neurons was regulated through GSK3β, we first examined whether knockdown of GSK3β alone in newborn neurons will lead to a cell positioning defect similar to that observed in NRP2-silenced newborn neurons. Indeed, knockdown of GSK3β in newborn neurons phenocopies the NRP2-mediated cell positioning defect. The shGSK3β-expressing neurons migrated farther into the GCL toward the molecular layer as compared with the shCTR-expressing neurons (Fig. 3, F and G).
We then investigated whether the shGSK3β-mediated mispositioning of newborn neurons is a consequence of enhanced neuronal maturation. Similar to the mispositioned shNRP2-expressing neurons, the mispositioned shGSK3β-expressing neurons are still DCX-positive at 14 DPI (Fig. 4A). Both control and GSK3β-silenced newborn neurons also show no significant differences in dendritic morphology as demonstrated by comparable total dendritic length (Fig. 4B) and total branch number (Fig. 4C) with the control cells. These results suggest that GSK3β possibly acts downstream of NRP2 specifically in regulating cell positioning but not dendritic growth.
FIGURE 4.
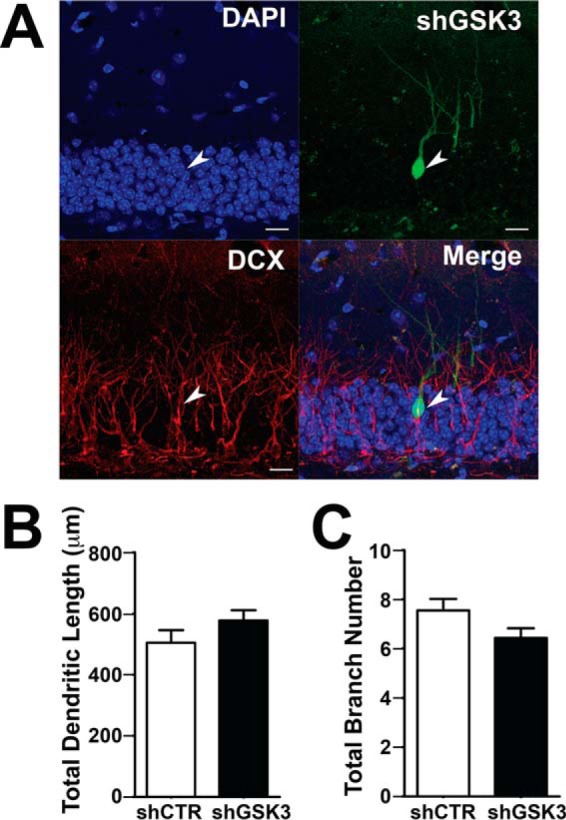
Knockdown of GSK3β does not affect dendritic arborization of newborn neurons. A, representative images of shGSK3β-expressing newborn neurons in the DG immunostained with anti-DCX antibody at 14 DPI (scale bar, 20 μm). B and C, graphs show quantification of total dendritic length (B) and total branch number (C) of control and shGSK3β-expressing newborn neurons at 28 DPI.
NRP2 Mediates Cell Positioning but not Dendritic Growth through GSK3β
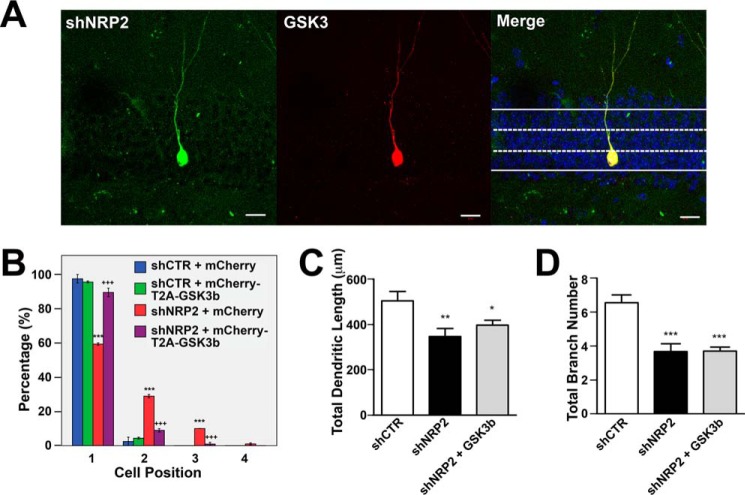
To determine whether GSK3β is a downstream mediator of NRP2 signaling, we performed rescue experiments in shNRP2-expressing neurons by overexpressing human full-length GSK3β. We co-injected retroviruses encoding shCTR or shNRP2 alone or together with retroviruses encoding mCherry (empty control vector) or mCherry-T2A-GSK3β (Fig. 5A). Neurons expressing shCTR + mCherry (blue bars in Fig. 5B) or shCTR + mCherry-T2A-GSK3β (green bars in Fig. 5B) showed cell positions comparable with control cells expressing shCTR alone (Figs. 1C and 3G). Knockdown of shNRP2 alone (shNRP2 + mCherry (red bars in Fig. 5B)) affected cell positioning, consistent with data shown in Figs. 1 and 2. Overexpression of GSK3β in shNRP2-silenced neurons (shNRP2 + mCherry-T2A-GSK3β (purple bars in Fig. 5B)) rescued cell positioning comparable to that of the control neurons (shCTR + mCherry) (blue bars in Fig. 5B). However, GSK3β overexpression did not rescue dendritic growth defects in NRP2-silenced neurons (Fig. 5, C and D). Therefore, GSK3β acts downstream of NRP2 specifically in regulating cell positioning of adult-born neurons.
FIGURE 5.
Overexpression of GSK3β rescues cell positioning phenotype of shNRP2-expressing adult-born neurons. A, representative images of newborn neurons in DG expressing both shNRP2 and GSK3β (mcherry-T2A-mCherry) at 28 DPI (scale bar, 20 μm). B, quantification of cell positioning of adult-born neurons co-infected with retrovirus combinations as indicated. Colored bars represent the percentages of cells found in the four layers as indicated. ***, p < 0.001, as compared with shCTR + mCherry, +++, p < 0.001, shNRP2 + mCherry T2A-GSK3b as compared with shNRP2 + mCherry, one-way ANOVA with Newman-Keuls' post hoc test. C and D, total dendritic length (C) and total branch number of adult-born neurons co-infected with retroviruses as indicated (D). *, p < 0.05, **, p < 0.01, ***, p < 0.001, as compared with shCTR, one-way ANOVA with Newman-Keuls' post hoc test.
Discussion
The present study shows that perturbation of NRP2 but not NRP1 signaling in adult-born neurons in DG induces ectopic positioning. The involvement of NRP2 or Sema3F in neuronal migration leading to correct cell positioning during embryonic development has been reported. Cells differentiated from the whole neocortical area are mostly confined to the surface of the telencephalon through ventral tangential migration in wild type embryo, but they spread over the deep medial region of the telencephalon in NRP2 and Sema3F mutant embryo (21). Migration of ganglionic eminence cells is also regulated by Sema3F/NRP2 interactions in the developing neocortex (22). On the other hand, NRP1 silencing impaired radial migration of cortical neurons to cortical plate during embryonic development (23). However, NRP1-silenced newborn neurons in adult DG show a decrease in total neurite length (13) but no changes in cell positioning (Fig. 1). These contradictory observations on migration and cell positioning between embryonic and adult-born neurons with disrupted NRP1 signaling indicate region-specific differences in cortex and hippocampus and distinct regulatory events at different developmental stages.
Protein expression and localization patterns were demonstrated to influence region specific-ligand binding as well as cell- and region-specific functions. For example, a chemokine, SDF1, and its receptor, CXCR4, play important roles in the tangential migration pathway of granule cells, likely through their specific complementary expression pattern, typical of chemokine and its receptor, in the target area for granule cell migration (24). Class 3 Semaphorins and their receptors are also differentially expressed in different cell types and in distinct regions that are essential for their influence on specific cell migration patterns. During development, Class 3 Semaphorins and their receptors regulate the migrations of striatal and cortical interneurons. Migrating interneurons expressing NRP1 and NRP2 are directed to the cortex, but interneurons lacking these receptors migrate to the striatum (25). The striatal cells express Sema3A and Sema3F, which may create an exclusion zone to prevent NRP1- and NRP2-expressing interneurons migrating into the striatum. In the adult brains, cells at entorhinal cortex express Sema3A, forming a Sema3A gradient in the outer two-thirds of the molecular layer that gradually declines toward the granule cell layers (26). The granule cells in DG do not express detectable levels of Sema3A. However, Sema3F proteins are expressed in DG granular cells, hilus, and medial and lateral entorhinal cortex and in their axonal projection to the DG (27). These differential expression patterns exhibited by Sema3A and Sema3F suggest possible differences in functions, including our observations demonstrating that Sema3F but not Sema3A signaling regulates the migration and cell positioning of adult newborn neurons through NRP2.
Newly generated neurons in adult DG are known to be functionally integrated into the pre-existing granule cell layer (28). These newly generated neurons form functional synapses with entorhinal cortical projections as well as with pre-existing neurons in the CA3 area (29, 30). Several proteins have been shown to be involved in the positioning of the DG newborn neurons, including DISC-1, phospholipase C-β1, GABAA receptor and NMDA receptor (3, 6, 10). However, it is still unclear how migration of newborn neurons in adult DG into different layers within GCL and into the molecular layer will affect their functions. Moreover, there is a distinct population of granule-like cells termed semilunar granule cells residing in the inner molecular layer that are normal and exhibit important functions (31).
Although the relevance of the cell positioning of adult-born neurons and the physiological implications are largely unknown, a few recent studies demonstrated learning and memory functions possibly related to the positioning of these newborn neurons in the DG. DISC1 knockdown in adult-born neurons resulted in dendritic structural abnormalities, mispositioning of newborn neurons, as well as severe cognitive and affective deficits (9). Fitzsimons et al. (32) showed glucocorticoid receptor knockdown-induced abnormal dendritic complexity, ectopic positioning of newborn granular cells, and impaired memory consolidation for contextual fear conditioning. Phospholipase C-β1 knock-out mice exhibited abnormal migration of adult-born neurons, and these mice showed a deficit in hippocampal dependent location recognition task (33). Because the mispositioned neurons in these studies also exhibit other morphological defects, it is still unclear whether these memory deficits are attributed by the mispositioning phenotype alone and/or other morphological phenotypes such as dendritic defects. Therefore, having a suitable model system that affects one but not the other morphological phenotypes will be essential to determine the contribution from each of these phenotypes to specific functions of these adult newborn neurons.
As shown in the present study, GSK3β can be activated via NRP2 pathway. However, it is still unclear whether NRP2 can activate GSK3β directly or through other signaling pathways. GSK3β regulates a wide range of cellular events, largely due to its broad range of substrates. These substrates, including transcription factors such as cyclic AMP-response element-binding protein (CREB), SMAD1, and c-Jun, as well as kinases such as focal adhesion kinase (FAK), PI3K, and Cdk5, are known to regulate GSK3β activity (14, 34–36). We previously showed that NRP1 or NRP2 can activate FAK and Cdk5 pathways (13), but both pathways are not involved in NRP2-dependent cell positioning of newborn neurons in the adult brains (13). Thus, it remains to be determined whether any other specific pathways or some unidentified kinases recruited to NRP2-Plexin at the cell membranes are directly involved in the activation of GSK3β. Although our unpublished data4 show that NRP1 stimulation by Sema3A can also activate GSK3β, the knockdown of NRP1 did not affect neuronal cell positioning (13).
Our results suggested that dendritic growth and cell positioning of adult-born neurons are regulated by distinct mechanisms. We reported that both NRP1 and NRP2 knockdown induce similar dendritic deficits (13), but only NRP2 knockdown disrupted cell positioning of adult-born neurons in the DG. GSK3β is specifically responsible for cell positioning but not dendritic development regulated by NRP2, indicating the existence of independent regulation of these two distinct processes. Future work will be required to determine how NRP1 and NRP2 utilize different downstream proteins and pathways to activate different processes, as well as functional analysis such as electrophysiology coupled with optogenetic approach to determine and map whether these mispositioned newborn neurons of normal dendritic arborization can integrate into the existing granule cell layer and make functional synapses that are comparable with those correctly positioned cells. Subsequent studies will have to determine how these distinct morphological defects contribute to different memory deficits.
In conclusion, we identified a new role for NRP2 in cell positioning of adult newborn neurons in the DG, acting via GSK3β. NRP2 mediates GSK3β-dependent neuronal positioning of newborn neurons in the adult neurogenic region, independent of its effect on dendritic development. This finding would contribute toward understanding the etiology of neuronal migration-related brain disorders.
Experimental Procedures
Animals
All animal procedures and applicable regulations of animal welfare were in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines and approved by SingHealth IACUC, Singapore. Adult (5–6-week-old) female C57Bl/6 mice were purchased from the SingHealth Experimental Medicine Centre, Singapore, and housed in the Specific Pathogen Free animal facility at Duke-NUS Graduate Medical School, Singapore. The surgical and postsurgical procedures were performed as described previously (13, 37).
Construction, Production, and Stereotaxic Injection of Engineered Retroviruses
Engineered self-inactivating murine retroviruses were used to express GFP specifically in proliferating cells and their progeny as described previously (13, 38). GFP expression was under the control of EF1α promoter, and shRNA was co-expressed under the control of human U6 promoter in the same vector. shRNAs against mouse NRP1 or NRP2 and shRNA with scrambled control sequence were described previously (13). shRNA against mouse GSK3β is CATGAAAGTTAGCAGAGAT (15). Retroviral constructs encoding for human cDNA of GSK3β fused to mCherry via a T2A linker were driven by CAG promoter. Knockdown efficiencies of each shRNA were demonstrated in previous studies (13, 15).
Cell Culture and Immunoblotting
HEK293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and were transfected with the indicated plasmids using the calcium phosphate method. Hippocampal neurons were isolated from the hippocampi of embryonic rats from timed-mated Sprague-Dawley as described previously (39, 40). The following primary antibodies were used for Western blotting analysis: rabbit anti-phospho-GSK3β (Ser-9) (Cell Signaling Technology, 1:1000), mouse anti-Tubulin (Sigma, 1:10000), and rabbit anti-GSK3β (Cell Signaling Technology, 1:1000). Goat anti-DCX (Santa Cruz Biotechnology, 1:500) antibody was used for immunohistochemistry.
Confocal Imaging and Analysis
Coronal brain sections (40 μm) prepared from viral injected mice were used for morphological analysis. Images were acquired on a Zeiss LSM 710 confocal system (Carl Zeiss, Singapore) and analyzed using Zeiss Zen software. Cell positioning of neurons in adult DG was analyzed according to previously published methods (3–5). Specifically, granule cell layer was divided into three equal layers. Cells located within each layer were determined. Cells located on the border of two layers were classified according to the majority percentage of cell bodies in each layer. To prevent biased quantification, all counting and analysis were done blindly. The dendritic structure of newborn neurons was analyzed according to previously published methods (13, 37). A total of 25–30 neurons from 4–6 animals per experimental group were analyzed. Statistical significance (p < 0.05) was assessed using Student's t test between two groups. GraphPad Prism software was used for all statistical analyses. Statistical significance between three or more groups was analyzed using a one-way analysis of variance (ANOVA) with Newman-Keuls' post hoc tests.
Author Contributions
T. N. and J. R. R. designed and performed most of the in vitro and in vivo experiments, analyzed data, and wrote the manuscript. C. H. H. H. performed Western blotting experiments and analysis. B. C. performed experiments and analyzed part of the data. J. Z and Z. Z provided critical inputs to the experimental design. E. L. K. G. initiated and directed the entire study, designed experiments, performed some in vivo experiments, analyzed data, and wrote the manuscript.
Acknowledgments
We thank W. Y. Leong for technical support and members of the Goh lab for sharing reagents and expertise.
This work was supported by Abbott Nutrition, the Academic Center of Excellence (ACE) research award from GlaxoSmithKline (GSK), and the National Research Foundation Singapore under its Competitive Research Program (Grant NRF 2008 NRF-CRP 002-082) (to E. L. K. G.). The authors declare that they have no conflicts of interest with the contents of this article.
T. Ng, C. H. H. Hor, B. Chew, J. Zhao, Z. Zhong, J. R. Ryu, and E. L. K. Goh, unpublished data.
- DG
- dentate gyrus
- GCL
- granular cell layer
- GSK3β
- glycogen synthase kinase-3β
- DPI
- days post-infection
- ANOVA
- analysis of variance.
References
- 1. Alvarez-Buylla A., and Garcia-Verdugo J. M. (2002) Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lledo P. M., Alonso M., and Grubb M. S. (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193 [DOI] [PubMed] [Google Scholar]
- 3. Duan X., Chang J. H., Ge S., Faulkner R. L., Kim J. Y., Kitabatake Y., Liu X. B., Yang C. H., Jordan J. D., Ma D. K., Liu C. Y., Ganesan S., Cheng H. J., Ming G. L., Lu B., and Song H. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Espósito M. S., Piatti V. C., Laplagne D. A., Morgenstern N. A., Ferrari C. C., Pitossi F. J., and Schinder A. F. (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kempermann G., Gast D., Kronenberg G., Yamaguchi M., and Gage F. H. (2003) Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130, 391–399 [DOI] [PubMed] [Google Scholar]
- 6. Duveau V., Laustela S., Barth L., Gianolini F., Vogt K. E., Keist R., Chandra D., Homanics G. E., Rudolph U., and Fritschy J. M. (2011) Spatiotemporal specificity of GABAA receptor-mediated regulation of adult hippocampal neurogenesis. Eur. J. Neurosci. 34, 362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koyama R., Tao K., Sasaki T., Ichikawa J., Miyamoto D., Muramatsu R., Matsuki N., and Ikegaya Y. (2012) GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat. Med. 18, 1271–1278 [DOI] [PubMed] [Google Scholar]
- 8. Whissell P. D., Rosenzweig S., Lecker I., Wang D. S., Wojtowicz J. M., and Orser B. A. (2013) γ-Aminobutyric acid type A receptors that contain the delta subunit promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. 74, 611–621 [DOI] [PubMed] [Google Scholar]
- 9. Zhou M., Li W., Huang S., Song J., Kim J. Y., Tian X., Kang E., Sano Y., Liu C., Balaji J., Wu S., Zhou Y., Zhou Y., Parivash S. N., Ehninger D., et al. (2013) mTOR inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron 77, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Namba T., Ming G. L., Song H., Waga C., Enomoto A., Kaibuchi K., Kohsaka S., and Uchino S. (2011) NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via Disrupted-In-Schizophrenia 1 (DISC1). J. Neurochem. 118, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayala R., Shu T., and Tsai L. H. (2007) Trekking across the brain: the journey of neuronal migration. Cell 128, 29–43 [DOI] [PubMed] [Google Scholar]
- 12. Tran T. S., Kolodkin A. L., and Bharadwaj R. (2007) Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 23, 263–292 [DOI] [PubMed] [Google Scholar]
- 13. Ng T., Ryu J. R., Sohn J. H., Tan T., Song H., Ming G. L., and Goh E. L. (2013) Class 3 semaphorin mediates dendrite growth in adult newborn neurons through Cdk5/FAK pathway. PLoS One 8, e65572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hur E. M., and Zhou F. Q. (2010) GSK3 signalling in neural development. Nat. Rev. Neurosci. 11, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chew B., Ryu J. R., Ng T., Ma D., Dasgupta A., Neo S. H., Zhao J., Zhong Z., Bichler Z., Sajikumar S., and Goh E. L. (2015) Lentiviral silencing of GSK-3β in adult dentate gyrus impairs contextual fear memory and synaptic plasticity. Front. Behav. Neurosci. 9, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eickholt B. J., Walsh F. S., and Doherty P. (2002) An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J. Cell Biol. 157, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chadborn N. H., Ahmed A. I., Holt M. R., Prinjha R., Dunn G. A., Jones G. E., and Eickholt B. J. (2006) PTEN couples Sema3A signalling to growth cone collapse. J. Cell Sci. 119, 951–957 [DOI] [PubMed] [Google Scholar]
- 18. Shelly M., Cancedda L., Lim B. K., Popescu A. T., Cheng P. L., Gao H., and Poo M. M. (2011) Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 71, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doble B. W., and Woodgett J. R. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jope R. S. (2003) Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24, 441–443 [DOI] [PubMed] [Google Scholar]
- 21. Ito K., Kawasaki T., Takashima S., Matsuda I., Aiba A., and Hirata T. (2008) Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J. Neurosci. 28, 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamamaki N., Fujimori K., Nojyo Y., Kaneko T., and Takauji R. (2003) Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J. Comp. Neurol. 455, 238–248 [DOI] [PubMed] [Google Scholar]
- 23. Chen G., Sima J., Jin M., Wang K. Y., Xue X. J., Zheng W., Ding Y. Q., and Yuan X. B. (2008) Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 11, 36–44 [DOI] [PubMed] [Google Scholar]
- 24. Bagri A., Gurney T., He X., Zou Y. R., Littman D. R., Tessier-Lavigne M., and Pleasure S. J. (2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129, 4249–4260 [DOI] [PubMed] [Google Scholar]
- 25. Marín O., Yaron A., Bagri A., Tessier-Lavigne M., and Rubenstein J. L. (2001) Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science 293, 872–875 [DOI] [PubMed] [Google Scholar]
- 26. Giger R. J., Pasterkamp R. J., Heijnen S., Holtmaat A. J., and Verhaagen J. (1998) Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J. Neurosci. Res. 52, 27–42 [DOI] [PubMed] [Google Scholar]
- 27. Tran T. S., Rubio M. E., Clem R. L., Johnson D., Case L., Tessier-Lavigne M., Huganir R. L., Ginty D. D., and Kolodkin A. L. (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462, 1065–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kempermann G., Gast D., and Gage F. H. (2002) Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52, 135–143 [DOI] [PubMed] [Google Scholar]
- 29. Gu Y., Arruda-Carvalho M., Wang J., Janoschka S. R., Josselyn S. A., Frankland P. W., and Ge S. (2012) Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 15, 1700–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumamoto N., Gu Y., Wang J., Janoschka S., Takemaru K., Levine J., and Ge S. (2012) A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 15, 399–405, S391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larimer P., and Strowbridge B. W. (2010) Representing information in cell assemblies: persistent activity mediated by semilunar granule cells. Nat. Neurosci. 13, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fitzsimons C. P., van Hooijdonk L. W., Schouten M., Zalachoras I., Brinks V., Zheng T., Schouten T. G., Saaltink D. J., Dijkmans T., Steindler D. A., Verhaagen J., Verbeek F. J., Lucassen P. J., de Kloet E. R., Meijer O. C., et al. (2013) Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol. Psychiatry 18, 993–1005 [DOI] [PubMed] [Google Scholar]
- 33. Manning E. E., Ransome M. I., Burrows E. L., and Hannan A. J. (2012) Increased adult hippocampal neurogenesis and abnormal migration of adult-born granule neurons is associated with hippocampal-specific cognitive deficits in phospholipase C-β1 knockout mice. Hippocampus 22, 309–319 [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi T., Hino S., Oue N., Asahara T., Zollo M., Yasui W., and Kikuchi A. (2006) Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions. Mol. Cell. Biol. 26, 898–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koga T., Suico M. A., Shimasaki S., Watanabe E., Kai Y., Koyama K., Omachi K., Morino-Koga S., Sato T., Shuto T., Mori K., Hino S., Nakao M., and Kai H. (2015) Endoplasmic reticulum (ER) stress induces Sirtuin 1 (SIRT1) expression via the PI3K-Akt-GSK3β signaling pathway and promotes hepatocellular injury. J. Biol. Chem. 290, 30366–30374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morfini G., Szebenyi G., Brown H., Pant H. C., Pigino G., DeBoer S., Beffert U., and Brady S. T. (2004) A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 23, 2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao N., Ma D., Leong W. Y., Han J., VanDongen A., Chen T., and Goh E. L. (2015) The methyl-CpG-binding domain (MBD) is crucial for MeCP2's dysfunction-induced defects in adult newborn neurons. Front. Cell. Neurosci. 9, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ge S., Goh E. L., Sailor K. A., Kitabatake Y., Ming G. L., and Song H. (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shivaraj M. C., Marcy G., Low G., Ryu J. R., Zhao X., Rosales F. J., and Goh E. L. (2012) Taurine induces proliferation of neural stem cells and synapse development in the developing mouse brain. PLoS One 7, e42935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Su C. T., Yoon S. I., Marcy G., Chin E. W., Augustine G. J., and Goh E. L. (2015) An optogenetic approach for assessing formation of neuronal connections in a co-culture system. J. Vis. Exp. 96, e52408. [DOI] [PMC free article] [PubMed] [Google Scholar]