Abstract
Background
Mitosis is a process of cell division resulting in two genetically equivalent daughter cells. Excessive proliferation of cells due to mitosis is the hallmark in pre cancer and cancer.
Aims
This study was conducted to count the number of mitotic figures in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma in both Hematoxylin and Eosin (H&E) and Crystal Violet stained sections. Also the overall number of mitotic figures with both stains were compared along with the evaluation of staining efficacy of both the stains.
Methods and material
The present study was conducted on 20 specimens each of the three categories. These were further divided into two groups for staining with H&E and with 1% Crystal Violet respectively. Images were captured and analyzed using image analysis software Dewinter Biowizard 4.1.
Results
Comparison of mitotic figure count in three categories in sections stained with both stains showed statistically significant difference (p < 0.001). The mean number of mitotic figures seen in Crystal Violet reagent were significantly higher as seen in H&E stain (p < 0.001). The overall diagnostic efficacy of Crystal Violet was 87.6%. Crystal Violet scored over H&E stain and also helped to better appreciate metaphases in Squamous cell carcinoma and telophases in dysplasia.
Conclusion
Number of mitotic figures progressively increase with the advancement of the pathology. Use of 1% Crystal Violet provides better appreciation of mitotic figures and can be employed as a selective stain in routine histopathology.
Keywords: Crystal Violet, Mitotic figures, Oral epithelial dysplasia, Squamous cell carcinoma
1. Introduction
Normal growth and maintenance of oral mucosa requires that birth, differentiation and loss of epithelial cells are regulated and coordinated. It is the disruption of normal growth control mechanisms that leads to neoplastic transformation of epithelium and the development of carcinomas.1
Preneoplasia is arguably the most important disease entity of modern man.2 Head and neck cancer annually, represents 650,000 new cases and 350,000 deaths worldwide.3 Oral cancer in India constitutes about 10% of all cancer cases and oral squamous cell carcinoma represents 90–95% of them.4 This ranks as the sixth most common cancer worldwide5, 6 and the third most common form of malignancy in developing countries.7 This emphasizes the importance of early identification of precursor lesions that will develop into carcinomas.8
Cell division is required to maintain tissue integrity.9 Dysregulation of cell cycle machinery is a fundamental hallmark of cancer progression10 and hyper-proliferation is thought to be an early, marker of disorderly growth.11 Mitosis is a process wherein a mother cell divides exactly into two identical daughter cells. The various phases of mitosis are prophase, metaphase, anaphase and telophase, some of which are seen in tissue sections.9 Mitotic figures are defined as atypical if they demonstrate an abnormal chromosomal distribution or an excessive number of mitotic spindles with a multipolar morphologic appearance.12
Errors in identifying a mitotic cell can weaken the reliability of histological grading due to the loose use of morphologic criteria. Combination of stains and modification of the existing histochemical techniques can overcome these problems. Crystal Violet is a basic dye which has a high affinity for the highly acidic chromatin of mitotic cells.9 The stain was primarily developed for the quantitative analysis of mitotic figures in developing brain, but lends itself to any tissue where mitotic counts are required.13
Screening for rare mitotic cells in a tumor cell population requires intensive effort at fairly high magnifications to ensure correct identification and classification of mitotic cells.14 Some authors have sought to remove this problem by using quantitative methods like point counting applying to prognostic evaluation of oral cancer.15
The present study was therefore undertaken to evaluate the mitotic figures in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma with Crystal Violet staining over routine H&E staining in order to judge its reliability in early detection of oral premalignancy and malignancy.
2. Materials and methods
The study was conducted on tissues/wax blocks of clinically and histologically proved cases of oral epithelial dysplasia and squamous cell carcinoma. A total of 20 specimens each of normal oral mucosa, oral Epithelial Dysplasia and Squamous Cell Carcinoma (SCC) comprised the study population. Each specimen was further divided as to be used for two different staining procedures. After routine staining procedure with Hematoxylin and Eosin (H&E); preparation for Crystal Violet (CV) staining was done.
3. Crystal Violet staining
3.1. Preparation of stain – Hucker and Conn (1928)16
Dissolve 2 g of Crystal Violet in 20 ml of 95% alcohol. Add 80 ml of 1% aqueous ammonium oxalate. Dissolve using minimum of heat. Cool, filter and store in amber colored bottle. The solution was used as 1% aqueous Crystal Violet for staining using the Fraser FJ modified13 procedure that has been illustrated in Table 1.
Table 1.
Crystal Violet staining procedure (Fraser FJ method modified).21
| Xylene | 3 changes 15 min each |
| Alcohol | Hydrate through graded alcohols (100%, 90% and 80%) |
| Water | Wash in water |
| Hydrolyze | 1 N HCl at 56–60 °C for 12 min |
| Distilled water | Wash in three changes of distilled water |
| Alkaline water | Wash in two changes of alkaline water |
| 1% aqueous Crystal Violet | Stain for 30 min in Crystal Violet |
| 1% acid alcohol | Dip for 5–10 s to differentiate |
| Alcohol | Dehydrate quickly through graded alcohols (80%, 90% and 100%) |
| Xylene | Clearing for 10–15 min |
| DPX | Mounting |
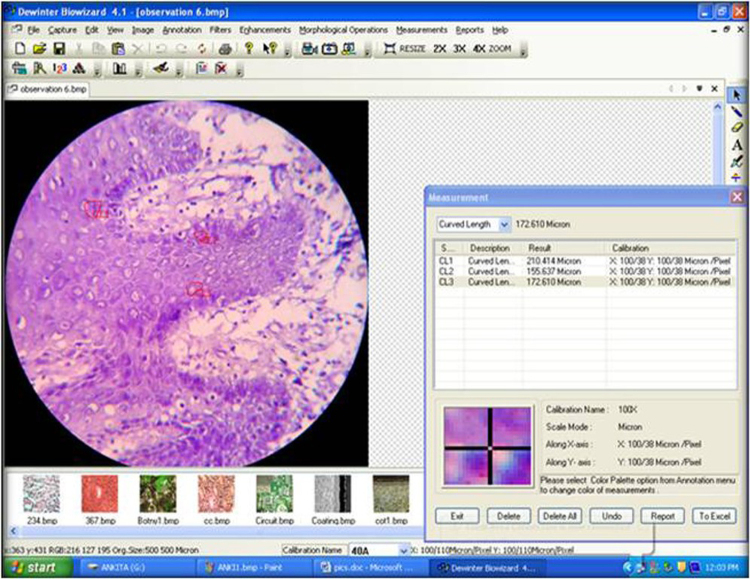
For analysis, images were captured using digital camera and trinocular research microscope with a 40× objective in both Hematoxylin and Eosin and Crystal Violet stained sections. The actual counting was done manually using the Dewinter Biowizard 4.1 after accurate calibration. Images were captured, stored and arranged according to the study groups. Microscopic fields were selected starting from most mitotically rich area and then moving the stage to the next field, and continuing the selection to include a minimum of 3 fields from each section. Each selected field included both basal and parabasal cell compartments. In sections pertaining to squamous cell carcinoma the counting was also performed in islands, cords and sheets of tumor cells infiltrating the connective tissue. The mitosis counting was done using Dewinter-Biowizard 4.1 on the fields stained with both Hematoxylin and Eosin and Crystal Violet stains (Fig. 1).
Fig. 1.
Method for counting mitosis using Dewinter Biowizard 4.1 in section stained with Crystal Violet stain.
The criteria given by Van Deist et al.9 was used to assign a structure as a mitotic figure in this study:
-
1)
The nuclear membrane must be absent indicating that cells have passed the prophase.
-
2)
Clear, hairy extensions of nuclear material (condensed chromosomes) must be present; either clotted (beginning metaphase), in a plane (metaphase/anaphase) or in separate clots (telophase).
-
3)
Two parallel, clearly separate chromosome clots to be counted as if they are separate.
MITOTIC COUNT is expressed as the mitotic count per field and the mitotic count per square millimeter using the image analysis software Dewinter Biowizard 4.1.
The mitotic count per field was calculated as:
The mitotic count per square millimeter was calculated as follows:
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical Analysis Software.
4. Results
The number of mitotic figures for all the cases in three groups with both the stains have been listed in Table 2. Comparative analysis of mitotic figure count in three types of specimens showed statistically significant difference for both unit high power field grid area and for unit area (mm2). Observations revealed no mitotic figures for normal group, while oral dysplasia had significantly lower mean mitotic figure count as compared to oral squamous cell carcinoma. The mean mitotic figure count in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma in sections stained with Crystal Violet (Table 3) were higher as compared to that in H&E (Table 4).
Table 2.
Number of mitotic figures per case in normal mucosa, oral epithelial dysplasia and squamous cell carcinoma with differential stains.
| S. No. | Normal oral mucosa |
Oral epithelial dysplasia |
Oral squamous cell carcinoma |
|||
|---|---|---|---|---|---|---|
| Mitotic figures (H&E) | Mitotic figures (Crystal Violet) | Mitotic figures (H&E) | Mitotic figures (Crystal Violet) | Mitotic figures (H&E) | Mitotic figures (Crystal Violet) | |
| 1 | 0 | 0 | 5 | 7 | 4 | 5 |
| 2 | 0 | 0 | 3 | 6 | 2 | 6 |
| 3 | 0 | 0 | 3 | 4 | 3 | 7 |
| 4 | 0 | 0 | 2 | 5 | 4 | 5 |
| 5 | 0 | 0 | 3 | 4 | 7 | 8 |
| 6 | 0 | 0 | 3 | 7 | 5 | 9 |
| 7 | 0 | 0 | 5 | 8 | 5 | 8 |
| 8 | 0 | 0 | 5 | 5 | 4 | 6 |
| 9 | 0 | 0 | 2 | 5 | 5 | 8 |
| 10 | 0 | 0 | 2 | 4 | 5 | 9 |
| 11 | 0 | 0 | 2 | 4 | 6 | 7 |
| 12 | 0 | 0 | 4 | 5 | 8 | 9 |
| 13 | 0 | 0 | 3 | 4 | 8 | 10 |
| 14 | 0 | 0 | 3 | 6 | 10 | 14 |
| 15 | 0 | 0 | 4 | 4 | 5 | 9 |
| 16 | 0 | 0 | 2 | 5 | 7 | 10 |
| 17 | 0 | 0 | 7 | 8 | 8 | 9 |
| 18 | 0 | 0 | 5 | 7 | 13 | 16 |
| 19 | 0 | 0 | 6 | 8 | 7 | 9 |
| 20 | 0 | 0 | 4 | 5 | 10 | 14 |
Table 3.
Comparison of mean mitotic figure count in three types of sections stained with Crystal Violet.
| S.No. | Group | Count/hpf grid (n = 20) |
Count/mm2 (n = 20) |
||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 1. | Normal oral mucosa | 0 | 0 | 0 | 0 |
| 2. | Oral epithelial dysplasia | 5.55 | 1.47 | 344.72 | 91.19 |
| 3. | Oral SCC | 8.90 | 2.92 | 552.80 | 181.25 |
| F | 113.611 | 113.611 | |||
| P | <0.001 | <0.001 | |||
Table 4.
Comparison of mean mitotic figure count in three types of sections stained with H&E.
| S.No. | Group | Count/hpf grid (n = 20) |
Count/mm2 (n = 20) |
||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 1. | Normal oral mucosa | 0 | 0 | 0 | 0 |
| 2. | Oral epithelial dysplasia | 3.65 | 1.46 | 226.71 | 90.74 |
| 3. | Oral SCC | 6.30 | 2.68 | 391.30 | 166.30 |
| F | 64.536 | <0.001 | |||
| P | 64.536 | <0.001 | |||
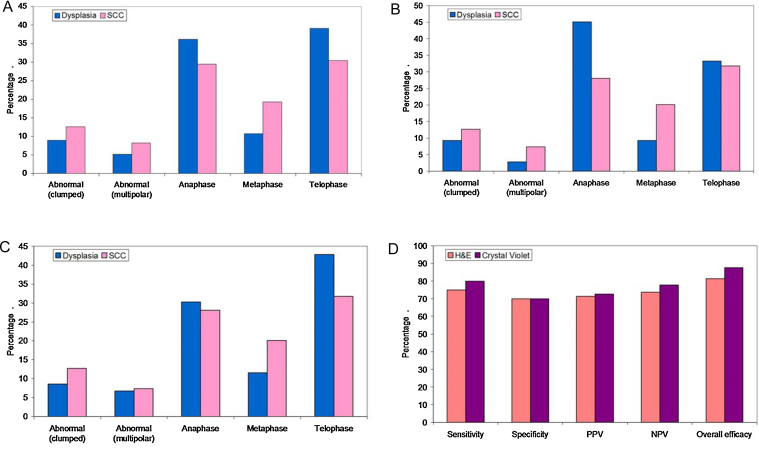
Also, irrespective of the stain used SCC group had significantly higher proportion of abnormal clumped (12.6%), abnormal multipolar (8.2%) and metaphase stages (19.3%) as compared to dysplasia group (8.9%, 5.2%, 10.7% respectively) while Anaphase (36.2%) and Telophase (39.1%) stages were significantly more common in dysplasia group (29.5%, 30.5%) (Fig. 2A).
Fig. 2.
Comparison of types of mitotic figures in Oral Epithelial Dysplasia and Squamous Cell carcinoma irrespective of the stain (A), with H&E (B), with Crystal Violet (C). Also comparison of staining efficacy of mitotic figures with both stains (D).
With H&E stain it was found that SCC group had significantly higher proportion of abnormal (multipolar) (9.2%) and metaphase (18.1%) stage cells as compared to dysplasia group. Anaphase (45.1%) stage was significantly higher in dysplasia group as compared to SCC group (Fig. 2B). However with CV stain SCC group had significantly higher proportion of metaphase (20.1%) stage cells as compared to dysplasia group. Telophase (42.8%) stage was significantly higher in dysplasia group as compared to SCC group (Fig. 2C).
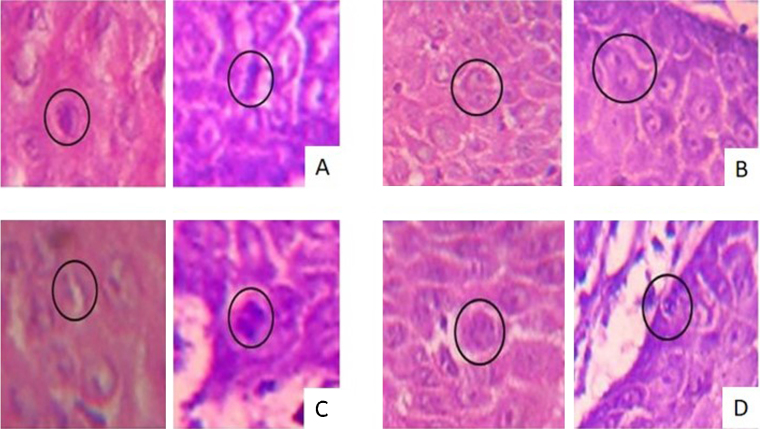
The overall diagnostic efficacy of Crystal Violet was 87.6% while that of H&E stain was 81.3% (Fig. 2D). Although most extensively used in histology H&E stain could help to decide the cut-off of >4.5 per high power field and >279.5/mm2 to differentiate between oral dysplasia and oral squamous cell carcinoma as compared to Crystal Violet which serves to decide the cut-off of >6.5 per high power field and >465.84/mm2. The various types of mitotic figures have also been illustrated in Fig. 3A–D.
Fig. 3.
Photomicrographs showing different types of mitotic figures in H&E and Crystal Violet stained sections (400×). Metaphase (A), Telophase (B), Anaphase (C), multipolar mitosis (D).
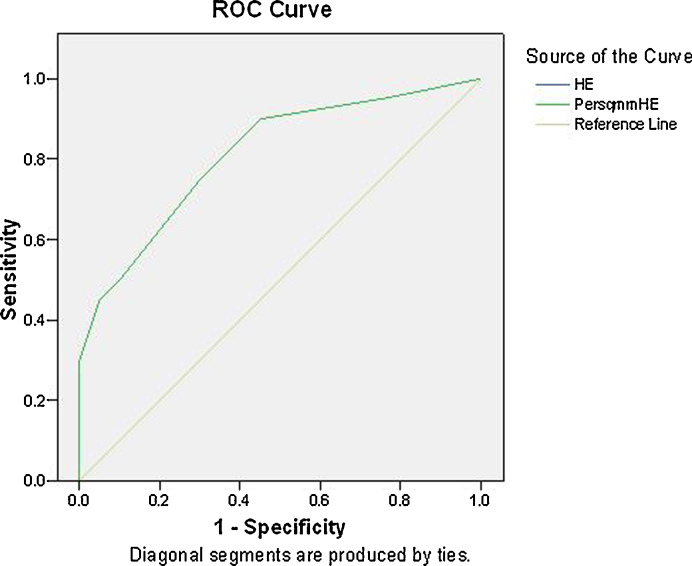
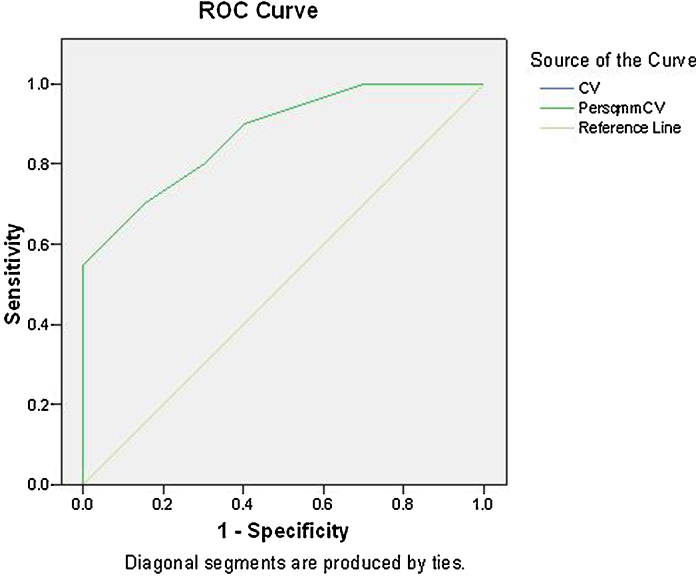
For the significance of mitotic counts the ROC curve analysis was performed for both the stains. The area under ROC curve for H&E stain was found to be 0.813, thereby indicating a diagnostic efficacy of 81.3% (Fig. 4). For Crystal Violet stain the area under ROC curve was 0.876, thereby indicating a diagnostic efficacy of 87.6% (Fig. 5).
Fig. 4.
ROC curve to analyse the diagnostic efficacy of mitotic figure count in sections stained with H & E stain.
Fig. 5.
ROC curve to analyse the diagnostic efficacy of mitotic figure count in sections stained with Crystal violet stain.
5. Discussion
The global increase in frequency and mortality, as well as the poor prognosis of head and neck squamous cell carcinoma, has intensified current research efforts in the field of prevention and early detection of this disease.17 Mitosis, the cell generating process in humans, runs at the nuclei level and, as such, there is an interest in studying the nuclei of cells with the purpose of detecting cancerous cells.18 The reliability and reproducibility of mitotic counts has recently also been improved by elaboration of quantitative methods19 of which image processing favors the detection of mitotic figures to achieve successful discrimination between mitoses and other objects such as artifacts and nuclei of inflammatory cells.20
Methodological errors are of great concern when establishing mitotic activity.21 Hence, as much as possible standardized protocols were used in counting mitotic figures, including analysis of multiple microscopic fields in representative areas of the tumor. The mitotic figures were counted and the result in each sample was expressed as the number of mitoses per high power field (hpf) and also in 1 square millimeter of the tumor tissue (mitoses/mm2). Mitosis per high power field may be quite variable as the microscopic objective used (numeric aperture, field diameter at specimen level) are difficult to control and so the results cannot be compared between different laboratories. Therefore, counting per mm2 reduced the need for further standardization and gave appreciable results between paucicellular and densely cellular tumor areas.22, 23 The resistance to counting mitoses is understandable from a psychological point of view, as a formal mitosis count takes 3–4 min and requires even more time when the quality of histology is poor.21 Given the tedious and subjective nature of manual scoring, computerized image analysis systems appear to be an attractive alternative.24 Also digitization helped to store images of proliferative areas which may be useful to compare the pretreatment and post treatment biological potential and predict prognosis.
Increases in mitotic index in dysplasia and carcinoma as compared to normal oral mucosa indicate an important kinetic change, possibly representing increased stem cell turnover.25 Also volume corrected mitotic index (mitotic figures per square mm of neoplastic tissue in the microscopic field) is considered as the best predictor of prognosis.26
The mitotic cells were numerous in basal and parabasal cell layers along with suprabasal layers in oral epithelial dysplasia whereas mitotic figures were distributed all throughout the superficial epithelium as well as nests, cords and sheets of infiltrating epithelial cells in squamous cell carcinoma. Also, in neoplastic tissues, the number of prophases is greatly reduced relative to metaphase stage which is of considerably longer duration. The relative number of anaphase stages shows also a tendency to decrease. This could be attributed to the fact that in cancer cells the relative duration of prophase stages is greatly reduced which would mean that the formation of the spindle is more rapid. The abnormal mitotic figures are due to an abnormal increase in the number of centrioles or to a lack of coordination between the chromosomal cycle and the division of centrioles.27, 28
The search for scientific publications related to the comparison of various types of mitotic figures seen in oral epithelial dysplasia and SCC with H&E or Crystal Violet stains could not reveal any substantial data and to the best of our knowledge our findings are the first of its kind. It is therefore proposed that since the type of mitotic figures found are directly related to the stage of progression of the pathology therefore, higher number of abnormal mitotic figures and metaphase cells with either stains could be due to the accumulation of these types of mitotic figures in SCC. Also, higher anaphases/telophases in dysplasia indicate the completion of cell cycle which is not that rapid as in SCC.
It has also been speculated that mitosis may go on to completion or even begin within excised tissue in the absence of oxygen29 and the regulation of malignant cell growth is subject to systemic, regional and cellular factors, such as oxygen supply, capacity for protein synthesis and endocrine stimulation of tumor cells.30 Hence it is difficult to comment and dictate that one particular stage will always be present with one type of the stain.
However, the finding is strong statistically and so it is proposed that with CV higher metaphases can be linked to its better identifiability and higher telophases is attributed to attraction of CV toward highly acidic lamins of nuclear membrane that appears to reform during telophase.31 Of great relevance is the fact that “it might be not the abundance of mitotic figures so much as the abnormality of those figures that is relevant to tumor growth and patient survival”.32
Numerous studies showing prognostic value of tumor proliferation using mophometric, flow cytometric, nucleotide radiolabelling, immunohistochemical, morphologic techniques,33 autoradiographic determination of thymidine labeling9 have been published. Physiological markers for mitotic cells like protein kinases and accumulation of different molecules as cyclins, dyenins in different stages of mitosis34 can also be used for studying mitotic frequency. Special stains like gallocyanin, Nissl stain, Giemsa and toluidine blue, Feulgen have also been employed for studying mitotic figures.9, 13, 35 Switching over to a modified histochemical stain that is cost effective and statistically reliable in its potential would be a wise option and Crystal Violet proved to be promising in this regard.
The higher sensitivity, PPV and NPV of Crystal Violet could be explained due to its basic nature and high affinity for highly acidic chromatin of mitotic cells9 and production of stable intermediaries on reaction being a metachromatic dye.36 The overall higher diagnostic efficacy of Crystal Violet could also be linked to the modification of staining methodology by incorporating the use of 1 N HCl at 60 °C thereby causing increase in contrast due to reduced staining of cytoplasm, which is likely to be the consequence of reduced RNA content following hydrolysis.13, 37, 38This delineated the normal and abnormal types of mitotic figures distinctly in the superficial epithelium as well as in tumor islands. Also the sections stained with Crystal Violet or other routine basic dyes freely and rapidly dissolve in lower alcohols.16 Therefore, these were dehydrated rapidly through alcohols to prevent excessive loss of dye.
6. Conclusion
In our study, it was observed that mitotic activity is readily accessible, reproducible as it can be performed on routine sections and is a reliable parameter of proliferative activity as it corresponds to actual cell divisions.39 Mitotic counts have been also considered as an important tool in histopathological grading which in turn facilitates easy diagnosis and treatment planning. Mitotic figure counting using image analysis reduces time involved in estimations while maintaining high degree of accuracy.40 Crisp staining with 1% Crystal Violet further improves the procedure with identification of mitotic figures in intense blue color outstanding loudly against the background of resting cells.13
Therefore, implying 1% Crystal Violet along with the use of image analysis better focuses to count mitotic figures and substantially differentiate the number and types of mitotic figures in different study groups thereby validating its necessity and usefulness in routine histopathology.
Conflict of interest
The authors have none to declare.
Acknowledgement
The authors thank all the staff members, colleagues and technicians of Department of Oral and Maxillofacial Pathology and Microbiology, Kothiwal Dental College and Research Centre, Moradabad for their kind support.
References
- 1.Thomson P.J., Potten C.S., Appleton D.R. Characterization of epithelial cell activity in patients with oral cancer. Br J Oral Maxillofac Surg. 1999;37(October (5)):384–390. doi: 10.1054/bjom.1999.0147. [DOI] [PubMed] [Google Scholar]
- 2.Berman J.J., Moore G.W. Image analysis software for the detection of preneoplastic and early neoplastic lesions. Cancer Lett. 1994;77:103–109. doi: 10.1016/0304-3835(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 3.Chuang A.Y., Chuang T.C., Chang S. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44(October (10)):915–919. doi: 10.1016/j.oraloncology.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R., Tiwari R.K., Reddy P.P., Ahuja Y.R. Risk assessment of oral cancer in patients with pre-cancerous states of the oral cavity using micronucleus test and challenge assay. Oral Oncol. 2008;44(April (4)):354–360. doi: 10.1016/j.oraloncology.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Speight P.M., Farthing P.M., Bouquot J.E. The pathology of oral cancer and precancer. Curr Diagn Pathol. 1996;3:165–176. [Google Scholar]
- 6.Zini A., Czerninski R., Sgan-Cohen H.D. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299–305. doi: 10.1111/j.1600-0714.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 7.Tumuluri V., Thomas G.A., Fraser I.S. The relationship of proliferating cell density at the invasive tumour front with prognostic and risk factors in human oral squamous cell carcinoma. J Oral Pathol Med. 2004;33:204–208. doi: 10.1111/j.0904-2512.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 8.Sudbo J., Reith A. Which putatively pre-malignant oral lesions become oral cancers? Clinical relevance of early targeting of high-risk individuals. J Oral Pathol Med. 2003;32:63–70. doi: 10.1034/j.1600-0714.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 9.Ankle M.R., Kale A.D., Charantimath S. Comparison of staining of mitotic figures by hematoxylin and eosin- and crystal violet stains, in oral epithelial dysplasia and squamous cell carcinoma. Indian J Dent Res. 2007;18(3):101–105. doi: 10.4103/0970-9290.33784. [DOI] [PubMed] [Google Scholar]
- 10.Todd R., Hinds P.W., Munger K. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002;13(1):51–61. doi: 10.1177/154411130201300106. [DOI] [PubMed] [Google Scholar]
- 11.Liu S.C., Klein-Szanto A.J.P. Markers of proliferation in normal and leukoplakic oral epithelia. Oral Oncol. 2000;36:145–151. doi: 10.1016/s1368-8375(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 12.Lau S.K., Weiss L.M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum Pathol. 2009;40:757–768. doi: 10.1016/j.humpath.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Fraser F.J. A selective stain for mitotic figures, particularly in the developing brain. Stain Technol. 1982;57(July (4)):219–224. doi: 10.3109/10520298209066712. [DOI] [PubMed] [Google Scholar]
- 14.Dooley W.C., Roberts J., Allison D.C. Acid Giemsa technique for rapid identification of mitotic cells. J Histochem Cytochem. 1989;37(October (10)):1553–1556. doi: 10.1177/37.10.2778310. [DOI] [PubMed] [Google Scholar]
- 15.Giardina C., Caniglia D.M., D’Aprile M. Nuclear morphometry in Squamous Cell Carcinoma (SCC) of the tongue. Oral Oncol Eur J Cancer. 1996;32B(March (2)):91–96. doi: 10.1016/0964-1955(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 16.Bancroft J.D., Gamble M. 6th ed. Elsevier; China: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- 17.Mehrotra R., Yadav S. Oral squamous cell carcinoma: etiology, pathogenesis and prognostic value of genomic alterations. Indian J Cancer. 2006;43(April–June (2)):60–66. doi: 10.4103/0019-509x.25886. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre A.N. Nuclei shape analysis: a statistical approach. Image Anal Stereol. 2008;27:1–10. [Google Scholar]
- 19.Kronqvist P., Kuopio T., Collan Y. Morphometric grading in breast cancer: thresholds for mitotic counts. Hum Pathol. 1998;29(December (12)):1462–1468. doi: 10.1016/s0046-8177(98)90017-x. [DOI] [PubMed] [Google Scholar]
- 20.ten Kate T.K., Belien J.A.M., Smeulders A.W.M., Baak J.P.A. Method for counting mitoses by image processing in Feulgen stained breast cancer sections. Cytometry. 1993;14:241–250. doi: 10.1002/cyto.990140302. [DOI] [PubMed] [Google Scholar]
- 21.Skaland I., van Diest P.J., Janssen E.A.M., Gudlaugsson E., Baak J.P.A., HonCausa Prognostic differences of World Health Organization-assessed mitotic activity index and mitotic impression by quick scanning in invasive ductal breast cancer patients younger than 55 years. Hum Pathol. 2008;39(April (4)):584–590. doi: 10.1016/j.humpath.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Baak J.P.A. Mitosis counting in tumors. Hum Pathol. 1990;21(July (7)):683–685. doi: 10.1016/0046-8177(90)90026-2. [DOI] [PubMed] [Google Scholar]
- 23.Simpson J.F., Dutt P.L., Page D.L. Expression of mitoses per thousand cells and cell density in breast carcinomas: a proposal. Hum Pathol. 1992;23(June (6)):608–611. doi: 10.1016/0046-8177(92)90314-s. [DOI] [PubMed] [Google Scholar]
- 24.Tolbert P.E., Shy C.M., Allen J.W. Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res. 1992;271(February (1)):69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 25.Birchall M.A., Schock E., Harmon B.V., Gobe G. Apoptosis, mitosis, PCNA and bcl-2 in normal, leukoplakic and malignant epithelia of the human oral cavity: prospective, in vivo study. Oral Oncol. 1997;33(November (6)):419–425. doi: 10.1016/s0964-1955(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 26.Imai Y., Sasaki T., Fujibayashi T. Volume-corrected mitotic index as a prognostic factor in oral squamous cell carcinomas. Oral Oncol. 2001;37(January (1)):72–76. doi: 10.1016/s1368-8375(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 27.Timonen S., Therman E. The changes in the mitotic mechanism of human cancer cells. Cancer Res. 1950;10(July (7)):431–439. [PubMed] [Google Scholar]
- 28.Jadhav K.B., Ahmed Mujib B.R., Gupta N. Crystal violet stain as a selective stain for the assessment of mitotic figures in oral epithelial dysplasia and oral squamous cell carcinoma. Indian J Pathol Microbiol. 2012;55(July–September (3)):283–287. doi: 10.4103/0377-4929.101731. [DOI] [PubMed] [Google Scholar]
- 29.Scully R.E. Mitosis counting I. Hum Pathol. 1976;7(4):481–482. doi: 10.1016/s0046-8177(76)80062-7. [DOI] [PubMed] [Google Scholar]
- 30.Donhuijsen K. Mitosis counts: reproducibility and significance in grading of malignancy. Hum Pathol. 1986;17(November (11)):1122–1125. doi: 10.1016/s0046-8177(86)80417-8. [DOI] [PubMed] [Google Scholar]
- 31.Gandor D.W., Meyer J. A simple two-dye basic stain facilitating recognition of mitosis in plastic embedded tissue sections. Stain Technol. 1988;63(March (2)):75–81. doi: 10.3109/10520298809107165. [DOI] [PubMed] [Google Scholar]
- 32.Lee T.K., Myers R.T., Marshall R.B., Bond M.G., Kardon B. The significance of Mitotic Rate: a retrospective study of 127 thyroid Carcinomas. Hum Pathol. 1985;16(October (10)):1042–1046. doi: 10.1016/s0046-8177(85)80282-3. [DOI] [PubMed] [Google Scholar]
- 33.Klijanienko J., El-Naggar A.K., de Braud F. Tumor vascularization, mitotic index. Histopathologic grade, and DNA ploidy in the assessment of 114 head and neck squamous cell carcinomas. Cancer. 1995;75(April (7)):1649–1656. doi: 10.1002/1097-0142(19950401)75:7<1649::aid-cncr2820750715>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Pollard T.D., Earnshaw W.C. 2nd ed. Saunders Elsevier; China: 2008. Cell Biology. [Google Scholar]
- 35.Rao R.S., Patil S., Agarwal A. Comparison and evaluation of mitotic figures in oral epithelial dysplasia using crystal violet and Feulgen stain. J Contemp Dent Pract. 2014;15(May (3)):273–277. doi: 10.5005/jp-journals-10024-1527. [DOI] [PubMed] [Google Scholar]
- 36.Kramer H., Windrum G.M. The metachromatic staining reaction. J Histochem Cytochem. 1955;3(May (3)):227–237. doi: 10.1177/3.3.227. [DOI] [PubMed] [Google Scholar]
- 37.van Straaten H.W.M., Hekking J.W., Drukker J. A HCl-Toluidine blue staining procedure specific for nuclei, mitotic figures and axons in GMA embedded embryonic neural tissue. Stain Technol. 1987;62(September (5)):360–362. doi: 10.3109/10520298709108024. [DOI] [PubMed] [Google Scholar]
- 38.Deitch A.D. A method for the cytophotometric estimation of nucleic acids using methylene blue. J Histochem Cytochem. 1964;12(June):451–461. doi: 10.1177/12.6.451. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph P., Peters J., Lorenz D., Schmidt D., Parwaresch R. Correlation between mitotic and Ki-67 labeling indices in paraffin-embedded carcinoma specimens. Hum Pathol. 1998;29(November (11)):1216–1222. doi: 10.1016/s0046-8177(98)90248-9. [DOI] [PubMed] [Google Scholar]
- 40.Scragg M.A., Johnson N.W. Epithelial cell kinetics – a review of methods of study and their application to oral mucosa in health and disease Part A. Methods for studying cell proliferation and some sources of variation. J Oral Pathol. 1980;9(November (6)):309–341. doi: 10.1111/j.1600-0714.1980.tb00389.x. [DOI] [PubMed] [Google Scholar]