Abstract
Liquiritigenin is a chiral flavonoid present in plant based food, nutraceuticals, and traditional medicines. It is also an important ingredient present in licorice. The purpose of this study is to explore the pharmacological activity of racemic liquiritigenin utilizing several in vitro assays with relevant roles in colon cancer and diabetes. Where possible, the pure enantiomers were tested to identify the stereospecific contribution to the activity. In vitro antioxidant, anticancer, anti-inflammatory activities (cyclooxygenase inhibition), antidiabetic activities (alpha-amylase and alpha-glucosidase inhibition) as well as cytochrome P450 (CYP450) inhibitory activities were assessed. Racemic liquiritigenin demonstrated a dose-dependent inhibition of alpha-amylase enzyme whereas its pure enantiomers did not. Racemic liquiritigenin showed moderate antiproliferative activity on a HT-29 (human colorectal adenocarcinoma) cancer cell line that was dose-dependent and potent inhibitory effects on the cyclooxygenase-2 enzyme. The flavonoid did not inhibit the activity of cytochrome CYP2D6 over the concentration range studied but was a potent antioxidant. The current study demonstrated the importance of understanding the stereospecific pharmacological effects of liquiritigenin enantiomers in alpha-amylase inhibition.
Keywords: Liquiritigenin, Flavonoid, Chiral, Stereospecific, Pharmacology
INTRODUCTION
Flavonoids are a group of polyphenolic compounds of low molecular weight (200-600 g/mol) (1) that present a common benzo-γ-pyrone structure. Flavanones are a class of flavonoids that are almost uniquely present in citrus fruits (2,3,4,5). Additionally, flavanones are also present in plant based foods (6,7,8,9,10,11), multiple nutraceuticals, natural health products, and traditional medicines in widely varying concentrations (12,13,14), Some flavanones present a unique structural feature known as chirality, which distinguishes them from other classes of flavonoids. Liquiritigenin (Fig. 1) belongs to the chiral flavanone family (15) and is an important ingredient present in Glycyrrhiza glabra (liquorice) (16) and some Chinese medicinal herbs. Liquiritigenin has been reported as an important bioactive ingredient of the popular Chinese herb Shao Yao Gan Cao Tang for its analgesic and anti-inflammatory properties (17).
Fig. 1.
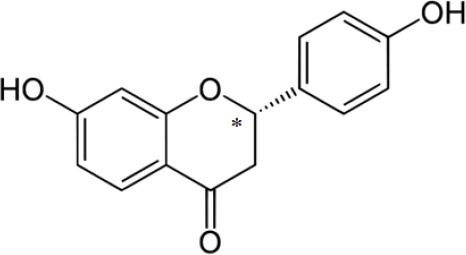
Chemical structure of liquiritigenin. Chiral center is denoted by *.
It has also been identified as an important constituent of heartwood of Dalbergia odorifera T. Chen (HEF), a Chinese medicine which demonstrates antidiabetic effects (18).
To more thoroughly understand liquiritigenin’s pharmacological activity, as well as its therapeutic and toxic effects, especially in light of the lipophilicity, a stereospecific HPLC method (16) has been developed and validated to separate and isolate liquiritigenin’s pure enantiomers and examine for the first time the stereoselective liquiritigenin in various biological assays. Previous studies have suggested some pharmacological activity for liquiritigenin including anticancer, anti-inflammatory, antidiabetic, and antioxidant activities (19,20,21,22). However, the role of each enantiomer in the pharmacological activity has not been fully evaluated. This study describes and compares the antidiabetic, antioxidant, cyclooxygenase (COX) inhibitory, and anticancer activities of liquiritigenin and its enantiomers where possible.
MATERIALS AND METHODS
Materials
Materials used in the current study and their respective suppliers are given in section below.
Racemic liquiritigenin from Extrasynthese (Genay, France), HPLC grade acetonitrile and water from J. T. Baker (Phillipsburg, USA), Phosphoric acid from Aldrich Chemical Co. Inc. (Milwaukee, USA), Silastic® laboratory tubing from Dow Corning Corporation, (Midland, USA), Intramedic® polyethylene tubing from Becton Dickinson Primary Care Diagnostics, Becton Dickinson and Company (Sparks, USA), dimethyl sulfoxide (DMSO), poly(ethylene glycol) (PEG) 400, phosphate buffered saline (PBS), alpha-amylase from porcine pancreas type VI-B, 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), and 4-nitrophenyl alpha-D-glucopyranoside from Sigma-Aldrich (St. Louis, USA), amylase HR reagent from Megazyme International Ireland (Wicklow, Ireland), the COX inhibitor screening assay kit (catalog No. 560131) and the antioxidant assay kit (catalog No. 709001) from Cayman Chemical Company (Ann Arbor, USA), and the Vivid P450 CYP2D6 blue screening kit from (Life Technologies; Burlington, Canada).
Cell culture
HT-29 (human colorectal carcinoma) cells were obtained from the American Type Culture Association (ATCC, Manassas, USA). Trypsin-Ethylenediaminetetraacetic acid (EDTA), trypan blue, phosphate-buffered saline (PBS), 4-methylumbelliferone, resazurin, cell culture tested sodium carbonate, HEPES, beta-glucosidase, sodium pyruvate, McCoy’s 5A medium, penicillin, streptomycin, and insulin were purchased from Sigma (St. Louis, USA). Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 Ham (DMEM/F-12) without phenol red and RMPI 1640 medium were purchased from Gibco Industries Inc. (Langley, USA). Fetal bovine serum (FBS) was procured from Equitech-Bio Inc. (Kerrville, USA).
Separation and collection of pure enantiomers
The limited commercial availability of pure liquiritigenin enantiomers made necessary the manual separation and collection of pure enantiomers using the analytical methods described earlier (16). The collection of the pure enantiomers was achieved following chiral chromatographic separation of multiple injections of racemic liquiritigenin. The racemic mixture of the flavonoid is readily available from commercial sources. Multiple injections of racemic liquiritigenin were performed via HPLC. Separation was carried out isocratically at ambient temperature (25 ± 1°C), with a flow rate of 0.6 mL/min and ultraviolet (UV) detection at 210 nm. The analytical column used was a Chiralpak® AD-RH (150 × 4.6 mm i.d., 5 μm particle size, Chiral Technologies Inc., West Chester, USA) and the mobile phase consisted of acetonitrile, water and acetic acid (50:50:0.05 v:v:v) (16). Enantiomeric peaks were collected in sequence using separate 50 mL tubes and were subsequently dried to completion under nitrogen. This labour intensive process combined with the relatively high quantities required for use in pharmacodynamic assays and the need to perform circular dicroism (CD) spectroscopy on some of the enantiomers also limited the number of stereospecific pharmacological studies completed. CD spectra obtained for liquiritigenin enantiomers were consistent with previously published data (23).
Antidiabetic activity
Alpha-glucosidase inhibition assay
Liquiritigenin was dissolved in DMSO and serially diluted to concentrations between 0 and 200 μg/mL. 160 μL of 100 mM phosphate buffer (pH 6.8), 25 μL of 20 mM para-nitrophenyl-alpha-D-glucopyranoside (PNPG) in phosphate buffer, and 10 μL of the flavonoid in DMSO was added to a 96-well plate (10 μL DMSO was added to the control wells). The plate was incubated at 30 °C for 5 min and then 10 μL of the buffer containing 0.02 mg/mL of enzyme was added to each well. Additional incubation for 5 min followed and 20 μL of 3.25 M sodium hydroxide was added to each well to stop the reaction. Alpha-glucosidase inhibition was determined through the colorimetric assay described by Tadera, et al. (24). The assay uses para-nitrophenyl-alpha-D-glucopyranoside (PNPG), which is hydrolyzed specifically by alpha-glucosidase into a yellow colored product (para-nitrophenol). The absorbance of liberated para-nitrophenol was measured at 410 nm.
Alpha-amylase inhibition assay
Racemic liquiritigenin was dissolved in methanol at concentrations between 0 and 200 μg/mL. Subsequent experiments with liquiritigenin used methanolic dilutions of the racemates and enantiomers at concentrations from 0 to 1 μg/mL. To assess the inhibition of alpha-amylase, a colorimetric assay was adapted and modified from work previously developed by Tadera, et al. (24). A synthetic substrate, non-reducing-end blocked para-nitrophenyl maltoheptaoside (BPNPG7) commercially prepared as amylase HR reagent, which is hydrolyzed specifically by alpha-amylase into para-nitrophenyl maltosaccharide is utilized. The alpha-glucosidase present in the assay then converts the new substrate into para-nitrophenol which has a yellow colour and absorbance at 410 nm was immediately read at room temperature (23 ± 1°C) using the Synergy HT Multi-well plate reader and Gen5TM data analysis software (Biotek® Instruments Inc., Winnoski, USA). Inhibition (%) was calculated as 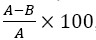 , where, A was the average absorbance of the control wells and B was the absorbance of the wells containing flavonoids.
, where, A was the average absorbance of the control wells and B was the absorbance of the wells containing flavonoids.
In vitro cyclooxygenase-1 and -2 inhibitory assay
Cyclooxygenase (COX) Inhibitor Screening Assay Kit was utilized to assess COX-1 and COX-2 inhibitory activity of liquiritigenin. On the day of the experiment, racemic liquiritigenin was dissolved in DMSO to yield concentrations of 1, 10, 50, and 100 μg/mL. Standards and samples used in the assay were prepared two days in advance. On day 1, the reaction buffer, COX-1 (bovine), COX-2 (human recombinant), heme, arachidonic acid, hydrochloric acid, and stannous chloride (SnCl2) were prepared according to the manufacturer’s instructions. Inactivated enzymes (COX-1 and COX-2) were used to generate background values; active enzymes were used to asses 100% initial activity for COX-1 and COX-2. All samples prepared this day were stored at 4 °C. On day 2, the reagents for the assay were prepared and COX inhibitory activity was assessed. The inhibitory activity of the compounds was tested individually for COX-1 and COX-2. The wash buffer, prostaglandin (PG) standard, PG screening AChE tracer, and PG screening antiserum were prepared following the manufacturer’s instructions. COX reactions were performed in serial dilutions for the background samples, the 100% initial activity samples, and the COX inhibitor samples. Controls, standards, and samples were placed in 96-well plates and incubated overnight at room temperature. On day 3, the plate was developed using Ellman’s reagent for 60 – 90 min. The COX inhibitor activity of the compounds was measured using Synergy HT multi-well plate reader (Biotek ® Instruments Inc., Winnoski, USA) using Gen 5 software from Biotek ®. Absorbance was read at 405 – 420 nm. The COX inhibitor activity of the samples was compared to the percentage of standard bound/maximum bound (%B/B0). The COX inhibitor screening assay kit directly measures the level of PGF2α produced from PGH2 after SnCl2 reduction. PGF2α is quantified via enzyme immunoassay (EIA) using a broadly specific antibody that binds PG compounds. This assay includes both bovine COX-1 and human recombinant COX-2 enzymes in order to screen isozyme-specific inhibitors. For more details see the manufacturer’s instruction manual. The inhibition of COX was expressed as percentage of COX activity.
In vitro antioxidant activity assay
The Cayman antioxidant assay kit was used to measure the total antioxidant capacity of liquiritigenin. On the day of the experiment, racemic liquiritigenin was dissolved in DMSO to yield concentrations of 1, 10, 50, and 100 μg/mL. The assay buffer, chromogen, trolox (a water soluble tocopherol analogue), and hydrogen peroxide were prepared on the day of the experiment following the manufacturer’s instructions. A trolox standard curve was constructed using a serial of dilutions. Controls, standards, and treatments at different concentrations (1 – 100 μg/mL) were placed in 96-well plates and hydrogen peroxide was used to start the oxidative reaction. The anti-oxidant activity of the compounds was measured using Synergy HT multi-well plate reader (Biotek ® Instruments Inc., Winnoski, USA) using Gen 5 software from Biotek ® after five min of exposure to hydrogen peroxide. Absorbance was read at 750 nm to decrease interference. The antioxidant capacity of the samples was compared to that of trolox.
The kit measured the total antioxidant capacity based on the ability of the antioxidants in the sample to inhibit the oxidation of ABTS+ to ABTS•+ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)). The antioxidant capacity expressed as trolox equivalent antioxidant capacity (TEAC) of racemic liquiritigenin was measured. For more details see the manufacturer’s instruction manual.
In vitro anticancer cytotoxicity assay
Counted HT-29 cells were seeded at a density of 5 × 104 per well in a 96-well plate, then incubated at 37 °C in a 5% CO2 atmosphere for 24 h. On the day of the experiment, racemic liquiritigenin, was dissolved in DMSO and diluted with the corresponding media to yield concentrations of 1, 5, 10, 50, 100, and 250 μg/mL of each enantiomer. Old media were aspirated from the wells, and cells were treated with media containing liquiritigenin racemate at different concentrations (1 – 250 μg/mL); DMSO in media and media alone were used as negative control and blank, respectively. Plates were incubated at 37 °C in a 5% CO2 atmosphere. After 72 h incubation, the 96-well plates were removed from the incubator; 20 μL of 10% alamar Blue (resazurin) fluorescent dye was added to the control and treatment groups in the 96-well plates and the plate was incubated for an additional 3 h at the same conditions. After that, the 96-well plates were placed in a darkened environment for 30 min at room temperature; then placed into Synergy HT multi-well plate reader using Gen 5 software from Biotek®. Fluorescence was read at an excitation of 530 nm and an emission of 590 nm. The percent cell viability (relative to control) was measured for each cell line exposed to varying concentrations of racemic liquiritigenin.
CYP2D6 inhibition assay
CYP2D6 inhibitory activity of racemic liquiritigenin was assessed using a Vivid P450 CYP 2D6 blue screening kit. Stock solutions of racemic liquiritigenin were prepared in methanol at concentrations of 0.01, 0.1, 1, 10, 50, and 100 μM. Utilizing a 96-well plate, 40 μL of flavonoid, quinidine (positive CYP2D6 inhibitor control), or blank methanol (solvent control) was added to each well. Twenty minutes of incubation followed with 50 μL of pre-mixture (containing Baculosomes® reagent, the regeneration system and reaction buffer) or 50 μL of reaction buffer only as a background control at room temperature (25 °C). To start the reaction, 10 μL of a mixture of Vivid substrate and NADP+ was added to each well. Fluorescence changes were monitored by immediate reading of the plate on a Synergy HT multiwell plate reader with Gen5 data analysis software. Excitation and emission wavelengths were 415 and 460 respectively. Monitoring occurred immediately after the start of the reaction and continued every min for 60 min at room temperature (23 ± 1 °C) as stipulated by the Vivid P450 kit protocol. The final methanol volume in the reactions was ≤ 1%.
Statistical analysis
Data were presented as mean and standard error of the mean (mean ± SEM). Student’s t-test was used to compare unpaired samples using Excel software. A P-value < 0.05 was considered statistically significant.
RESULTS
Antidiabetic activity
Alpha-glucosidase inhibition
Inhibition of alpha-glucosidase by racemic liquiritigenin was screened using a colorimetric assay via measurement of para-nitrophenol at 410 nm. In ongoing studies in our laboratory we have determined that this enzyme was inhibited by structurally similar stilbene compounds (25). The results are reported in Fig. 2. Racemic liquiritigenin showed weak and variable alpha-glucosidase inhibitory activity with apparent induction at the higher concentrations. The lack of reproducible concentration dependent alpha-glucosidase inhibitory activity by any of the tested racemic flavonoids made it an unattractive assay for further enantiospecific investigation.
Fig. 2.
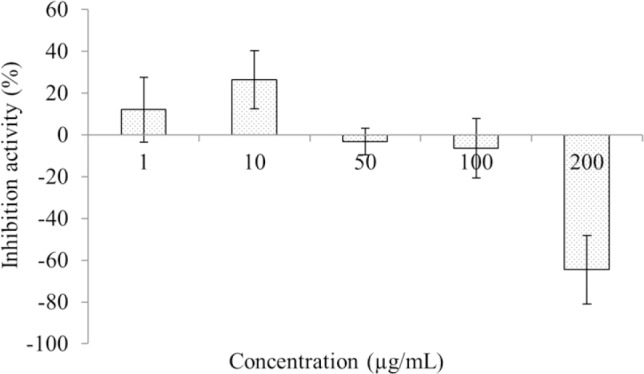
Alpha-glucosidase percent inhibition of racemic liquiritigenin using a colorimetric assay via measurement of para-nitrophenol at 410 nm (n = 6; Mean ± SEM).
Alpha-amylase inhibition
The liquiritigenin racemate was initially assessed for alpha-amylase inhibitory activity at the concentration range of 1 – 200 μg/mL (Fig. 3). Weak inhibition was observed at 1 μg/mL. Subsequent ascending concentration point showed induced activity of the enzyme. It was again decided to repeat the alpha-amylase inhibition assay again at the lower concentration range of 0.01 – 0.75 μg/mL.
Fig. 3.
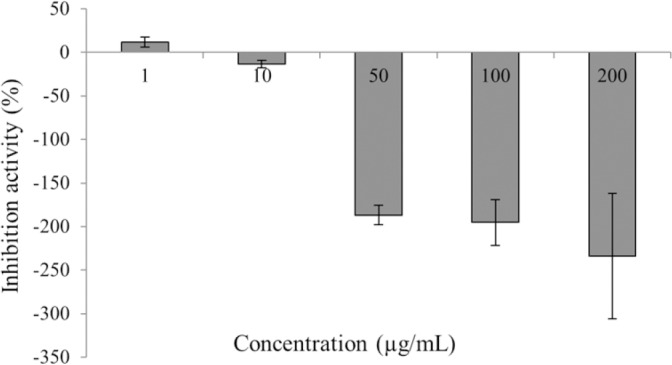
Alpha-amylase percent inhibition of racemic liquiritigenin using a colorimetric assay via measurement of paranitrophenol at 410 nm (n = 6; Mean ± SEM).
Alpha-amylase inhibition activity of racemic liquiritigenin and its enantiomers was measured over the concentration range of 0.01 – 0.75 μg/mL (Fig. 4). The dose-dependent decrease of enzyme inhibition demonstrated by the liquiritigenin racemate is apparent; however this dose-dependency is not statistically significant. Greater percent inhibition at the two lowest concentration points (15% at 0.01 μg/mL and 12% at 0.05 μg/mL) was noted when compared to the lowest concentration point (11% at 1 μg/mL) of the higher concentration range (1 – 200 μg/mL) assay. This observation is not noted in the pure enantiomers, which demonstrated significantly higher alpha-amylase inhibition at several concentrations than racemic liquiritigenin (Fig. 4).
Fig. 4.
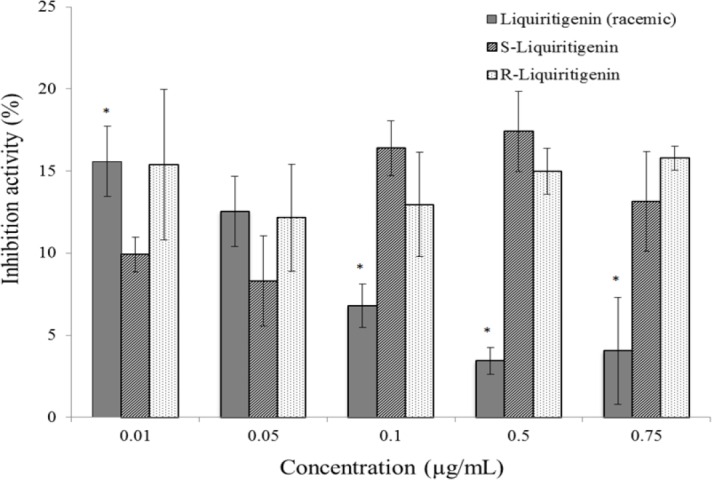
Alpha-amylase percent inhibition of racemic liquiritigenin and its pure enantiomers (n = 6; mean ± SEM) using a colorimetric assay via measurement of para-nitrophenol at 410 nm. *Represents a significant difference between racemic liquiritigenin and its enantiomers (P < 0.05).
In vitro cyclooxygenase activity
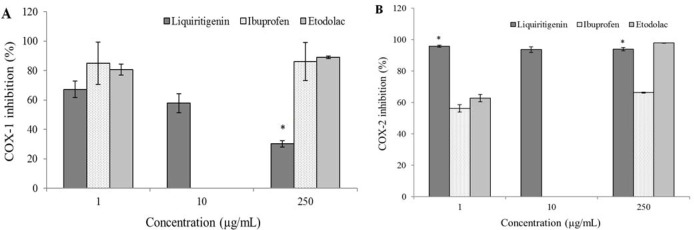
The assay measured COX inhibition by the quantification of PGF2α produced by stannous chloride reduction of PGH2. Ibuprofen was used as a positive control for the COX-1 assay due to its inhibitory activity towards both COX-1 and COX-2. For the COX-2 assay, ibuprofen and etodolac were employed as positive controls although etodolac is suggested to be a more selective COX-2 inhibitor.
Racemic liquiritigenin was assessed for its COX-1 and COX-2 inhibitory activity using a commercially available ELISA assay. The results are presented in Fig. 5. Liquiritigenin racemate showed COX-1 inhibitory activity approximately equivalent to ibuprofen (positive control) at 1 μg/mL, with decreasing inhibition at the remaining consecutive concentrations. At 250 μg/mL racemic liquiritigenin demonstrated poor COX-1 inhibition compared to the positive controls (ibuprofen and etodolac). Potent COX-2 inhibition was apparent, with percent inhibition approaching 100% at the lowest concentration tested (1 μg/mL) as well as both subsequent concentrations (10 and 250 μg/mL).
Fig. 5.
(A) cyclooxygenase-1 (COX-1), and (B) cyclooxygenase-2 (COX-2) inhibition after addition of racemic liquiritigenin, ibuprofen and etodolac activity using a commercially available ELISA assay (n = 3, mean ± SEM). Values are expressed as percentage (%). *Represents a significant difference between racemic liquiritigenin and ibuprofen or etodolac (P < 0.05).
In summary, racemic liquiritigenin demonstrated COX-1 and COX-2 inhibitory activity. COX-1 inhibitory activity results were characteristic of decreasing inhibition with increasing concentration of the racemates. COX-2 inhibition assay results consistently demonstrated potent and COX-2 selective inhibition over the concentration range tested by racemic liquiritigenin.
In vitro antioxidant activity
The assay reported here measures direct antioxidant activity via the formation of the radical cation ABTS•+ from the oxidation of ABTS by the radical ferryl myoglobin. Inhibitory activity is assessed by the test compound ability to prevent the oxidation of ABTS to ABTS•+ compared to the positive control trolox. ABTS•+ is quantified spectrophotometrically with UV detection.
As shown in Fig. 6, racemic liquiritigenin did not show antioxidant activity greater than baseline over the concentration range tested (1 – 100 μg/mL). Due to the difficulty in procuring and collecting pure enantiomers as well as the non-enzymatic nature of the antioxidant assay, further enantiospecific testing was not conducted.
Fig. 6.
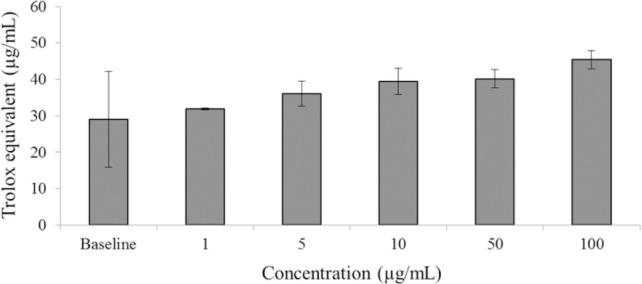
Antioxidant capacity (trolox equivalent) of racemic liquiritigenin (n = 4, mean ± SEM) compared to DMSO alone (baseline). Measured by direct antioxidant activity via the formation of the radical cation ABTS•+ (2,2’-azinobis (3-ethylbenzothiazoline-6-sulphonic acid)) from the oxidation of ABTS, quantified with UV spectrophotometry.
In vitro cytotoxicity activity
Anticancer activity of liquiritigenin has been observed (22). Screening for antiproliferative activity using the alamar Blue (resazurin) fluorescent cell viability measurement is relatively simple and accurate. The non-fluorescent dye resazurin can be metabolized by viable cells, whereas non-viable cells cannot. Resorufin is the fluorescent metabolite of resazurin, and can be detected using a plate reader. Relative number of viable cells can be determined using this measurement.
The anti-proliferative activity of racemic liquiritigenin on HT-29 cancer cell line is shown in Fig. 7. This cell line was chosen specifically because pharmacokinetic studies characterization revealed elimination and excretion predominately by non-renal routes (liver and feces) which could present high concentrations in the colon. Additionally, other flavonoids studied in our laboratory have been observed to have particular antiproliferative activity against liver and colon cancer cell lines. Racemic liquiritigenin showed moderate antiproliferative activity in a dose-dependent manner. The screen for anticancer activity utilizing an alamar Blue anti-proliferative assay of HT-29 cells showed moderate to poor decreases in cell viability for racemic liquiritigenin in a dose-dependent manner. Thus, this particular assay is not suitable for pursuit in further investigating the enantiospecific pharmacologic activity of liquiritigenin.
Fig. 7.
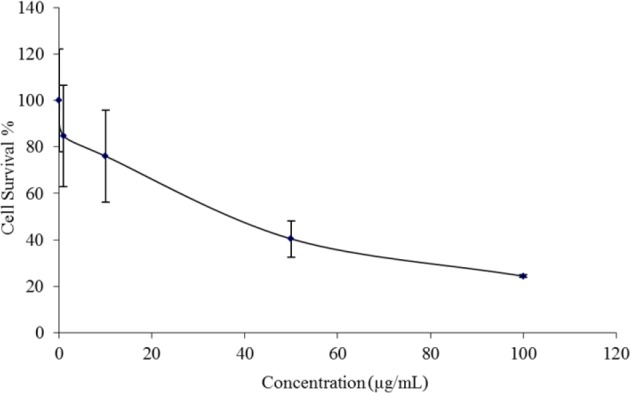
HT-29 cell viability after administration of racemic liquiritigenin at different concentrations. Measured via cell counting after alamar Blue antiproliferative assay.
CYP 2D6 Inhibition
Pharmacokinetic drug interactions include alterations in drug metabolism. A common culprit of this type of reaction can be a compound which inhibits or induces drug metabolizing enzymes in the CYP450 family. CYP2D6 is a CYP450 enzyme responsible for the metabolism of many commonly used drugs (26). Flavonoids from several subclasses have been shown to inhibit various subtypes of CYP450 drug metabolizing enzymes (27). Liquiritigenin racemates were assessed for their inhibitory activity of CYP2D6 (Fig. 8). Here in our studies, no significant inhibition of CYP2D6 was observed at any concentration of liquiritigenin. Further, neither inhibition nor induction appeared in a dose-dependent manner, leaving weak activity and high variability the hallmarks of the assay.
Fig. 8.
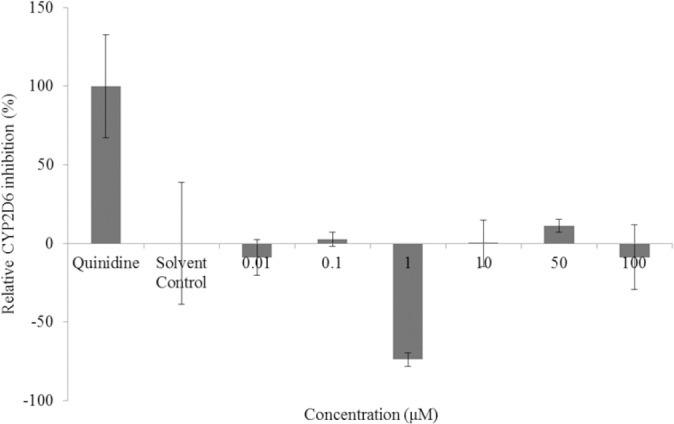
CYP2D6 inhibition (%) of liquiritigenin racemate, quinidine (positive control), and blank methanol (solvent control) from 0.01 to 100 μM (n=3, mean ± SEM). Assessed using a Vivid P450 CYP2D6 blue screening kit and quantified by fluorescence.
DISCUSSION
In vitro studies of the pharmacological activity of the stereoisomers of liquiritigenin are necessary since differences in the stereochemistry of compounds can impact their disposition, metabolism, and elimination by the body and it is also likely that enantiomers produce different pharmacological effects in biological systems depending on their stereochemical configuration. As was stated in the introduction, racemic liquiritigenin has been reported to demonstrate multiple pharmacological effects. However, as far as we know, there are no studies examining the pharmacological contribution of liquiritigenin enantiomers compared to its racemate. Structurally similar chiral flavonoids such as Naringenin (28), hespertein (29), eriodictyol (30), taxifolin (30), pinocembrin and pinostrobin (31) have been examined by our group and demonstrated some stereoeselectivity in their disposition and pharmacological effects.
Due to the ready availability of commercial racemic flavonoids, in vitro studies can be of significant utility in exploratory pharmacology for the screening of the pharmacological activity of these compounds; if the desired pharmacological effect is obtained with the racemate, more in-depth studies can be performed with the pure enantiomers in the future. It has been previously reported that for some racemic xenobiotics, a detrimental effect is apparent when given as a mixture while the pure stereoisomer may produce a desired effect, i.e. the teratogenic effect of thalidomide results from DNA intercalation of the S-enantiomer in the racemate, but R-thalidomide does not produce such an effect.
However, some chiral drugs like ibuprofen that have a carbon chiral centre, are usually marketed as the racemic mixture because of the expense and time consuming nature of making the pure active S-enantiomeric compound. Furthermore, some studies have demonstrated that an isomerase converts R-ibuprofen into the active S-enantiomer, making the production of pure S-ibuprofen, which is costly, unnecessary.
Therefore, in vitro studies of each stereoisomer of liquiritigenin are needed. Although the isolation of stereoisomers is possible with the validated HPLC method (16), the cost resources of isolating them is often a limiting factor to extensive studies in an academic laboratory. In addition, it is a time consuming process, taking between two to six weeks to collect the amount of individual enantiomers necessary for one single assay. Consequently, as many assays were performed with pure compounds as possible within the inherent time and cost constraints. In addition, for the stereoisomers that are commercially available, issues with stereochemical purity must be taken into consideration. Therefore, it is important to consider the commercial sources of the pure stereoisomers to ensure the purity of these compounds. In this study, one in vitro drug interaction assay and four in vitro pharmacologic activity models with relevance to chronic disease pathology were chosen to assess bioactivity in liquiritigenin, and explore the contribution of the flavonoid enantiomers to any observed pharmacological activity. Liquiritigenin racemates were tested first in each assay to confirm or refute reported activity. Assessment of alpha-amylase inhibition of racemic liquiritigenin as well as their pure enantiomers is reported for the first time. Alpha-amylase inhibition was seen by liquiritigenin racemate with a characteristic decline in enzyme inhibition as concentration increased. Interestingly, the decline in enzyme inhibition and apparent induction in enzyme activity was not seen in the pure enantiomers of each flavonoid. This suggests possible differential pharmacologic behaviour and/or differential physiochemical characteristics of racemate compared to its enantiomers. Here, we demonstrated moderate to weak alpha-glucosidase inhibition by racemic liquiritigenin. Since performing these experiments, alpha-glucosidase inhibition was observed by pure S-liquiritigenin extracted from a Chinese medicinal plant in one study (18). However, this was not compared to the racemates, which could have different enzyme inhibition characteristics as well as physiochemical properties than the pure enantiomers. A previous study reported liquiritigenin to possess overall moderate CYP2D6 inhibition (32). However, at 10 μM liquiritigenin showed around 60% activation of CYP2D6, similar to the effect seen at 1 μM in these studies. After screening racemic liquiritigenin for CYP2D6 inhibitory activity, the results suggest that liquiritigenin is likely not an inhibitor.
CONCLUSIONS
Overall, this study has demonstrated that liquiritigenin exhibits pharmacological activities in a variety of exploratory and well established in vitro assays. Interestingly, these investigations have revealed that chiral differences in the chemical structure of liquiritigenin may result in significant pharmacological effects. In the alpha-amylase antidiabetic assay, racemic liquiritigenin demonstrated a dose-dependent decline in the alpha-amylase inhibitory activity as the concentration increases, while its pure enantiomer did not. Liquiritigenin’s pure enantiomers demonstrated significantly higher alpha-amylase inhibition at several concentrations than racemic liquiritigenin. Further studies are necessary with the pure stereoisomers of liquiritigenin to elucidate their potential differences in anti-inflammatory and antioxidant activity.
The validated HPLC methods described elsewhere (16) was successfully used to isolate the stereoisomers of liquiritigenin that are not commercially available. Nonetheless, the expense and time needed to collect enough quantity of the compounds limited the number of investigations able to be conducted. A number of technical issues require further experimental scrutiny including: purity of the compounds obtained from commercial suppliers, and solubility.
ACKNOWLEDGEMENTS
The authors would like to thank Kuwait University for graduate scholarship awarded to Samaa Alrushaid.
REFERENCES
- 1.Haslam E. Cambridge University Press; 1998. Practical polyphenolics: from structure to molecular recognition and physiological action; p. 422. [Google Scholar]
- 2.Cook NC, Samman S. Flavonoids-Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7(2):66–76. [Google Scholar]
- 3.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, release 3.1 (2013). [Internet] 2013. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Flav/Flav3-1.pdf .
- 4.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem. 1999;47(9):3565–3571. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen SE, Freese R, Kleemola P, Mutanen M. Flavonoids in human urine as biomarkers for intake of fruits and vegetables. Cancer Epidemiol Biomarkers Prev. 2002;11(5):459–466. [PubMed] [Google Scholar]
- 6.Bugianesi R, Catasta G, Spigno P, D’Uva A, Maiani G. Naringenin from cooked tomato paste is bioavailable in men. J Nutr. 2002;132(11):3349–3352. doi: 10.1093/jn/132.11.3349. [DOI] [PubMed] [Google Scholar]
- 7.Le Gall G, DuPont MS, Mellon FA, Davis AL, Collins GJ, Verhoeyen ME, et al. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J Agric Food Chem. 2003;51(9):2438–2446. doi: 10.1021/jf025995e. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean ME, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48(7):2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- 9.Daigle DJ, Conkerton EJ, Sanders TH, Mixon AC. Peanut hull flavonoids: their relationship with peanut maturity. J Agric Food Chem. 1988;36(6):1179–1181. [Google Scholar]
- 10.Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57(2):235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 11.Krause M, Galensa R. Analysis of Enantiomeric Flavanones in Plant Extracts by High-Performance Liquid Chromatography on a Cellulose Triacetate Based Chiral Stationary Phase. Chromatographia. 1991;32(1):69–72. [Google Scholar]
- 12.Sayre CL, Davies NM. Quantification of three chiral flavonoids with reported bioactivity in selected licensed Canadian natural health products and US marketed dietary supplements. J Pharm Pharm Sci. 2013;16(2):272–278. doi: 10.18433/j3x01q. [DOI] [PubMed] [Google Scholar]
- 13.Corradini C, Borromei C, Cavazza A, Merusi C, De Rossi A, Nicoletti I. Determination of flavanones in citrus byproducts and nutraceutical products by a validated RP-HPLC method. J Liq Chromatogr Relat Technol. 2009;32(10):1448–1462. [Google Scholar]
- 14.Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68([22]-[24]):2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Yáñez JA, Andrews PK, Davies NM. Methods of analysis and separation of chiral flavonoids. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(2):159–181. doi: 10.1016/j.jchromb.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Sayre CL, Hopkins M, Takemoto JK, Davies NM. Chiral analytical method development of liquiritigenin with application to a pharmacokinetic study. Biomed Chromatogr. 2013;27(3):404–406. doi: 10.1002/bmc.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan P, Huang X, Zhong M, Sun M, Qin F, Zhang C. Simultaneous determination of eight major constituents in the traditional Chinese medicine Shaoyao-Gancao-Tang by UPLC-PDA. J Med Plants Res. 2010;4(24):2615–2621. [Google Scholar]
- 18.Zhao C, Liu Y, Cong D, Zhang H, Yu J, Jiang Y, et al. Screening and determination for potential α-glucosidase inhibitory constituents from Dalbergia odorifera T. Chen using ultrafiltration-LC/ESIMS(n) Biomed Chromatogr. 2013;27(12):1621–1629. doi: 10.1002/bmc.2970. [DOI] [PubMed] [Google Scholar]
- 19.Yu JY, Ha JY, Kim KM, Jung YS, Jung JC, Oh S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules. 2015;20(7):13041–13054. doi: 10.3390/molecules200713041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, He Y, Yu H, Ma F, Wu J, Zhang X. Liquiritigenin protects rats from carbon tetrachloride induced hepatic injury through PGC-1á pathway. Evid Based Complement Alternat Med 2015. 2015:649568. doi: 10.1155/2015/649568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine. 2014;21(4):415–422. doi: 10.1016/j.phymed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Lu J, Liu Y, Meng Q, Xie J, Wang Z, et al. Liquiritigenin induces tumor cell death through mitogen-activated protein kinase- (MPAKs-) mediated pathway in hepatocellular carcinoma cells. Biomed Res Int 2014. 2014:965316. doi: 10.1155/2014/965316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaffield W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides. Tetrahedron. 1970;26(17):4093–4108. [Google Scholar]
- 24.Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52(2):149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 25.Martinez SE, Sayre CL, Davies NM. Pharmacometrics of 3-methoxypterostilbene: a component of traditional chinese medicinal plants. Evid Based Complement Alternat Med 2013. 2013:261468. doi: 10.1155/2013/261468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58(3):521–590. doi: 10.1124/pr.58.3.6. [DOI] [PubMed] [Google Scholar]
- 27.Yáñez JA, Chemuturi NV, Womble SW, Sayre CL, Davies NM. New York: John Wiley & Sons Ltd; 2012. Flavonoid Pharmacokinetics: Methods of Analysis, Pre-Clinical and Clinical Pharmacokinetics, Safety, and Toxicology; pp. 281–319. [Google Scholar]
- 28.Torres CA, Davies NM, Yanez JA, Andrews PK. Disposition of selected flavonoids in fruit tissues of various tomato (lycopersicon esculentum mill.) Genotypes. J Agric Food Chem. 2005;53(24):9536–9543. doi: 10.1021/jf051176t. [DOI] [PubMed] [Google Scholar]
- 29.Yanez JA, Remsberg CM, Miranda ND, Vega-Villa KR, Andrews PK, Davies NM. Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm Drug Dispos. 2008;29(2):63–82. doi: 10.1002/bdd.588. [DOI] [PubMed] [Google Scholar]
- 30.Vega-Villa KR, Remsberg CM, Takemoto JK, Ohgami Y, Yanez JA, Andrews PK, et al. Stereospecific pharmacokinetics of racemic homoeriodictyol, isosakuranetin, and taxifolin in rats and their disposition in fruit. Chirality. 2011;23(4):339–348. doi: 10.1002/chir.20926. [DOI] [PubMed] [Google Scholar]
- 31.Sayre CL, Alrushaid S, Martinez SE, Anderson HD, Davies NM. Pre-Clinical pharmacokinetic and pharmacodynamic characterization of selected chiral flavonoids: pinocembrin and pinostrobin. J Pharm Pharm Sci. 2015;18(4):368–395. doi: 10.18433/j3bk5t. [DOI] [PubMed] [Google Scholar]
- 32.Qiao X, Ji S, Yu S-W, Lin X-H, Jin H-W, Duan Y-K, et al. Identification of key licorice constituents which interact with cytochrome P450: evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J. 2014;16(1):101–13. doi: 10.1208/s12248-013-9544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]