Abstract
Non-alcoholic fatty liver disease (NAFLD) is a burgeoning health problem that affects 1/3 of the adult population and an increasing number of children in developed countries. Oxidative stress and insulin resistance are the mechanisms that seem to be mostly involved in its pathogenesis. This study was conceived in a NAFLD rat model to evaluate the efficacy of both metformin (MTF) and N-acetylcysteine (NAC) with dietary control on biochemical and histologic liver manifestations. Rats were classified into nine groups; normal (I), NAFLD-induced by feeding high-fat diet (HFD; II) for 12 weeks, NAFLD switched to regular diet (RD; III), NAFLD-HFD or -RD treated with MTF in a dose of 150 mg/kg (IV, V), NAC in a dose of 500 mg/kg (VI, VII) or MTF+NAC (VIII, IX) respectively for 8 weeks. After 20 weeks, the rats in group II showed notable steatosis, lobular inflammation, fibrosis accompanied with elevated (P < 0.05) serum alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (γ-GT), cholesterol, triglycerides, LDL, VLDL, leptin, tumor necrosis factor (TNF-α), transforming growth factor (TGF-β1) and hepatic malondialdehyde (MDA) compared with group I. Meanwhile, hepatic superoxide dismutase (SOD), glutathione GSH with serum HDL, adiponectin were significantly decreased (P < 0.05). These changes were to a less extent in group III. MTF or NAC individually resulted in improvement of most of these biochemical and histological parameters. These improvements were more pronounced in the combined groups VIII and IX versus each drug alone. NAC supplementation concomitant with MTF could be beneficial for the treatment of NAFLD and prevention of nonalcoholic steatohepatitis (NASH).
Keywords: NAFLD, Metformin, N-acetylcysteine, Oxidative stress, Adipocytokines, TNF-α
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a worldwide public health problem, which affects 10–24% of the world’s population, and its prevalence is greater in obese individuals, ranging from 57.5 to 74.0% (1). NAFLD is the hepatic manifestation of the metabolic syndrome comprising hypertension, insulin resistance (IR), obesity and dyslipidemia (2). The classical supporting theory in NAFLD pathogenesis is the ’two-hit’ hypothesis (3). In the first hit, the healthy liver becomes steatotic; then, the second hit is elicited by oxidative stress and cytokine synthesis. This leads to exacerbation of IR, followed by oxidative stress and liver cells dysfunction, resulting in an inflammatory process, hepatocellular degeneration and fibrosis (3).
The current standard of care for NAFLD is weight loss, which if successful improves both metabolic parameters and liver histology (4). Based on evidence that IR is the key metabolic abnormality in NAFLD, it has been proposed that treatment with insulin-sensitizing agents might prove effective (5). The anti-diabetic drug “metformin” (MTF) is non-hepatotoxic and cost-effective; therefore, it is more suitable for long-term treatment (6). However, the evidence for its beneficial effects in NAFLD treatment is still limited and conflicting reports have been published (7,8). The mixed results, heterogeneous therapeutic approaches and small number of patients have limited its widespread use in clinical practice (8). Several studies have demonstrated that oxidative stress is a major player triggering the progression of steatosis to steatohepatitis (NASH) (9). N-acetylcysteine (NAC) is a precursor of glutathione, the most powerful antioxidant in the liver. Previous studies have reported improvement of liver histopathology and reduction of oxidative stress by NAC in NAFLD rats (10). Since NASH has a multilevel and multifactorial pathogenesis, Al-Busafi et al. (11) hypothesized that a drug combination might be a more appropriate therapeutic strategy. Accordingly, this study was conceived in NAFLD rats to explore whether the use of both MTF and NAC with dietary control has positive impacts, beyond their individual effects, on liver steatosis, inflammation, fibrosis and oxidative stress.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (120 g ± 20), were bred and maintained at the Schistosome Biology Supply Center of Theodor Bilharz Research Institute (TBRI), Egypt. Rats were housed under standard conditions of temperature (25°C), humidity, and lighting with free access to laboratory rat chow and tap water ad libitum. Animal experiments were conducted after approval by the institutional review board of TBRI.
Experimental design
A total of 108 rats were randomly divided into 9 groups; 12 rats each. Normal control (I) maintained on the standard chow diet, NAFLD-induced with high-fat diet [HFD-II; 25% fats + 1% cholesterol (Win lab Laboratory chemicals) + 0.25% bile salts (Alpha chemika, India)] for 12 weeks (12), NAFLD switched to regular diet (RD-III; from 13th–20th week), NAFLD-HFD or -RD treated orally with MTF (IV, V; 150 mg/kg, Glucophage®, Mina Pharm Egypt) or NAC (VI, VII; 500 mg/kg, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) or MTF+NAC (VIII, IX), respectively from the 13th – 20th week. At the end of the experiment, all rats were weighed and then killed with an intraperitoneal injection of ketamine (80 mg/kg) after overnight fasting. Blood samples were collected for biochemical assays. Livers were immediately removed and weighed after rinsed with ice-cold saline, and sampled for assessment of liver glutathione (GSH), superoxide dismutase (SOD), malonaldehyde (MDA), and for histological study. Liver weight index (%) was calculated as liver weight/body weight × 100.
Assay of biochemical and oxidative stress markers
Blood biochemical markers including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (γ-GT), alkaline phosphatase (ALP), triglycerides (TG), total cholesterol (TC) and high-density lipoprotein (HDL), were assayed spectrophotometrically using the available commercial kits. Low-density lipoprotein (LDL) was calculated using Friedewald formula: [LDL = TC - HDL - TG /5 (mg/dL)]. Very low-density lipoprotein (VLDL) was calculated by formula [VLDL=TC–(HDL+LDL (mg/dL)]. Serum tumor necrosis factor-α (TNF-α) and leptin (Assaypro, St. Louis, MO, USA), adiponectin (RayBiotech Inc., Norcross, GA, USA) and transforming growth factor-β1 (TGF-β1) levels (IBL International GMBH, Hamburg, Germany) were measured using mouse enzyme-linked immunosorbent assay kits. Liver tissues were homogenized (Pro Scientific Inc., U.S.A. homogenizer) in 4 volumes (w/v) of ice-cold 100 mM KH2 PO4 buffer pH 7.4 containing 1 mM EDTA, and centrifuged at 10.000 g for one hour at 4 °C. The supernatants were used for analysis of GSH content (13); SOD activity (14) and MDA level (15).
Histological studies
Liver tissues were fixed in 10% buffered formaldehyde, then embedded in paraffin wax and 5 μm-thick sections were stained with hematoxylin-eosin (H&E) and Masson’s trichrome. The count was done in high power microscopic fields at ×400 /section using the computerized image analysis system (Axiovision version 4.8, Zeiss Germany). Steatosis was assessed by a morphological semi-quantitative approach and graded as follows: mild = 5 – 30%, moderate = 30 – 60%, and severe > 60% of hepatocytes affected (16). The specimens were also examined for histological features including; ballooning degeneration, vacuolation (17), acidophilic necrosis, sinusoidal fibrosis and polymorph nuclear infiltration (18).
Statistical analysis
Data were expressed as mean ± SEM and analyzed using the one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. Statistical analyses were performed using the SPSS, software package version 16.0 (Chicago, IL, USA). P-values ≤ 0.05 were considered statistically significant.
RESULTS
Effect of treatments on hepatic metrology
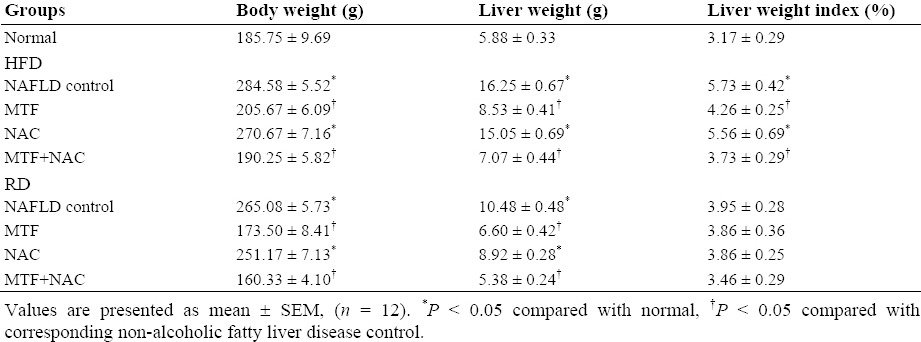
The body, liver weights and liver index in HFD (II) and RD (III) untreated groups were significantly higher (P < 0.05) compared with those of normal control group (Table 1). Compared with NAFLD - HFD or - RD, there was a significant decline in these parameters in groups treated with MTF alone or in addition to NAC, with a tendency for normalization in VIII and IX groups (Table 1).
Table 1.
Effect of metformin and N-acetylcysteine alone or in combination on liver metrology in non-alcoholic fatty liver disease induced rats.
Effect of treatments on lipid profile, liver function, and adipocytokine markers
Animals in HFD group showed an increase in TG, TC, LDL and VLDL (Table 2), as well as the serum levels of ALT, AST, γ-GT, ALP (Fig. 1), leptin, TNF-α, TGF-β1 (Fig. 2) with a significant decrease (P < 0.05) in HDL and adiponectin levels when compared to those of normal control. The return to the RD for two months reverted partly all these effects (Table 2, Figs. 1 and 2). Compared with the corresponding NAFLD control, all previous parameters were significantly improved (P < 0.05) in groups treated with MTF alone (IV, V) or in combination with NAC (VIII, IX).
Table 2.
Effect of metformin and N-acetylcysteine alone or in combination on lipid profile in non-alcoholic fatty liver disease induced rats.
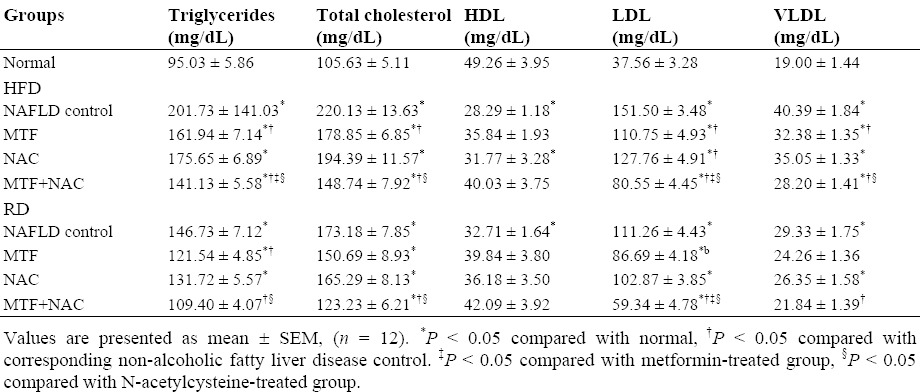
Fig. 1.
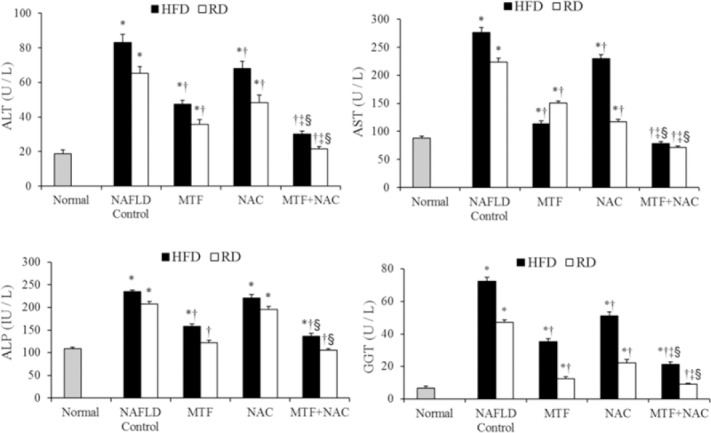
Effect of metformin and N-acetylcysteine alone or in combination on serum liver enzymes in non-alcoholic fatty liver disease -induced rats. Values are presented as mean ± SEM, (n = 12). *P < 0.05 compared with normal, †P < 0.05 compared with corresponding non-alcoholic fatty liver disease control. ‡P < 0.05 compared with metformin-treated group, §P < 0.05 compared with N-acetylcysteine -treated group.
Fig. 2.
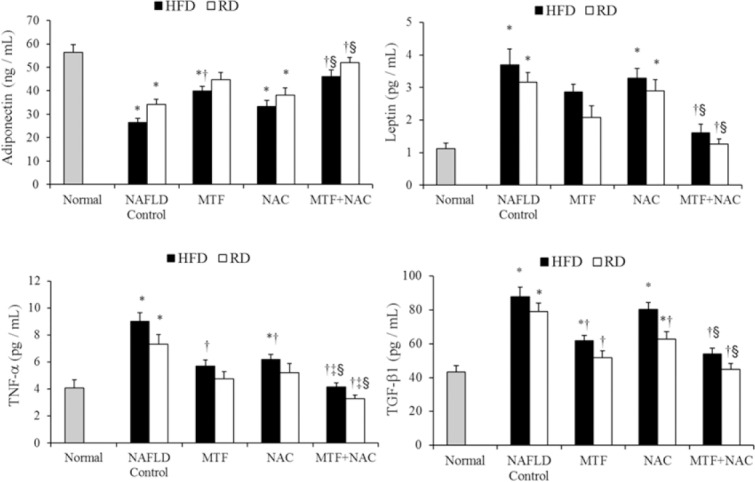
Effect of metformin and N-acetylcysteine alone or in combination on adipocytokine markers in non-alcoholic fatty liver disease -induced rats. Values are presented as mean ± SEM, (n = 12). *P < 0.05 compared with normal, †P < 0.05 compared with corresponding non-alcoholic fatty liver disease control. ‡P < 0.05 compared with metformin-treated group, §P < 0.05 compared with N-acetylcysteine -treated group.
Effect of treatments on oxidative stress and lipid peroxidation
Compared with normal control, the hepatic GSH content and SOD activity in HFD group were significantly decreased (P < 0.05), while MDA level was significantly increased (Table 3). These changes were to a less extent in RD group. Comparing to NAFLD - HFD or -RD groups, administration of either MTF or NAC alone or both significantly restored GSH content, increased SOD activity and decreased MDA level (Table 3) with a tendency for normalization in MTF + NAC (VIII, IX) treated groups respectively.
Table 3.
Effect of metformin and N-acetylcysteine alone or in combination on oxidative stress parameters and lipid peroxidation in non-alcoholic fatty liver disease induced rats.
Effect of treatments on liver histology
Liver sections of normal rats showed preserved lobular architecture with neither fat accumulation, nor inflammatory infiltration, nor significant hepatocyte ballooning (Fig. 3a). On the contrary, animals fed on HFD developed typical micro- or macrovesicular steatosis with abundant fat deposition, lobular infiltration by mononuclear cell and lymphocyte, hepatocytes with vacuolation, fat droplets accumulation, spotty and focal necrosis (Table 4, Fig. 3b). The return to the RD partially ameliorated histological findings, reaching minimum criteria (40%) for the diagnosis of steatosis (Fig. 3c).
Fig. 3.
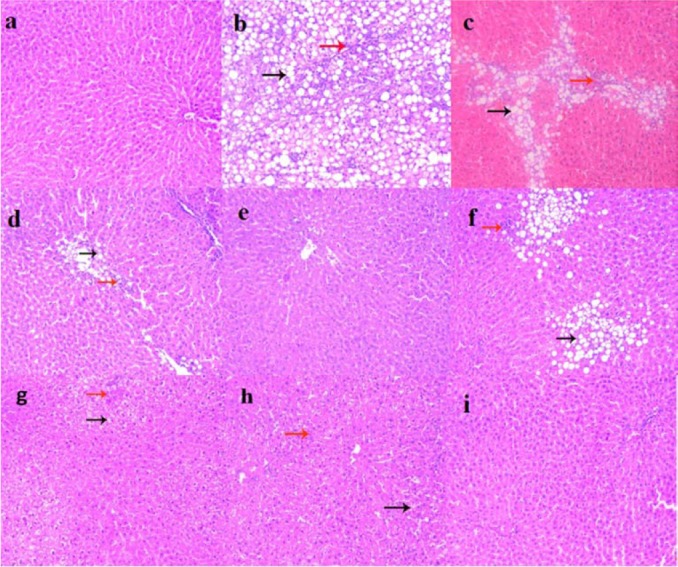
Liver sections showing: (a) normal control, intact hepatic lobular architecture with normal morphological appearance; (b) NAFLD-HFD, marked micro and macro steatotic changes (black arrow) with moderate interlobular inflammation (red arrow); (c) NAFLD-RD, mild micro and macro steatotic changes (black arrow) with mild interlobular inflammation (red arrow); (d) MTF-HFD, mild micro steatotic changes (black arrow) with mild interlobular inflammation (red arrow); (e) MTF-RD, improved steatotic changes and interlobular inflammation; (f) NAC-HFD, mild to moderate micro and macro steatotic changes (black arrow) with moderate interlobular inflammation (red arrow); (g) NAC-RD, mild micro steatotic changes (black arrow) and mild interlobular inflammation (red arrow); (h) MTF+NAC/HFD, scattered micro steatotic changes (black arrow) and mild interlobular inflammation (red arrow); (i) MTF+NAC/RD, improvement in steatotic changes and interlobular inflammation and almost normal morphological appearance. Mild = 5–30%, moderate = 30–60%, and severe > 60% of hepatocytes affected. The hematoxylin and eosin images were captured under magnification ×200.
Table 4.
Effect of metformin and N-acetylcysteine alone or in combination on histological parameters of liver in non-alcoholic fatty liver disease induced rats.
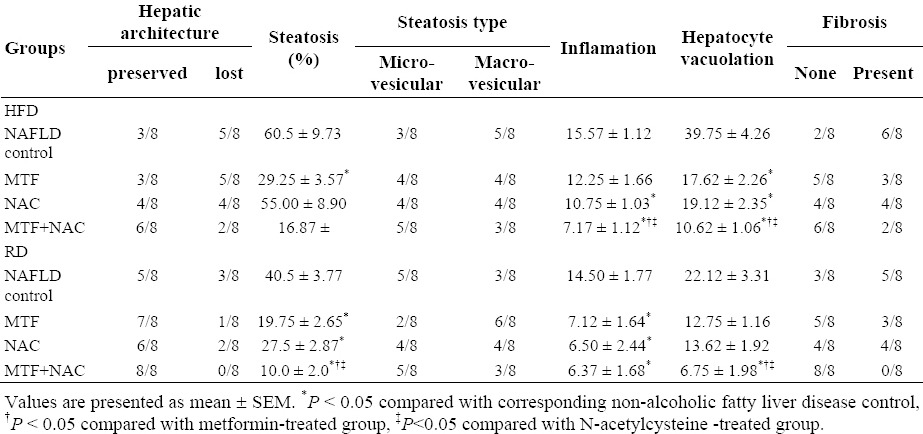
Treatment of rats maintained on HFD or switched to RD, with either MTF (Fig. 3d and 3e) or NAC (Figs. 3f and 3g) alone markedly attenuated steatosis, inflammation and hepatocytes vacuolation. In MTF + NAC combined group, steatosis and extension of lobular inflammation were relieved significantly over each individual treated group, and in all RD groups over those groups maintained on HFD (Table 4, Figs. 3h and 3i). No histological signs of cirrhosis were found in any of the studied groups.
DISCUSSION
To date, studies evaluating the effects of combined administration of MTF + NAC on liver biochemistry and histopathology in a NAFLD model are very limited. One study has reported a significant decrease in liver steatosis and fibrosis in NASH patients receiving MTF + NAC (19). In our study, we used an animal model to avoid the confounding variables of the uncontrolled pilot study of de Oliviera et al. (19) due to small series of patients, genetic heterogeneity, gender, and environmental factors including diet and lifestyle. Moreover, de Oliviera et al. (19) ignored assessment of oxidative stress markers, one of the key factors in NAFLD pathogenesis. In our study, the histological picture of a NAFLD rat model showed liver steatosis, lobular inflammatory infiltrates, hepatocytes necrosis and ballooning, and fibrosis. Other characteristic features of NAFLD /metabolic syndrome including; visceral obesity, dyslipidemia, elevated serum leptin, TNF-α, TGF-β1, oxidative stress and lipid peroxidation, increased serum ALT, AST and decreased adiponectin were also observed. These changes are in agreement with those of Seif el-Din et al. (20). Conceivably, control of diet is important for the prevention and intervention of NASH (21).
In this work, treatment of NAFLD - HFD with MTF alone or combined with NAC significantly decreased body, liver weights and hepatic index versus untreated NAFLD - HFD control. On the other hand, NAC alone did not affect these parameters significantly. This means that MTF alone acts by decreasing fat accumulation in the liver, and therefore decreases hepatic index. These data support the rationale of using MTF, which help reducing body weight in obese patients with /without diabetes and induces a significant reduction in total body and visceral fat (22,23). Weight loss during MTF treatment has been attributed to decreased net caloric intake (24), probably through appetite suppression, an effect largely independent of gastrointestinal side effects of MTF (25).
ALT and AST are biomarkers in the diagnosis of hepatic damage because they are released into the circulation after hepatocellular damage (26). In our study, the elevated transaminase serum levels, where AST /ALT values as > 3-fold the upper limit of normal, correlated strongly with NAFLD (27), are the result of leakage from damaged cells. The use of MTF /NAC alone or both, improved significantly these abnormalities. This positive effect on ALT and AST might account for the improvement of the liver histology and less fatty infiltration in hepatocytes as observed in this study.
In this study, all disturbances in lipid profile and adipocytokines were significantly improved in MTF - and partially in NAC - treated groups, and hepatic steatosis was significantly decreased. Obviously, the best results were achieved using the combined treatment regimen together with dietary control. Several authors have shown that MTF improves lipoprotein profiles with a decrease in TC and TG levels (28). Furthermore, in ob/ob mice, a model of hepatic steatosis, it has been shown that MTF reversed hepatomegaly, hepatic fat accumulation, and ALT abnormalities, by reducing hepatic TNF-α expression, which promotes hepatic lipid accumulation and ATP depletion (29).
Mitochondrial dysfunction is thought to play a central role in NAFLD as production of reactive oxygen species is increased and lead to inflammation, hepatocellular apoptosis, or in other words increased oxidative stress (30). In this study, treatment of NAFLD - HFD or RD groups with MTF or /and NAC restored hepatic GSH, SOD and reduced MDA level with a tendency for normalization using the combined treatment regimen.
Previous findings showed that the effect of MTF in counteracting adipose tissue expansion occurs not only through a direct inhibition of adipogenesis but also by modulating adipokine synthesis or secretion (31). Indeed, adiponectin induced by MTF directly stimulates adenosine monophosphate-activated protein kinase (AMPK), and prevents hepatic lipid accumulation by increasing β-oxidation of free fatty acids and/or by decreasing their de novo synthesis through stimulating of ATP-producing catabolic pathways (glycolysis, fatty acid oxidation, and mitochondrial biogenesis) and inhibiting ATP consuming anabolic processes (gluconeogenesis, glycogen, fatty acid, and protein synthesis) (6). NAC can stimulate GSH synthesis, promote detoxification, and act as a scavenger of free radicals, inhibit the production of TNF-α and attenuate inflammation, cellular damage, hepatocyte injury and fibrosis as shown in the histological part of this study. Herein and based on the above mentioned, the enhanced pharmacological effects using the combined treatment regimen could be due to the complementary action of both MTF and NAC.
CONCLUSION
Weight loss through diet control is an important step in NAFLD management. The use of NAC as an antioxidant and anti-inflammatory in addition to MTF as insulin sensitizer appear to be a valuable adjuvant therapy for NAFLD, beyond their individual effects, as evidenced by biochemical and histological results. This combination therapy is very encouraging for treatment of patients with NAFLD and in the prevention of NASH.
ACKNOWLEDGEMENTS
This work was supported by the internal research project 95 /A for basic and applied research, a grant from Theodor Bilharz Research Institute.
REFERENCES
- 1.Qin Y, Tian Y. Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats. Lipids Health Dis. 2010;9:78. doi: 10.1186/1476-511X-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steato-hepatitis. J Hepatol. 2005;42:S2–12. doi: 10.1016/j.jhep.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Medina J, Fernandez-Salazar LI, Garcia-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of non-alcoholic steatohepatitis. Diabetes Care. 2004;27:2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 4.Patel AA, Torres DM, Harrison SA. Effect of weight loss on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2009;43:970–974. doi: 10.1097/MCG.0b013e3181b57475. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterol. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 6.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–38. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol. 2007;13:5127–5132. doi: 10.3748/wjg.v13.i38.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Busafi SA, Bhat M, Wong P, Ghali P, Deschenes M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepat Res Treat 2012. 2012 doi: 10.1155/2012/947575. doi: 10.1155/2012/947575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zulet MA, Barber A, Garcin H, Higueret P, Martinez JA. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr. 1999;1:36–42. doi: 10.1080/07315724.1999.10718825. [DOI] [PubMed] [Google Scholar]
- 13.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–342. [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Toledo-Pereyra LH. Monoclonal antibody to intercellular adhesion molecules-1 as an effective protection for liver ischeamia and perfusion injury. Transplant Proc. 1993;25:3325–3327. [PubMed] [Google Scholar]
- 18.Poo JL, Hernande-Pando R, Diliz H, Morales L, Marine E, Panduro A, et al. Semiquantitative histologic evaluation of the liver in patients after liver transplantion. Transplant Proc. 1992;24:1973–1975. [PubMed] [Google Scholar]
- 19.de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159–165. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 20.Seif el-Din SH, El-Lakkany NM, El-Naggar AA, Hammam OA, Abd El-Latif HA, Ain-Shoka AA, et al. Effects of rosuvastatin and/or â-carotene on non-alcoholic fatty liver in rats. Res Pharm Sci. 2015;10:275–287. [PMC free article] [PubMed] [Google Scholar]
- 21.Botezelli JD, Mora RF, Dalia RA, Moura LP, Cambri LT, Ghezzi AC, et al. Exercise counteracts fatty liver disease in rats fed on fructose-rich diet. Lipids Health Dis. 2010;9:116. doi: 10.1186/1476-511X-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve- Smith L. Metformin-induced resumption of normal menses in 39 of 43 (91%) previously amenorrheic women with the polycystic ovary syndrome. Metabolism. 1999;48:511–519. doi: 10.1016/s0026-0495(99)90113-0. [DOI] [PubMed] [Google Scholar]
- 23.Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, et al. Effect of long term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767–2774. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 24.Meneghini LF, Orozco-Beltran D, Khunti K, Caputo S, Damçi T, Liebl A, et al. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011;96(11):3337–3353. doi: 10.1210/jc.2011-1074. [DOI] [PubMed] [Google Scholar]
- 25.Haupt E, Knick B, Koschinsky T, Liebermeister H, Schneider J, Hirche H. Oral antidiabetic combination therapy with sulphonylureas and metformin. Diabete et Metabolisme. 1991;17:224–231. [PubMed] [Google Scholar]
- 26.Naik SR, Panda VS. Antioxidant and hepato-protective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393–399. doi: 10.1111/j.1478-3231.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 27.AL-Dosari M, ALqasoumi S, Ahmad M, AL-yahya M, Ansari MN, Rafatullah S. Effect of Beta vulgaris L. on cholesterol rich diet-induced hypercholesterolemia in rats. Farmacia. 2011;59:669–678. [Google Scholar]
- 28.Rizos CV, Milionis HJ, Kostapanos MS, Florentin M, Kostara CE, Elisaf MS, et al. Effects of rosuvastatin combined with olmesartan, irbesartan, or telmisartan on indices of glucose metabolism in Greek adults with impaired fasting glucose, hypertension, and mixed hyperlipidemia: a 24-week, randomized, open-label, prospective study. Clin Ther. 2010;32:492–505. doi: 10.1016/j.clinthera.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic sp spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 30.Chitapanarux T, Tienboon P, Suwalee P, Donrawee L. Open-labeled pilot study of cysteine-rich whey protein isolate supplementation for nonalcoholic steatohepatitis patients. J Gastroenterol Hepatol. 2009;24:1045–1050. doi: 10.1111/j.1440-1746.2009.05865.x. [DOI] [PubMed] [Google Scholar]
- 31.Huypens P, Quartier E, Pipeleers D, Van De Casteele M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Europ J Pharmacol. 2005;518:90–95. doi: 10.1016/j.ejphar.2005.06.016. [DOI] [PubMed] [Google Scholar]


