Abstract
Melissa officinalis L. is a medicinal plant with a large variety of pharmacological effects and traditional applications. This study aimed to evaluate the protective and antioxidant activities of the extract of M. officinalis aerial parts on human umbilical vein endothelial cells (HUVECs) under oxidative stress induced by H2O2. Cells were incubated with H2O2 (0.5 mM, 2 h) after pretreatment with M. officinalis extract (25-500 μg/mL). Cell viability was evaluated by 3-(4, 5- Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The concentration of hydroperoxides and ferric reducing antioxidant power (FRAP) were measured in intra- and extra-cellular fluids. Pretreatment of HUVECs with M. officinalis extract at the concentrations of 100-500 μg/mL improved the cell viability after exposure to H2O2 significantly. It also decreased hydroperoxides concentration and increased FRAP value in both intra- and extra-cellular fluids. The results revealed antioxidant and cytoprotective effects of M. officinalis against H2O2-induced oxidative stress in HUVECs. Due to the valuable antioxidant activity, this plant extract may have potential benefits for the prevention of cardiovascular diseases associated with oxidative stress.
Keywords: Melissa officinalis L., HUVECs, Oxidative stress, Antioxidant
INTRODUCTION
Oxidative stress plays a great role in the pathophysiological mechanisms involved in cardiovascular diseases. Increased production of free radicals and oxidative cellular damages has been implicated in various cardiovascular diseases such as atherosclerosis, high blood pressure, heart failure, diabetes and hypercholesterolemia (1). Oxidative stress is mainly caused by the production of large amounts of reactive oxygen species (ROS) and reduction of antioxidant capacity. Superoxide anion, hydroxyl radicals, lipid radicals and hydrogen peroxide (H2O2) are the examples of ROS. In the vasculature, ROS cause inactivation of nitric oxide, oxidation of low density lipoprotein (LDL), inflammation and impairment of endothelial function associated with hypertension and atherosclerosis (2,3,4).
Antioxidants are substances that protect endothelial cells against oxidative toxicity by preventing ROS formation, neutralizing the free radicals and inhibiting oxidation reactions (5,6,7). A growing body of evidence has shown a relation between supplementation with antioxidants and reduction in morbidity and mortality from cardiovascular diseases. Antioxidants may improve endothelial function through prevention from lipid peroxidation in LDL, enhancement of endothelium-dependent vasodilatory responses and modulation of endothelium-leukocyte interactions (8,9,10).
Melissa officinalis L. (lemon balm) is a traditional herbal medicine with a large variety of effects such as spasmolytic, analgesic, hypnotic, hepatoprotective, antibacterial, anticancer, antioxidant and antiviral activities (11,12,13,14,15). Lemon balm grows in central and southern Europe, North America, and in Asia Minor. In Iranian traditional medicine, it is used to treat a wide range of diseases including fever, headaches, influenza, bloating, indigestion, colic, nausea, anemia, dizziness, fainting, weakness, asthma, bronchitis, amenorrhea, heart failure, arrhythmia, insomnia, epilepsy, depression, neurological disorders, wounds and injuries (16,17). Lemon balm contains many antioxidant compounds including flavonoids such as naringin and hesperidin and also hydroxycinnamic acid derivatives such as rosmarinic acid, m-coumaric acid and caffeic acid (18,19).
In this study, protective effect of hydroalcoholic extract obtained from the aerial parts of M. officinalis was investigated in human umbilical vein endothelial cells (HUVECs) under oxidative stress induced by H2O2. To assure the safety of M. officinalis extract on HUVECs, the toxicity of this herbal extract was also evaluated.
MATERIALS AND METHODS
Chemicals
Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from GIBCO Life Technologies (Paisley, Scotland). Fetal bovine serum (FBS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit was purchased from Bioidea Co. (Tehran, Iran). The assay kits for measurement of hydroperoxides and ferric reducing antioxidant power (FRAP) were purchased from Hakiman Shargh Research Co. (Isfahan, Iran). All other chemicals were from Merck Co., Germany.
Plant material and preparation of extract
The aerial parts of M. officinalis were collected from Hasan Abad Jarghooyeh in the Isfahan Province during May 2014. After authentication, a voucher specimen (No. 3395) was deposited at the Herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences. For preparation of hydroalcoholic extract, the air-dried aerial parts of M. officinalis were powdered and extracted three times with ethanol:water (70:30) using maceration process at room temperature for 72 h. After filtration and evaporation of solvent under reduced pressure using a rotary evaporator, the obtained extract was freeze-dried and the residue was stored at -20 °C. The yield of the plant extract was 16.8 % (w/w). The extract was dissolved in dimethyl sulfoxide (DMSO) 0.8% and diluted with cell culture medium to get different concentrations as per requirement.
Determination of total phenolic content
The total phenolic content of M. officinalis extract was estimated using Folin-Ciocalteu assay. Briefly, the plant samples were mixed with sodium bicarbonate (20%) and diluted Folin’s reagent. After 2 h, the absorbance was measured at 765 nm. Each experiment was assayed in triplicate. The total phenolic content was estimated using a standard curve obtained from different concentrations of gallic acid as the standard reference (20).
Cell culture
Human umbilical vein endothelial cells were obtained from the National Cell Bank of Iran (Pasteur Institute of Iran, Iran, Tehran,). The cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Cultures were maintained under normal culture condition (5% CO2 and 95% humidified air at 37 °C).
Cell viability evaluation
MTT assay was used to determine the effect of M. officinalis extract on viability of HUVECs. This assay was based on the reduction of tetrazolium salt by mitochondrial dehydrogenases of viable cells and performed for evaluation of both probable cytotoxic and cytoprotective effects of M. officinalis extract in this research (21).
At first, the probable cytotoxicity of the extract (25 to 1000 μg/mL) on normal HUVECs was assessed using MTT method. Briefly, at the end of 24 h incubation period, HUVECs were treated with different concentrations of freshly prepared M. officinalis extract and incubated for an additional 24 h at 37 °C. After washing out with phosphate buffered saline (PBS), new medium containing MTT reagent was added to each well and incubated for 3 h at 37 °C. MTT reaction with living cells creates insoluble foramazan crystals with purple color. After dissolution of foramazan crystals by adding DMSO, the absorbance was measured at 570 nm by a microplate reader (BioTek Instruments, PowerWave XS, Wincoski, USA).
The cytoprotective effect of M. officinalis extract on HUVECs was also evaluated under oxidative stress induced by hydrogen peroxide. The cells were pre-incubated with the extract (25 to 500 μg/mL) or vitamin C (100 μg/mL as the standard reference) for 24 h at 37 °C. After removal of the medium and washing out with PBS, HUVECs were exposed to H2O2 0.5 mM for 2 h. The rest of the experiment was completed as above. The cells without any exposure to the extract or H2O2 were considered as negative control. The cells with exposure to vitamin C were used as the positive control. The viability of treated samples was assessed by comparison of the absorbance of different concentrations of the samples with negative control according to the following formula and each experiment was tested in triplicate (21):
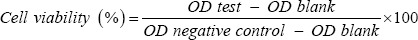
Measurement of extra- and intra-cellular hydroperoxides concentration
The effects of M. officinalis extract on intra- and extra-cellular level of hydroperoxides were evaluated based on the ferrous ion oxidation by xylenol orange reagent (FOX-1) (22). The FOX-1 reagent containing ammonium ferric sulfate in aqueous medium with sorbitol was prepared according to the instructions of the kit manufacturer.
The HUVECs were pretreated with different concentrations of the extract and exposed to the H2O2. Then 10 μL of supernatant of the cells or the cell lysates from each well was mixed with 190 μL of FOX-1 reagent. After incubation for 30 min at 40 °C, absorbances of the samples were determined using a microplate reader/spectrophotometer at 540 nm. The hydroperoxides content of the samples were estimated as H2O2 equivalents from a H2O2 standard curve.
Measurement of cell-free and intra- and extra-cellular ferric reducing antioxidant power
The effects of M. officinalis extract on intra- and extra-cellular FRAP was determined by Benzie and Strain method (23). This method measures the total antioxidant capacity based on the reduction of ferric-tripyridyltriazine (TPTZ) complex to ferrous form by spectrophotometric assay.
After pretreatment of HUVECs with different concentrations of M. officinalis extract, the cells were exposed to oxidative stress by H2O2.
The FRAP reagent containing TPTZ/ferric chloride/acetate buffer was freshly prepared according to the instructions of the kit manufacturer. For each well, 10 μL of the supernatant of the cells or the cell lysates was added to 200 μL of FRAP reagent.
The FRAP level was also evaluated on samples without cells. These samples contained different concentrations of M. officinalis extract. The reaction mixture was incubated for 40 min at 40 °C and absorbance was measured at 570 nm using a microplate reader/spectrophotometer.
The FRAP value of samples was calculated using a standard curve of FeSO4.7H2O.
Statistical analysis
Data were presented as mean ± standard error of mean (SEM) in triplicate experiments. Statistical analysis was done by one-way analysis of variance (ANOVA) followed by Tukey post-hoc test (SPSS software version 16.0). P value < 0.05 was considered as the criteria for significant differences.
RESULTS
Total phenolic content
The total phenolic content of the hydroalcoholic extract of M. officinalis was estimated as 28.84 ± 4.04 mg gallic acid equivalent/g of the dried aerial parts of the plant.
Effect of M. officinalis extract on human umbilical vein endothelial cells viability
In order to evaluate the probable cytotoxicity of M. officinalis extract on HUVECs, the cell viability was measured by MTT assay. There was no inhibitory effect on HUVECs viability after exposure to M. officinalis extract at the concentrations of 25 - 500 μg/mL for 24 h. However, the concentration of 1000 μg/mL of the extract significantly decreased the HUVECs viability (Fig. 1).
Fig. 1.
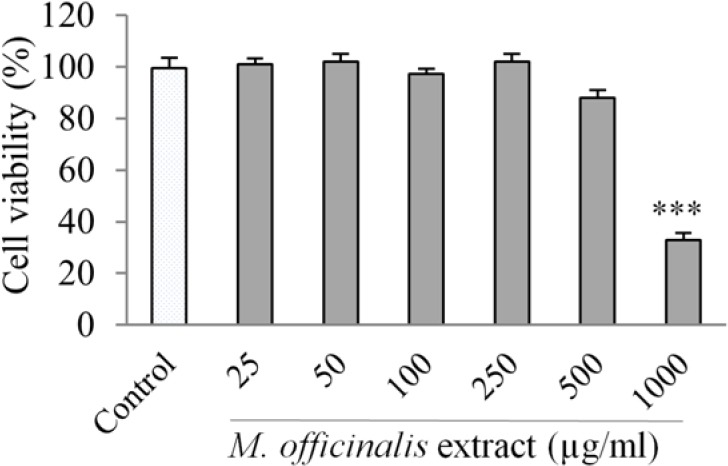
Effect of M. officinalis extract on human umbilical vein endothelial cell viability determined by MTT assay. Cells were incubated with different concentrations of the extract (25-1000 μg/mL) for 24 h. Values are the means ± SEM of three independent experiments. ***P < 0.001 versus control (untreated cells).
Cytoprotective effect of M. officinalis extract against H2O2-induced oxidative stress
Fig. 2 shows the cytoprotective effect of M. officinalis extract against the oxidative damage induced by H2O2 using the MTT method.
Fig. 2.
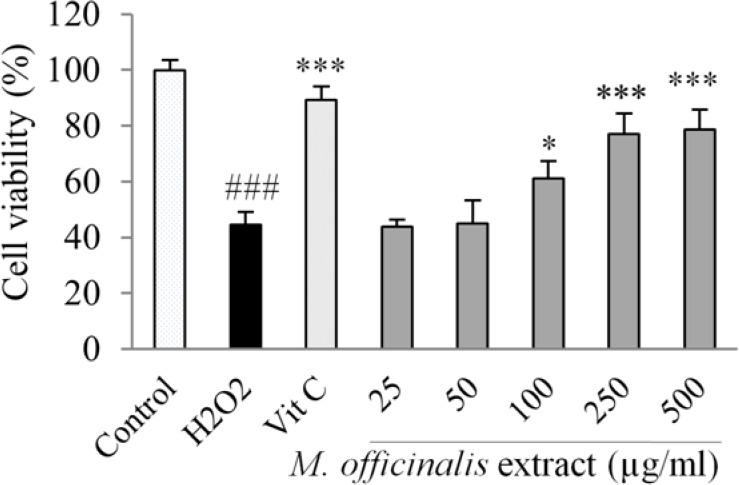
Effect of M. officinalis extract on human umbilical vein endothelial cells viability in H2O2-induced oxidative stress. Cells were incubated with H2O2 (0.5 mM, for 2 h) after pretreatment with the extract (25-500 μg/mL) or vitamin C (100 μg/mL). The cell viability was determined by the MTT assay. Values are the means ± SEM of three independent experiments. ###P < 0.001 versus control (untreated cells), *P < 0.05 and ***P < 0.001 versus H2O2 stimulated cells.
The exposure of HUVECs to 0.5 mM H2O2 for 2 h remarkably reduced cell viability (P < 0.001).
Pretreatment of HUVECs with M. officinalis extract at the concentrations of 100-500 μg/mL significantly decreased the cell death resulted from the exposure to H2O2. The protective effect was not observed at concentrations of 25 and 50 μg/mL of the extract.
Effects of M. officinalis extract on intra- and extra-cellular concentration of hydroperoxides
The effects of M. officinalis extract on intra- and extra-cellular hydroperoxides concentration estimated as H2O2 equivalents by FOX1 method are shown in Fig. 3. The levels of hydroperoxides were markedly reduced in intra- and extra-cellular fluids after pretreatment of the HUVECs with M. officinalis extract at the concentrations of 100-500 μg/mL compared with the control group.
Fig. 3.
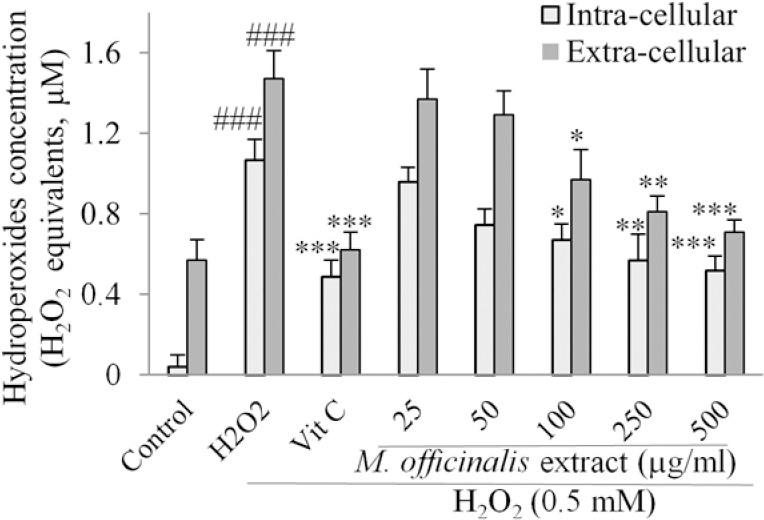
Effect of M. officinalis extract on intra- and extra-cellular concentration of hydroperoxides in human umbilical vein endothelial cells measured as H2O2 equivalents determined by ferrous ion oxidation by xylenol orange reagent method. Cells were incubated with H2O2 (0.5 mM, for 2 h) after pretreatment with the extract (25-500 μg/mL) or vitamin C (100 μg/mL). Values are the means ± SEM of three independent. ###P < 0.001 versus control (untreated cells), *P < 0.05, **P < 0.01 and ***P < 0.001 versus H2O2 stimulated cells.
Effects of M. officinalis extract on cell-free and intra- and extra-cellular ferric reducing antioxidant power value
In cell-free assay, the FRAP value of the M. officinalis extract which was expressed as equivalence of ferrous sulphate showed increasing trend in total antioxidant capacity with the extract concentrations (Fig. 4). In cell-based assay, the FRAP value was markedly increased after pre-treatment with the extract at the concentration range of 100-500 μg/mL in intra-cellular fluids compared with the control group. Pre-incubation with the extract at the concentrations of 50 - 500 μg/mL also increased the total antioxidant capacity in extra-cellular fluids in a concentration-dependent manner (Fig. 5).
Fig. 4.
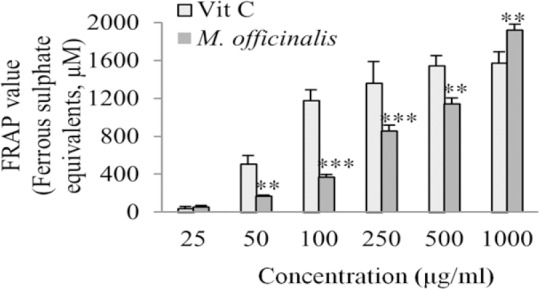
Ferric reducing antioxidant power values of different concentrations of M. officinalis extract and vitamin C (25-1000 μg/mL) determined as ferrous sulphate equivalents. Values are the means ± SEM of three independent experiments. **P<0.01 and ***P<0.001 versus vitamin C control group at the same concentration.
Fig. 5.
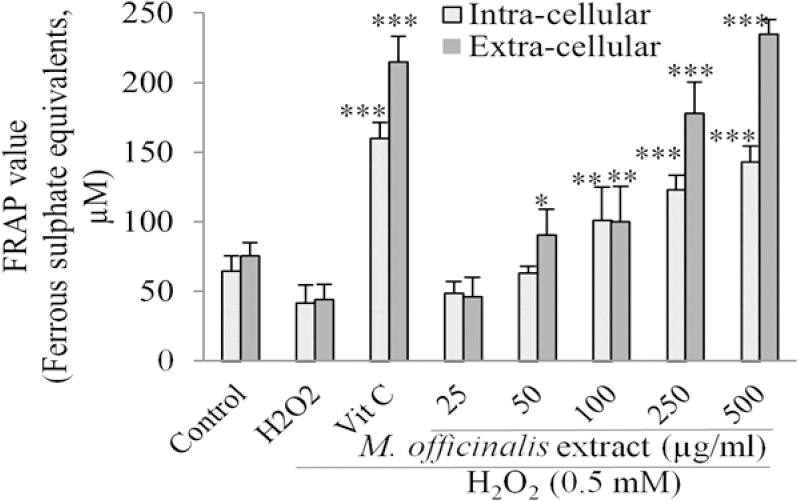
Effect of M. officinalis extract on intra- and extra-cellular ferric reducing antioxidant power value in human umbilical vein endothelial cells determined as ferrous sulphate equivalents. Cells were incubated with H2O2 (0.5 mM, for 2 h) after pretreatment with the extract (25-1000 μg/mL) or vitamin C (100 μg/mL). Values are the means ± SEM of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 versus H2O2 stimulated cells.
DISCUSSION
This investigation explored the antioxidant effect of M. officinalis hydroalcoholic extract as well as its cytoprotective effect against H2O2 -induced toxicity in HUVECs. The plant extract protected the cells against oxidative stress at the concentrations of 100-500 μg/mL. The extract decreased concentration of hydroperoxides in intra- and extra-cellular fluids at the concentration range of 100-500 μg/mL and increased FRAP value in intra-cellular fluid at the concentrations of 100-500 μg/mL and in extra-cellular fluid at the concentrations of 50-500 μg/mL.
The role of endothelial cells in the regulation of physiological functions of vascular system has been elucidated in many investigations and studies as well as the role of oxidative stress in the impairment of endothelium function (1). In the present study, HUVEC as a normal cell type with good homogeneity, rapid expansion property and well-characterized surface markers was used in a model of oxidative stress on endothelial cells (24).
We also used FRAP assay for evaluation of antioxidant effects of M. officinalis extract in the present investigation. This assay is a simple, inexpensive and reproducible method for measuring antioxidant power in both in vitro and biological fluids (23).
M. officinalis has earlier been revealed to have antioxidant activities (11,18,19). However, the present study was planned to confirm this property at the cellular level. The detected cytoprotective effect against oxidative injury of HUVECs is possibly due to its various antioxidant phytochemicals. Rosmarinic acid has been identified as the major component of M. officinalis extract (18). This polyphenolic compound is a caffeic acid derivative with favorable cardiovascular effects such as antioxidant, anti-inflammatory, anti-apoptotic, cyto- and cardio-protective activities (25). Rosmarinic acid is a superoxide scavenger which can inhibit LDL oxidation in human aortic endothelial cells (26). The structure-activity relationship studies have shown the role of an ortho dihydroxyphenyl group and the conjugated double bond in the C3 carbon chain in its scavenging activity (27). The antioxidant effect of rosmarinic acid also contributes in its anti-angiogenesis activity through reducing the expression of H2O2 -dependent vascular endothelial growth factor, decreasing release of IL-8 from endothelial cells and inhibiting the proliferation, migration, adhesion, and tube formation of HUVECs (27).
Caffeic acid, another antioxidant phenolic compound in the extract of M. officinalis, has potential to reduce the risk of cardiovascular disorders by inhibiting the production of ROS (28). It can improve resistance of the cells against oxidative stress by inhibition of lipid peroxidation and reduction of glutathione depletion (29). Hesperidin is another phenolic constituent of M. officinalis with cytoprotective effect which has increased the cell viability by providing powerful antioxidant protection against paraquat and H2O2 -induced oxidative stress (30). The major component s of the essential oil of the lemon balm leaf, citronellal and citral are also antioxidant compounds with superoxide scavenging activity (31).
CONCLUSION
In conclusion, the results of this study revealed that hydroalcoholic extract of M. officinalis aerial parts has the potential to protect HUVECs under oxidative stress induced by H2O2. Due to the presence of various antioxidant phytochemicals, the plant extract may have potential benefits in human health through preventing harmful oxidative injuries.
ACKNOWLEDGEMENTS
This research as a project No. 393659 has been financially supported by Vice Chancellor of Research of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 2.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 3.Schulz E, Anter E, Keaney JF., Jr Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11:1093–1104. doi: 10.2174/0929867043365369. [DOI] [PubMed] [Google Scholar]
- 4.Safaeian L, Ghasemi-Dehkordi N, Haghjoo Javanmard S, Namvar H. Antihypertensive and antioxidant effects of a hydroalcoholic extract obtained from aerial parts of Otostegia persica (Burm.) Boiss. Res Pharm Sci. 2015;10:192–199. [PMC free article] [PubMed] [Google Scholar]
- 5.Houston MC. The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Altern Ther Health Med. 2013;19:32–49. [PubMed] [Google Scholar]
- 6.Safaeian L, Haghjoo Javanmard S, Mollanoori Y, Dana N. Cytoprotective and antioxidant effects of human lactoferrin against H 2 O 2 -induced oxidative stress in human umbilical vein endothelial cells. Adv Biomed Res. 2015;4:188. doi: 10.4103/2277-9175.164010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safaeian L, Haghjoo Javanmard S, Ghanadian M, Seifabadi S. Cytoprotective and antioxidant effects of Echium amoenum anthocyanin-rich extract in human endothelial cells (HUVECs) Avicenna J Phytomed. 2015;5:157–166. [PMC free article] [PubMed] [Google Scholar]
- 8.Safaeian L, Ghannadi A, Javanmard SH, Vahidian MH. The effect of hydroalcoholic extract of Ferula foetida stems on blood pressure and oxidative stress in dexamethasone-induced hypertensive rats. Res Pharm Sci. 2015;10:326–334. [PMC free article] [PubMed] [Google Scholar]
- 9.Carr A, Frei B. The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Radic Biol Med. 2000;28:1806–1814. doi: 10.1016/s0891-5849(00)00225-2. [DOI] [PubMed] [Google Scholar]
- 10.Praticò D. Antioxidants and endothelium protection. Atherosclerosis. 2005;181:215–224. doi: 10.1016/j.atherosclerosis.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.De Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR. Melissa officinalis L. essential oil: antitumoral and antioxidant activities. J Pharm Pharmacol. 2004;56:677–681. doi: 10.1211/0022357023321. [DOI] [PubMed] [Google Scholar]
- 12.Hajhashemi V, Safaei A. Hypnotic effect of Coriandrum sativum, Ziziphus jujuba, Lavandula angustifolia and Melissa officinalis extracts in mice. Res Pharm Sci. 2015;10:477–484. [PMC free article] [PubMed] [Google Scholar]
- 13.Guginski G, Luiz AP, Silva MD, Massaro M, Martins DF, Chaves J, et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behavior. 2009;93:10–16. doi: 10.1016/j.pbb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Sadraei H, Ghannadi A, Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74:445–452. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 15.Bolkent S, Yanardag R, Karabulut-Bulan O, Yesilyaprak B. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: A morphological and biochemical study. J Ethnopharmacol. 2005;99:391–398. doi: 10.1016/j.jep.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Zargari AI. Medicinal plants. Vol. 1. Tehran: Tehran University Press; 1990. pp. 77–81. [Google Scholar]
- 17.Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res Pharm Sci. 2014;9:107–114. [PMC free article] [PubMed] [Google Scholar]
- 18.Dastmalchi K, Dorman H.J.D, Oinonena PP, Darwis Y, Laakso I, Hiltunen R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT- Food Sci Technol. 2008;41:391–413. [Google Scholar]
- 19.Pereira RP, Fachinetto R, De Souza Prestes A, Puntel RL, Santos da Silva GN, Heinzmann BM, et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem Res. 2009;34:973–983. doi: 10.1007/s11064-008-9861-z. [DOI] [PubMed] [Google Scholar]
- 20.Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106:929–936. [Google Scholar]
- 21.Ma ZC, Hong Q, Wang YG, Tan HL, Xiao CR, Liang QD, et al. Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. Int J Radiat Biol. 2011;87:130–140. doi: 10.3109/09553002.2011.523510. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Chen YC, Chang YC, Wang LW, Lin YC, Chiang YL, et al. Human umbilical vein endothelial cells protect against hypoxic-ischemic damage in neonatal brain via stromal cell-derived factor 1/C-X-C chemokine receptor type 4. Stroke. 2013;44:1402–1409. doi: 10.1161/STROKEAHA.111.000719. [DOI] [PubMed] [Google Scholar]
- 25.Petersen M, Simmonds MS. Rosmarinic acid. Phytochem. 2003;62:121–125. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 26.Pearson DA, Frankel EN, Aeschbach R, German JB. Inhibition of endothelial cell-mediated oxidation of low-density lipoprotein by rosemary and plant phenolics. J Agric Food Chem. 1997;45:578–582. [Google Scholar]
- 27.Huang SS, Zheng RL. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;239:271–280. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Li PG, Xu JW, Ikeda K, Kobayakawa A, Kayano Y, Mitani T, et al. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2005;28:369–377. doi: 10.1291/hypres.28.369. [DOI] [PubMed] [Google Scholar]
- 29.Nardini M, Pisu P, Gentili V, Natella F, Di Felice M, Piccolella E, et al. Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress in U937. Free Radic Biol Med. 1998;25:1098–1105. doi: 10.1016/s0891-5849(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 30.Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–4761. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 31.Kreis P, Mosandl A. Chiral compounds of essential oils. Part XVI. Enantioselective multidimensional gas chromatography in authenticy control of balm oil (Melissa oficinalis L.) Flavour Fragr J. 1994;9:249–256. [Google Scholar]


