Abstract
Lead belongs to the heavy metal group and is considered as an environmental contaminant. Acute or chronic contact to lead can change the physiological function of human organs. One of the most important disorders following the lead exposure is neurotoxicity. Lead neurotoxicity consists of the neurobehavioral disturbances like cognitive impairment. The aim of the current study is to evaluate the possible protective effect of vitamin C (Vit C), vitamin B12 (Vit B12), omega 3 (ω-3), or their combination on the lead-induced memory disorder. Adult wistar rats were orally administered Vit C (120 mg/kg/day) or Vit B12 (1 mg/kg/day) or ω-3 (1000 mg/kg/day) or their combination for 3 weeks in groups of 7 animals each. Then lead acetate (15 mg/kg/day) was injected intraperitoneally for one week to all pretreated animals. The control group received normal saline as a vehicle while the positive control for cognitive impairment received just lead acetate. At the end of treatments animal memories were evaluated in Object Recognition Task. The results showed, although 15 mg/kg lead acetate significantly declines the memory-evaluating parameters, pretreatment with Vit C, Vit B12, ω-3, or their combination considerably inverted the lead induced reduction in discrimination (d2) index (P < 0.001) and recognition (R) index (P < 0.001, P < 0.05, P < 0.05, and P < 0.001, respectively). Our findings indicate while lead acetate impairs spatial memory in rat, administration of Vit C, Vit B12, ω-3, or their combination prior to the lead exposure inhibits the lead induced cognitive loss. There was no remarkable difference in this effect between the used supplements.
Keywords: Vitamin C, Vitamin B12, Omega-3, Lead, Memory impairment, Object Recognition Task
INTRODUCTION
Lead is a highly toxic heavy metal and a major environmental pollution. The lead contamination can occur from different sources such as paint, glazed ceramics, water, food containers, cigarettes, and cosmetics. It is well documented that chronic exposure to lead can cause its accumulation in the bone, muscle, liver, kidneys, hematopoietic system and the brain followed by the toxicity events. Neurotoxicity is a popular disorder from the lead exposure which involves both central and peripheral nervous system. Lead-induced neurotoxicity triggers a wide range of structural and behavioral changes in the nervous system (1,2). Cognitive impairment is one of the most common deterioration occurs from the lead neurotoxicity (3).
Although the alteration of vital enzyme activity, receptors like glutamate, neuro-transmitters like acetylcholine, calcium homeostasis, and DNA damage are considered as the mechanisms of lead toxicity (2), increase in generation of reactive oxygen species (ROS) and suppression of cell antioxidant capacity play a key role in the lead-induced structural and behavioral neurotoxicity. Suppression of cell antioxidant capacity can permanently impair the integrity of cell membrane, DNA and other macromolecules, finally causes cell apoptosis or cell death known as neurodegenerative effects (4). Therefore, the antioxidant agents could potentially protect the neuronal damage against to the lead toxicity (5).
There are several agents possess antioxidant property or scavenging effect of ROS. Some of the well-known antioxidant that is freely accessible for human is ascorbic acid (Vit C) and the fish oil (ω-3). Also the vitamin B12 (Vit B12, cobalamin) is one of the most important supplements need for the normal function of neurons in the nervous system (6).
Vit C, a six carbon lactate, is a water soluble vitamin found in the dietary sources such as citrus fruits, grape fruits, berries, cabbage, tomatoes, pepper, and leafy vegetables (7).
The ω-3 polyunsaturated fatty acid, consist of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), is mainly present in fish, shell fish, and sea mammals and also finds in land animals and plants (8,9).
Vit B12, a water soluble vitamin, exists in the egg, milk, meat, and fish. It plays a regulatory role in the CNS enzymes activity as a co-factor and causes their correct metabolic function (10).
The aim of the current study was firstly to identify the cognitive impairment of repeated lead exposure and secondly to evaluate the possible protective effect of Vit C, Vit B12, ω-3 or their combination on the lead-induced memory disorder using Object Recognition Task (ORT).
MATERIALS AND METHODS
Animals
The experiments were carried out on male rats (220 ± 20 g) bred in School of Pharmacy animal house, Isfahan, Iran. The animals had free access to food and drinking water during the experiment and were kept at a constant room temperature (25 ± 5 °C), under a 12 h light/dark cycle.
All experimental procedures were conducted in the light phase of the cycle. All animal experiments were approved by the Ethics Committee of Isfahan University of Medical Science and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Lead acetate was purchased from Sigma-Aldrich Co. (USA). Vit C, Vit B12, and ω-3 were gifts from Daroupakhsh Co. (Iran). The solutions of drugs were prepared freshly every day.
Apparatus and objects
The ORT apparatus consisted of a circular arena, 83 cm in diameter and 40 cm high wall was made of white polyvinyl chloride. Two different sets of objects consist of a massive aluminum cube (10.0 × 5.0 × 7.5 cm) and a massive aluminum cube with a tapering top (13.0 × 8.0 × 8.0 cm) were used. Each object was available in triplicate. The objects could not be displaced by rats.
Drug administration
The animals were divided in different groups each 7 rats. The following schedules were used to treat the animals: Control group: Animals were daily gavaged 3 mL distilled water for 3 weeks. Afterwards they received normal saline intraperitoneally (i.p) for 1 week. Lead acetate group: Animals were gavaged daily 3 mL distilled water for 3 weeks. Then 15 mg/kg (1) lead acetate was injected i.p for 1 week. Vit C, Vit B12, or ω-3 groups: Animals in these groups were fed 120 mg/kg Vit C (7), 1 mg/kg Vit B12 (11), or 1000 mg/kg ω-3 (12) by gavage for 3 weeks. After the administration of the last dose of the supplement each group was injected 15 mg/kg lead acetate i.p for 1 week. Combination group: Animals in this group received a combination of 120 mg/kg Vit C, 1 mg/kg Vit B12, and 1000 mg/kg ω-3 orally. They were then injected i.p with lead acetate at 15 mg/kg for 1 week.
Experimental procedure
During the lead administration period, the animals were transferred to the arena for procedure adaptation. For this purpose they were allowed to explore the apparatus (without any objects) twice daily for 5 min with 1 h intervals. Object recognition consisted of three clearly defined phases: a training session or first trial (T1), a 1-h training test interval, and a test session or second trial (T2). Each trial lasted for 5 min. The experimental procedure details and the calculation of important factors of memory assessment like d2, R index and the frequency of exploration have been previously explained in details (13).
Statistical analysis
Data are expressed as mean ± SEM. The values of memory evaluation factors were analyzed using one way analysis of variance (ANOVA). For multiple comparisons, the Tukey’s post hoc tests were used. The Graph pad prism 4 Software was employed for statistical analysis. P-values less than 0.05 were considered statistically significant.
RESULTS
Effect of lead acetate on memory indices
The daily i.p administration of lead acetate at 15 mg/kg for one week significantly decreased both d2 (Fig. 1, P < 0.001) and R (Fig. 2, P < 0.05) indices in trial T2. Also treatment with lead caused a mild decrease in frequency of exploration for the new object compared with familiar one in trial T2 (Fig. 3).
Fig. 1.
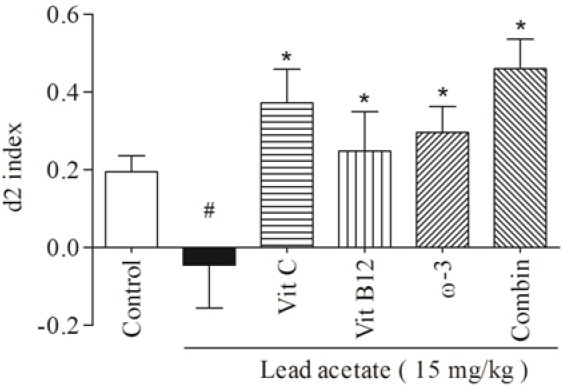
The comparative effects of vit C, vit B12, or ω-3 and their combination on the d2 indices in trial T2. Data are presented as Mean ± SEM. (#P < 0.001, compared to control group; *P< 0.001, compared to lead acetate group). (d2) discrimination, (Vit C) vitamin C, (Vit B12) vitamin B12, (ω-3) omega-3, (Combin) combination therapy.
Fig. 2.
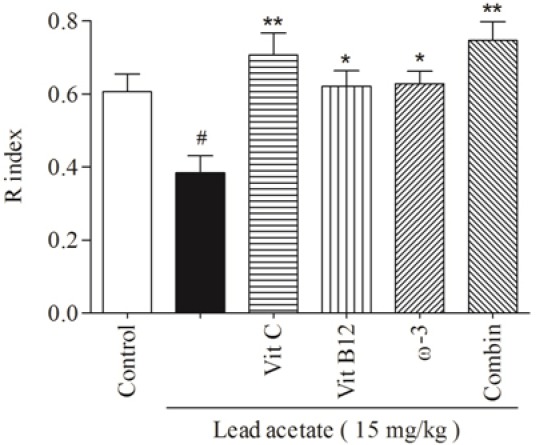
The comparative effects of vit C, vit B12, or ω-3 and their combination on the lead acetate decreased R indices in trial T2. Data are presented as Mean ± SEM. (#P < 0.05, compared to control group; *P < 0.05, **P < 0.001, compared to lead acetate group). (R) recognition, (Vit C) vitamin C, (Vit B12) vitamin B12, (ω-3) omega-3, (Combin) combination therapy.
Fig. 3.
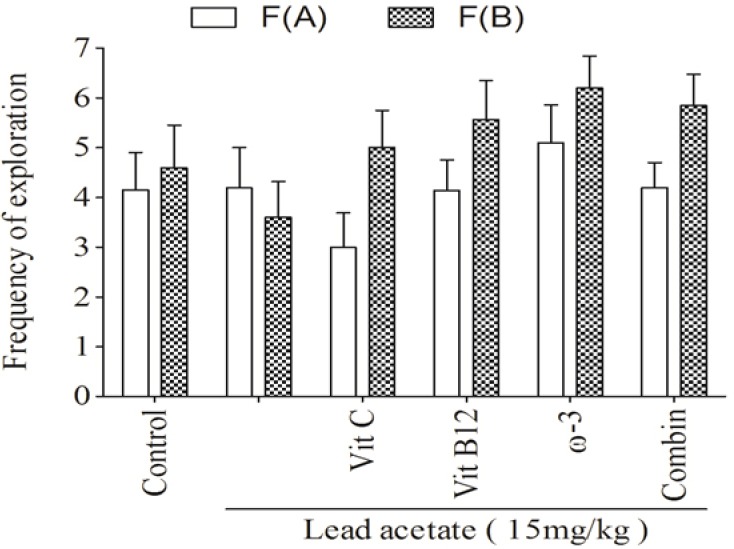
The effects of Vit C, Vit B12, or ω-3 and their combination on the frequency of exploration for old and new object in trial T2. (F(A)) Frequency of exploration for familiar object, (F(B)) Frequency of exploration for new object, (Vit C) vitamin C, (Vit B12) vitamin B12, (ω-3) omega-3, (Combin) combination therapy.
Effects of Vit C, Vit B12, ω-3 or their combination on d2 indices
Pre-treatment with 120 mg/kg Vit C for 3 weeks before lead administration could reverse the effect of lead element on d2 indices (P < 0.001). Administration of Vit B12 at 1 mg/kg for 3 weeks caused a significan increase (P < 0.001) in lead-induced d2 diminution. Also the group received ω-3 at 1000 mg/kg for 3 weeks showed significant improvement (P < 0.001) in d2 indices compared to the lead-administered group in trial T2 (Fig. 1).
Combination of Vit C (120 mg/kg), Vit B12 (1 mg/kg), and ω-3 (1000 mg/kg) orally administered for 3 weeks led to a remarkable increase (P < 0.001) in d2 indices compared to lead-treated animals (Fig. 1). Although different treatment, either alone or in combination, increased d2 indices in trial T2, but significant differences were not observed amongst treatments (P > 0.05) (Fig. 1).
Effects of Vit C, Vit B12, or ω-3 or their combination on R indices
Administration of 120 mg/kg Vit C for 3 weeks before lead administration showed considerable improvement (P < 0.001) in R indices of lead-treated animals. Pretreatment with Vit B12 at 1 mg/kg or ω-3 at 1000 mg/kg for 3 weeks showed remarkable increase (P < 0.05) in R index compared to those of lead-administered group in trial T2. The combination of tested supplements also significantly (P < 0.001) prevented the diminishing effect of lead on the R index (Fig. 2). Vit C, vit B12, ω-3, or their combination elevated the lead-induced reduction in R indices in trial T2; however, no significant differences were observed amongst (Fig. 2).
Effects of Vit C, Vit B12, or ω-3 or their combination on exploration frequencies
Although none of the treatments affected the frequency of old or new objects exploration, but the supplements showed a different pattern in the exploration frequency between the old and new objects. Lead acetate decreased the exploration frequency of new object but all supplements, alone or in combination, increased the exploration frequency of new object compared to the familiar one (Fig. 3).
DISCUSSION
During the development of industries in the world, exposure to lead element and consequently its toxicity has been increased worldwide. Central nervous system is the most important target for the lead toxicity. Lead-induced neurotoxicities include behavioral, morphological, and electrophysiological disruptions (1,14). In the present study, we evaluated the possible protective effects of Vit C, Vit B12, and ω-3 alone or in combination, on the lead-induced memory impairment in a rat model.
Following repeated doses of lead animals showed a reduction in d2 and R indices compared to that of the control animals. d2 index indicates the differences between new and old object exploration time while the R index indicates the exploration time that animals especially spend around the new object. This is a marker related to the memory of animal to the old object. The results showed that lead-treated group spent less time for exploration of the novel object than the familiar one in comparison with the control group. The lead also diminished the difference between exploration frequencies of the novel and old objects seen in the control group. Normally, the animals showed more exploratory behavioral in time and frequency to new object than the old one because they had a memory to the old object in trial T2. The memory destructive agent impaired the memory to old object and caused a reduction in differences between old and new object exploration. These data demonstrate that repeated exposure to lead causes destructive effects on the memory in rat.
The current results are in agreement with the large volume of experimental and clinical studies which demonstrated lead exposure impairs cognitive functions and destroys the memory and learning processes in human and animals (3,15,16). Several mechanisms have been proposed to explain the neurotoxicity of pb 2+. One of the most important mechanisms in lead-induced neurotoxicity is the triggering of lipid peroxidation and oxidative stress processes by accumulation of reactive oxygen species (16,17,18). In addition, lead binds to the thiol groups of biologic macromolecules like glutathione and decreases their reductive potencies and antioxidant activities (19). It has been reported that the initiating of apoptosis cascade, increasing inflammatory mediators, alteration in glutamate-induced neuroplasticity and changes in calcium homeostasis are some of other mechanisms involved in lead neurotoxicity (20,21,22,23,24).
The current study provided evidences that administration of 120 mg/kg Vit C, 1 mg/kg Vit B12, and 1000 mg/kg ω-3 for three weeks before lead exposure could clearly prevent the lead- induced memory impairments. Vit C, Vit B12, and ω-3 showed similar beneficial effects when administered in combination. Although each supplement improves the memory in the experimental groups, significant differences amongst treated groups were not observed.
The protective effect of Vit C on the lead-induced memory impairment is in agreement with the studies indicated that the Vit C could improve dementia induced by aging or anticholinergic agents in animals (7,25). It is well known that Vit C is highly concentrated in the central nervous system and possess the antioxidant properties. Indeed, Vit C scavenges the Pb2+ generated ROS (25,26,27).
There are several evidences representing Vit C anti-inflammatory effects by inhibiting the NF-kB, TNF-α, IL-1, and IL-6 (28), as well as its anti-apoptotic effect (29). With aforementioned mechanisms, Vit C protects the key pathways in the memory formation including the serotonergic, dopaminergic, and glutamatergic neurons against the lead-induced damages (30,31).
Vit B12 is actually a nerve supplement easily passing the blood brain barrier. Many studies have demonstrated that Vit B12 mediates recovery process in the nervous system and plays a protective role in the neurodegenerative disorders such as Parkinson’s, multiple sclerosis, and Alzheimer’s disease. Vit B12 possesses the antioxidant activity and is involved in the synthesis of phospholipids and myelin. It also shows anti-inflammatory, anti-apoptotic, and anti-necrotic effects (32,33,34,35). These mechanisms could explain the observed beneficial effects of Vit B12 in the present study.
Omega-3 fatty acids contain the most abundant polyunsaturated fatty acids that in the brain cause neuronal differentiation, neurite growth, synapses formation, and receptor biogenesis (36). The polyunsaturated fatty acids could play the pro-oxidant role in the oxidative tensions and encounter the lipid peroxidation of neuronal cells (37). It can also decrease the inflammatory factors, suppress neuronal apoptosis as well as enhance nitric oxide generation causing improvement in cerebrovascular endothelial function (38,39). It is well-known that the polyunsaturated fatty acids modulate neural functions including neurotransmitters especially acetylcholine, membrane fluidity, ion channel, enzyme regulation, and neurotrophin gene expression (35,40,41). There are some studies consistent with our results that evidenced the preventive or ameliorating effects of polyunsaturated fatty acids in the memory impairment induced by stress, aging or different neurotoxic agents (8,40,41,42).
CONCLUSION
In summary, the present study provided evidences that different neuro-influence agents, Vit C, Vit B12, and ω-3 prevent the memory impairment induced by lead element. The antioxidant, anti-inflammatory, and anti-neurodegeneration properties of these supplements could be supposed as their main mechanism of action in the reversing of lead-induced memory losses.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Pharm.D thesis NO. 393643 submitted by Saeedeh Alsadat Moosavirad which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran
REFERENCES
- 1.Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M. Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo. Neurosci Lett. 2002;329(1):45–48. doi: 10.1016/s0304-3940(02)00576-1. [DOI] [PubMed] [Google Scholar]
- 2.Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991;41(4):479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27(2):168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 4.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12(3):57–65. [PubMed] [Google Scholar]
- 5.Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning. Free Radic Biol Med? 2000;29(10):927–945. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 6.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 7.Shahidi S, Komaki A, Mahmoodi M, Atrvash N, Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76([1]-[2]):109–113. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Feng Z, Zou X, Jia H, Li X, Zhu Z, Liu X, et al. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: possible role of neuronal mitochondria metabolism. Antioxid Redox Signal. 2012;16(3):275–289. doi: 10.1089/ars.2010.3750. [DOI] [PubMed] [Google Scholar]
- 9.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18(4-5):383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 10.McCaddon A, Regland B, Hudson P, Davies G. Functional vitamin B12 deficiency and Alzheimer disease. Neurology. 2002;58(9):1395–1399. doi: 10.1212/wnl.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 11.Hobbenaghi R, Javanbakht J, Hosseini E, Mohammadi S, Rajabian M, Moayeri P, et al. Neuropathological and neuroprotective features of vitamin B12 on the dorsal spinal ganglion of rats after the experimental crush of sciatic nerve: an experimental study. Diagn Pathol. 2013;8:123. doi: 10.1186/1746-1596-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Pan JP, Zhang HQ, Wei-Wang, Guo YF, Na-Xiao, Cao XH, et al. Some subtypes of endocannabinoid/endovanilloid receptors mediate docosahexanoic acid-induced enhanced spatial memory in rats. Brain res. 2011;15(1412):18–27. doi: 10.1016/j.brainres.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini-Sharifabad A, Rabbani M, Sharifzadeh M, Bagheri N. Acute and chronic tramadol administration impair spatial memory in rat. Res Pharm Sci. 2016;11(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DR. Neonatal lead exposure in the rat: decreased learning as a function of age and blood lead concentrations. Toxicol Appl Pharmacol. 1975;32(3):628–637. doi: 10.1016/0041-008x(75)90126-x. [DOI] [PubMed] [Google Scholar]
- 15.Haider S, Shameem S, Ahmed SP, Perveen T, Haleem DJ. Repeated administration of lead decreases brain 5-HT metabolism and produces memory deficits in rats. Cell Mol Biol Lett. 2005;10(4):669–676. [PubMed] [Google Scholar]
- 16.Kuhlmann AC, McGlothan JL, Guilarte TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci Lett. 1997;233([2]-[3]):101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- 17.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11(2):114–127. [PubMed] [Google Scholar]
- 18.Adonaylo VN, Oteiza PI. Pb2+ promotes lipid oxidation and alterations in membrane physical properties. Toxicology. 1999;132(1):19–32. doi: 10.1016/s0300-483x(98)00134-6. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti C. Molecular targets of lead in brain neurotoxicity. Neurotox Res. 2003;5(3):221–236. doi: 10.1007/BF03033142. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Ji LD, Xu LH. Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3. Toxicol Lett. 2006;166(2):160–167. doi: 10.1016/j.toxlet.2006.06.643. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol. 2008;46(5):1488–1494. doi: 10.1016/j.fct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Yan HC, Yang B, Tong LS, Zou YX, Tian Y. Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J Negat Results Biomed. 2009;8:5. doi: 10.1186/1477-5751-8-5. doi: 10.1186/1477-5751-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerink RH, Klompmakers AA, Westenberg HG, Vijverberg HP. Signaling pathways involved in Ca2+- and Pb2+-induced vesicular catecholamine release from rat PC12 cells. Brain Res. 2002;957(1):25–36. doi: 10.1016/s0006-8993(02)03580-1. [DOI] [PubMed] [Google Scholar]
- 24.Silbergeld EK, Adler HS. Subcellular mechanisms of lead neurotoxicity. Brain Res. 1978;148(2):451–467. doi: 10.1016/0006-8993(78)90732-1. [DOI] [PubMed] [Google Scholar]
- 25.Parle M, Dhingra D. Ascorbic Acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93(2):129–135. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- 26.Santos IM, Tome Ada R, Saldanha GB, Ferreira PM, Militao GC, Freitas RM. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid Med Cell Longev. 2009;2(4):214–221. doi: 10.4161/oxim.2.4.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos LF, Freitas RL, Xavier SM, Saldanha GB, Freitas RM. Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol Biochem Behav. 2008;89(1):1–5. doi: 10.1016/j.pbb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Golde DW. Vitamin C suppresses TNFα-induced NFκB activation by inhibiting IêBá phosphorylation. Biochemistry. 2002;41(43):12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 29.Shah SA, Yoon GH, Kim HO, Kim MO. Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem Res. 2015;40(5):875–884. doi: 10.1007/s11064-015-1540-2. [DOI] [PubMed] [Google Scholar]
- 30.Binfare RW, Rosa AO, Lobato KR, Santos AR, Rodrigues AL. Ascorbic acid administration produces an antidepressant-like effect: evidence for the involvement of monoaminergic neurotransmission. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):530–540. doi: 10.1016/j.pnpbp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43(6):537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi M, Kashii S, Honda Y, Tamura Y, Kaneda K, Akaike A. Protective effects of methylcobalamin, a vitamin B12 analog, against glutamate-induced neurotoxicity in retinal cell culture. Invest Ophthalmol Vis Sci. 1997;38(5):848–854. [PubMed] [Google Scholar]
- 33.Masuda Y, Kokubu T, Yamashita M, Ikeda H, Inoue S. EGG phosphatidylcholine combined with vitamin B12 improved memory impairment following lesioning of nucleus basalis in rats. Life Sci. 1998;62(9):813–822. doi: 10.1016/s0024-3205(97)01183-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1 G93A transgenic mice. Neuropharmacology. 2008;54(7):1112–1119. doi: 10.1016/j.neuropharm.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease--but how and why. Prostaglandins Leukot Essent Fatty Acids? 2008;78(1):11–19. doi: 10.1016/j.plefa.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102(31):10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka K, Farooqui AA, Siddiqi NJ, Alhomida AS, Ong WY. Effects of docosahexaenoic acid on neurotransmission. Biomol Ther (Seol) 2012;20(2):152–157. doi: 10.4062/biomolther.2012.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008;49:133–143. doi: 10.1007/978-1-4020-8831-5_5. [DOI] [PubMed] [Google Scholar]
- 39.Sinn N, Howe PRC. Mental health benefits of omega-3 fatty acids may be mediated by improvements in cerebral vascular function. Biosci Hypotheses. 2008;1(2):103–108. [Google Scholar]
- 40.Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, et al. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem. 2002;81(5):1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 41.Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1â administration. J Lipid Res. 2004;45(6):1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10(2):136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]


