Abstract
The aim of this study was to produce a recombinant protein consisting of the catalytic and translocation domains of diphtheria toxin for its later application as a vaccine candidate against Corynebacterium diphtheria. To achieve this goal, at first, the amino acid sequence of DT386 was used for prediction of T and B cell epitopes using on-line servers. The DT386 coding sequence was synthesized and subcloned into the NcoI and XhoI sites of pET28a plasmid and recombinant pET28a plasmid was used to transform Escherichia coli BL21 (DE3) host cells. Afterwards, recombinant cells were selected and subjected to induction of expression by 1 mM isopropyl β-D-1-thiogalactopyranoside, (IPTG). Expression of the desired protein was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, and finally, the recombinant protein was purified using nickel affinity chromatography. The results of epitope prediction using on-line servers established the ability of DT386 for stimulation of immune system against diphtheria toxin. Restriction digestion of the recombinant plasmids using NcoI and XhoI enzymes confirmed the fidelity of cloning by producing a band of about 1200 bp. SDS-PAGE analysis following induction of expression and also purification step confirmed the expression of the desired protein by showing a band of about 45 kDa. In addition, Western blot analysis using anti-6X-His antibody confirmed the identity of the expected protein. In conclusion, in the present study we amplified and cloned the coding sequence of DT386 fragment, followed by its expression by E. coli BL21 (DE3) cells. Then, the expressed protein was purified and will be used for later studies of evaluation of its immunogenic properties.
Keywords: Diphtheria toxin, DT386, Vaccine candidate, Immunoinformatics
INTRODUCTION
Diphtheria toxin (DT) is a single chain protein with 535 amino acid residues exerting its cytolethal effects through inhibition of protein synthesis in susceptible cells (1). This toxin is categorized as AB toxins consisting of two different fragments; A and B. The B fragment is responsible for binding to the corresponding cells and following entering the cells A fragment would exert its cell toxicity (2). The catalytic domain of the A fragment is located at its N terminus (C domain: residues 1-193) and catalyzes transfer of ADP-ribosyl moiety of NAD+ to the eukaryotic polypeptide elongation factor 2 (EF2) leading to its inactivation and, finally, inhibition of protein synthesis. The B fragment, on the other hand, consists of two different domains: a translocation domain (T domain: residues 194-381) which enables the toxin to cross the cell membrane and to reach the cytosol, and a binding domain, (R domain: residues 382-535) that recognizes specific receptors on vulnerable cell surface (3).
The removal of the DT receptor-binding domain is expected to result in a truncated DT, unable to interact with its receptor on the surface of eukaryotic cells and, therefore, unable to bind to and kill the cells (3). It has been shown that this truncated form of diphtheria toxin is not cytotoxic (4).
On the other hand, it has been shown that most of the polyclonal B-cell response was directed against DT-A (5), meaning that, the truncated DT can induce neutralizing antibodies and prevent the pathogenesis of diphtheria, although cellular immunity reacted predominantly against the B subunit (5).
The cellular immunity is only involved in the elimination of the infected cells with diphtheria toxin after entering cells and killing them, while neutralizing antibodies can bind the free toxin, before entering cells, and prevent cell entering. Thus humoral immunity is more important than cellular immunity in toxin defense concept (6). It has been shown that anti-fragment A antibody, as an inhibitor of the enzymatic activity of fragment A, was more potent than antibody against traditional DT vaccine (toxoid) (7). Thus the high-affinity anti-fragment A antibodies may bind to fragment A and neutralize the toxin (7). Consequently, production of an immunogenic and non-toxic form of diphtheria toxin, help us to use as a candidate vaccine. Prediction of potential B- and T-cell epitopes, using immunoinformatics led to reduce time and to save resources for the development of vaccines, because these tools can survey the virulence genes and surface-associated proteins in silico, which consequently, lowers the cost needed for laboratory analysis of pathogen antigens (8). These information, facilitate the analyzing of sequence parts with potential immunogenicity, led to the development of new vaccines (8). Thus, the first step in this project was the prediction of B cell and T cell epitopes of this truncated form of DT.
After the establishment of the presence of immune system inducer epitopes, cloning, expression and purification of DT386 was performed as a vaccine candidate against Corynebacterium diphtheria.
In this regard, the DT386 coding sequence was cloned in pET28a expression vector and following induction of expression, the recombinant protein was subjected to affinity chromatography purification using Ni-NTA column.
MATERIALS AND METHODS
Materials
pET28a vector was obtained from Novagen, USA. The coding sequence of DT386 was synthesized by NedayeFan Company (Tehran, Iran), and received in pGH cloning vector. Ampicillin and kanamycin were obtained from sigma-aldrich company, USA. Fast Digest™ restriction endonucleases and GeneJET Plasmid Extraction Kit were purchased from Thermoscientific, USA. Luria-Bertani (LB) culture medium was purchased from HiMedia (Miller, India). All other chemicals were obtained from Sigma-Aldrich (Germany). All experiments were performed according to standard molecular biotechnology protocols (9) unless otherwise stated.
Prediction of the T cell and B cell epitopes of DT386
B-cell epitope prediction (BCPred) (10) and EpiJen (11) web-based servers have been used to predict the linear B-cell and T-cell epitopes of DT386 protein (GenBank: BAL14546.1). In order to predict the B-cell epitopes, BCpreds: B-cell epitope prediction server was used; first, the primary sequence (amino acid sequence in plain format) was added to the chart and BCpred was selected for fixed length epitope prediction. Furthermore, the epitope length was selected as 20 residues and 75% as specificity. On the other hand, EpiJen v1.0 server was used to predict the T-cell epitopes. In order to use this server, the protein sequence was inserted in plain format. Then, the HLA*0101 was selected as an allele and finally, the request was submitted.
Design and cloning of the DT386 protein
The coding sequence of DT386 was optimized for expression in Escherichia coli K12 using online databases. One 6x-His-tag coding sequence was added to the 3’ end of the gene. After obtaining the synthesized fragment, it was sub-cloned into the NcoI and XhoI sites of pET28a plasmid, and the fidelity of cloning was evaluated by restriction endonuclease digestion with the above mentioned enzymes, and also by DNA sequencing. The confirmed recombinant plasmid was designated as pET28-DT386 and used for later studies on expression of the DT386 fragment.
Expression of DT386 protein
The pET28-DT386 recombinant plasmid was used to transform E. coli BL21 (DE3) bacterium as a suitable expression host. One recombinant colony was cultured overnight in LB broth containing 15 μg/mL kanamycin, and used for inoculation of 200 mL medium containing 15 μg/mL kanamycin, until OD600 was reached 0.6. Afterwards, expression was induced using 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 4 h at 37 °C. Then, the cells were harvested by centrifugation at 7,000×g and 4 °C for 10 min, and the obtained pellets were assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12).
Purification of DT386 recombinant protein
The expressed DT386 protein was purified using Ni-NTA Purification system (Invitrogen, USA) as instructed by the manufacturer (13). Briefly, guanidine lysis buffer (pH 7.8) was added to bacterial samples, sonicated, and then the cell lysate was centrifuged at 4000 g for 15 min. The supernatant was applied to a column packed with Ni-NTA resin and incubated at room temperature for 30 min with gentle agitation. Afterwards, the column was washed once with denaturing binding buffer (pH 7.8) and then twice with the denaturing wash buffer (pH 6.0). Finally, the resin was washed four times with the native wash buffer (pH 8.0), and DT386 was eluted using native elution buffer (pH 8.0). Finally, the purified protein was subjected to SDS-PAGE analysis.
Western blot analysis
Authentication of the recombinant protein following both expression and purification procedures were performed by Western blotting. In this regard, protein bands were separated by 12% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membrane (Sigma, Germany). Then, the membrane was incubated overnight with blocking buffer (5% non-fat dry milk in TB) at 4 °C. Afterwards, it was washed three times with washing buffer containing 125 mM NaCl, 25 mM Tris -HCl and 0.1% of Tween 20, followed by incubation with anti-His tag antibody (1:1000 in TBS-Tween buffer) for 1.5 h at room temperature. Subsequently, the membrane was washed three times with the wash buffer and incubated with horseradish peroxidase (HRP) conjugated anti-mouse antibody (Roche, Germany) (diluted 1:5000) at room temperature for 1 h. Finally, the membrane was washed three times with the wash buffer and developed by 3,3-diaminobenzidine (DAB) solution (Sigma, Germany) containing 0.9 mg DAB powder dissolved in wash buffer and supplemented with 15 μL/mL H2O2 30%.
RESULTS
Prediction of T cell and B cell epitopes
The BCpred server predicted ten B cell epitopes in the protein sequence which were arranged as high to low score (Table 1). Furthermore, the results of predicted T cell epitopes by EpiJen server is shown in Table 2.
Table 1.
B cell epitope prediction using BCpred server. Amino acid sequence and the scores of predicted epitopes.
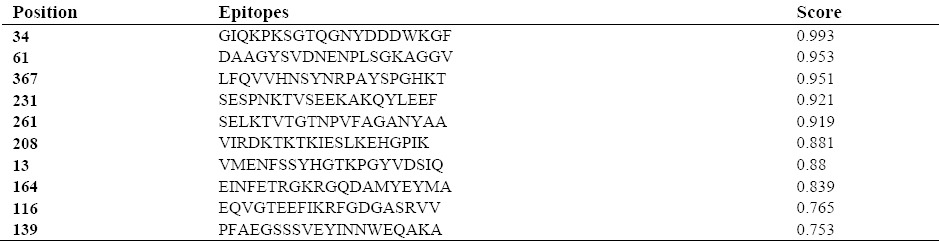
Table 2.
T cell epitope prediction using EpiJen server.
Cloning of the DT386 coding sequence
Digestion of recombinant pGH vector containing DT386 gene using NcoI and XhoI showed a band of approximately 1200 bps on 0.8% agarose gel which corresponds to the size of the desired gene. After sub-cloning of the DT386 fragment from the pGH vector into the pET28a plasmid, the obtained recombinant vector was evaluated by digestion with the similar enzymes used for cloning. As shown in Fig. 1, digestion of the recombinant pET28-DT386 plasmid using NcoI and XhoI enzymes showed two bands of about 5000 and 1200 bp corresponding to the pET28a plasmid and DT386 fragment, respectively. The fidelity of the cloning was also confirmed by DNA sequencing.
Fig. 1.
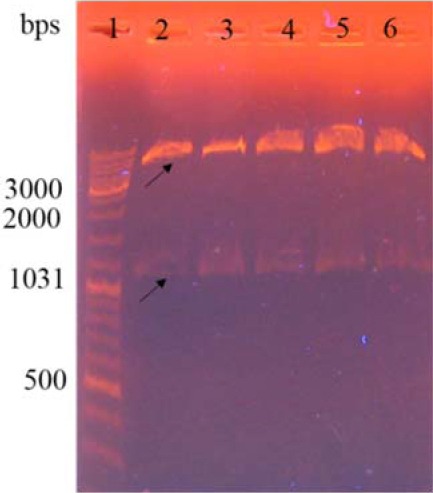
Agarose gel electrophoresis of the DT386 coding sequence. Digestion of the recombinant pET28a containing DT386 fragment by NcoI and XhoI restriction endonucleases. Lane 1: DNA marker, lanes 2 to 6: authenticated recombinant vectors.
Expression of DT386 protein
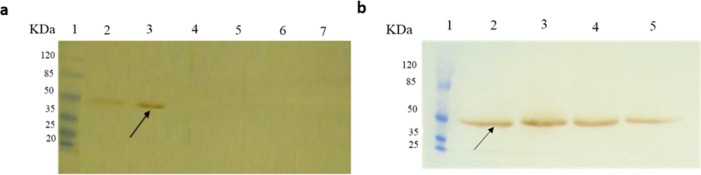
Following induction of DT386 protein expression, the obtained bacterial pellets were analyzed using 12% SDS-PAGE. As represented in Fig. 2, a band of about 45 kDa was observed on SDS-PAGE for the induced bacterial samples. However, this band was not observed in the cases of either non-induced recombinant bacteria, or induced bacteria transformed with the empty pET28a plasmid.
Fig. 2.
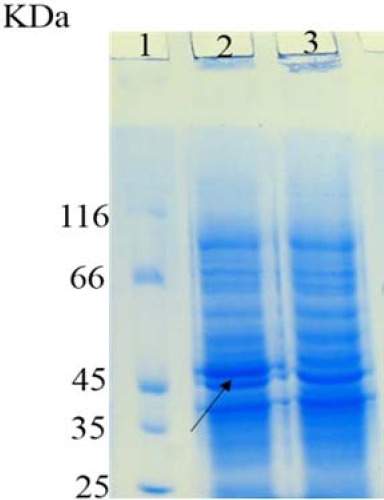
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of DT386 expression. Lane 1: protein marker, lane 2: induced E. coli BL21 (DE3) cells containing pET28a-DT386. Lane 3: un-induced E. coli BL21 (DE3) cells containing pET28a-DT386.
Purification of the recombinant proteins
Purification of the recombinant DT386 protein was performed using Ni-NTA agarose as described in the material and method section. Following purification, the samples were analyzed by 12% SDS-PAGE. As shown in Fig. 3, the hybrid (combined native and denaturing condition) purification procedure was effective for purification of DT36 protein.
Fig. 3.
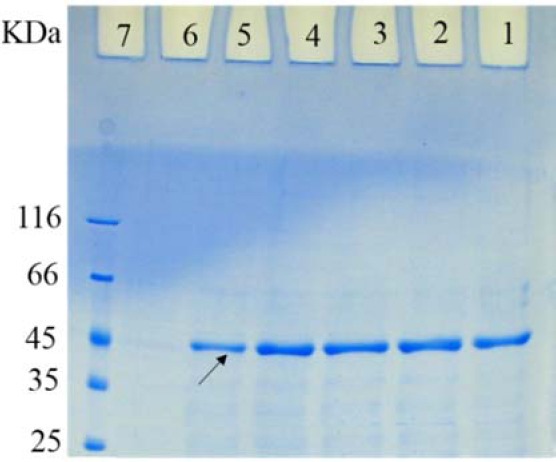
Sodium dodecyl sulfate polyacrylamide gel electrophoresis of the purified DT386. Lanes 1 to 6: elutes 1 to 6 of purified DT386, lane 7: protein marker.
Western blot analysis
The authenticity of the recombinant DT386 following induction of expression, and also after purification procedure was confirmed by Western blot analysis as represented by Fig. 4. As shown, specific bands of about 45 kDa were observed in case of induction of bacteria containing recombinant plasmid or following purification procedure.
Fig. 4.
Western blot analysis for evaluation of DT386 expression. (A) lane 1: protein marker, lane 2: un-induced E. coli BL21 (DE3) containing pET28a-DT386, lane 3: induced E.coli BL21 (DE3) containing pET28a-DT386, lane 4: uninduced E. coli BL21 (DE3) containing pET28a, lane 5: induced E.coli BL21 (DE3) containing pET28a, lane 6: uninduced E. coli BL21 (DE3), lane 7: induced E. coli BL21 (DE3). (B) lane 1: protein marker, lanes 2 to 5: elutes 1 to 4 of purified DT386.
DISCUSSION
Traditional anti-diphtheria vaccines have several serious disadvantages, mainly due to the complicated procedure of conversion of toxin to toxoid. Thus, exploring new antigen substances with sufficient efficacy for stimulating protective responses to diphtheria toxin is essential in anti-diphtheria vaccine development (14). Furthermore, using an appropriate non-toxic antigen for injection to susceptible animals in order to produce neutralizing antibodies for passive immunization eliminates the need for full length DT injection to horses and this resolves the ethical issues. It is noteworthy that another benefit of our product is its potential for induction of immune system for long period due to its high serum concentrations because of lacking the receptor binding domain.
Several studies have shown that human antibodies against diphtheria toxoid, react with both A and B subunits and monoclonal antibodies against both subunits can neutralize DT (15). For example, in a study performed by Autran et al, it was shown that most of the polyclonal B-cell response was directed against A subunit of DT; in contrast, T-cell clones reacted predominantly against B subunit (5). Furthermore, in the study of Rolf et al, a number of isolated hybridomas secreted anti-DTA monoclonal antibodies could immunoprecipitate native toxin and neutralize DT cytotoxicity. Furthermore, these anti-DTA monoclonal antibodies inhibited binding of the toxin to DT receptors on the surface of Vero cell (16). However, some studies have shown that most neutralizing antitoxins are against B subunit especially the R domain, inhibiting binding of the toxin to its receptor. Furthermore, a cysteine loop (residues 186-201) located between the A and B fragments, is a neutralizing epitope present in DT386 (17). Finally, it was shown in another study that rabbits immunized with amino acids 224-237, produced reacting antibodies against the natural toxin (18).
In one study, the R domain and DT168–220 (containing cysteine loop) were expressed separately in Escherichia coli. These fragments were fused with a modified version of immunoglobulin binding domain of protein A from Staphylococcus aureus to facilitate the purification and increase the immunogenicity of DT fragment. It has been shown that the immunogenicity of conventional toxoid is higher than these two produced fusion proteins and DT168–220 is more immunogenic than DTR (19).
In another study, a nontoxic DTA was genetically fused to the major surface protein antigen P1 (SpaP) and surface expressed in Streptococcus gordonii DL-1. The recombinant S. gordonii expressing double copies of DTA (SpaP-DTA2) induced a mucosal immunoglobulin A response and a weak systemic immunoglobulin G response (20).
We predicted ten B-cell epitopes using immunoinformatic tools which 60% of them were located at the A subunit of DT. Furthermore, two of these epitopes overlapped with residues 224-237 and 168–220 whose antigenic properties were proven in previous studies. This finding validated the prediction of B-cell epitopes using BCpred. For predicted T-cell epitopes by EpiJen server, the sequence of the recombinant protein passed four steps including proteasome cleavage, transporter associated with antigen processing (TAP transport), MHC binding and epitope selection. Finally, a set of peptides with antigenic properties remained. From 19 predicted T-cell epitopes using immunoinformatic tools, more than 60% were in B subunit of DT and these findings are in agreement with the results of previous studies in this regard. Finally, since the expressed protein in our study has the cysteine loop and contains more than 70% of the whole molecule, and the results of on-line server showed that this part of diphtheria toxin has either B cell or T cell epitopes, it can be used as a candidate for immunization against diphtheria.
Another aspect of protein vaccines is their fusion to an adjuvant peptide or protein in order to increase its immune response against the antigenic protein. For example, Savar et al, fused two truncated forms of Flagellin (FliC) of enteroaggregative Escherichia coli (EAEC) as a potent adjuvant to type 1 fimbrial FimH adhesion from uropathogenic Escherichia coli (UPEC) to form a recombinant vaccine against urinary tract infections (UTIs). Their results showed that truncated forms of FliC are capable of inducing Th1 and Th2 responses and fusion vaccine induced strong cellular and humoral immune responses (21). These results encouraged us to use these peptide adjuvants for production of candidate vaccines from DT386 in the future.
Because DT is produced in a prokaryotic host, using of a bacterial host is a convenient method for its expression. As shown in Ouyang et al study, the expression of this part of DT was successfully achieved in E. coli (22). Finally, according to the existence of a His-tag at the sequence of this recombinant protein, purification using nickel affinity chromatography was successfully performed.
CONCLUSION
In the present study, we produced a purified truncated DT with the potential of immunization against diphtheria according to the results of immunoinformatic analyzes. In the next steps, the purified recombinant protein will be used for evaluation of its antigenicity. In addition, it will be evaluated in fusion forms with truncated bacterial flagella.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Ph.D thesis NO. 193038 submitted by Fatemeh Shafiee which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran. The authors are also grateful to Mrs. Fatemeh Moazen for her technical assistance.
REFERENCES
- 1.Murphy JR. Corynebacterium diphtheriae. 1996 [PubMed] [Google Scholar]
- 2.Falnes PO, Sandvig K. Penetration of protein toxins into cells. Curr Opin Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Schulte W, Pink D, Phipps K, Zijlstra A, Lewis JD, et al. Sensitivity of cancer cells to truncated diphtheria toxin. PloS one. 2010;5:e10498. doi: 10.1371/journal.pone.0010498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramakrishnan S, Olson T, Bautch VL, Mohanraj D. Vascular endothelial growth factor-toxin conjugate specifically inhibits KDR/flk-1-positive endothelial cell proliferation in vitro and angiogenesis in vivo. Cancer Res. 1996;56:1324–1330. [PubMed] [Google Scholar]
- 5.Autran B, Triebel F, Viguier M, Jolivet M, Falmagne P, Debre P. Monoclonal B-cell response to diphtheria toxoid: evidence for cross-reactive epitopes. Immunology. 1987;60:531–538. [PMC free article] [PubMed] [Google Scholar]
- 6.Janeway CA, Travers P, Walport M, Shlomchik M. The immune system in health and disease. Immunobiology. 2001:39. [Google Scholar]
- 7.Cryz SJ, Welkos SL, Holmes RK. Immunochemical studies of diphtherial toxin and related nontoxic mutant proteins. Infect Immun. 1980;30:835–846. doi: 10.1128/iai.30.3.835-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patronov A, Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biol. 2013;3:120139. doi: 10.1098/rsob.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook JF, Russell DW. Molecular cloning. Cold spring Harbor Press, Cold Spring Harbor, NY. 2001:158–159. [Google Scholar]
- 10.EL‐Manzalawy Y, Dobbs D, Honavar V. Predicting linear B‐cell epitopes using string kernels. J Mol Recognit. 2008;21:243–255. doi: 10.1002/jmr.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doytchinova IA, Guan P, Flower DR. EpiJen: a server for multistep T cell epitope prediction. BMC Bioinformatics. 2006;7:131. doi: 10.1186/1471-2105-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golshani M, Rafati S, Jahanian-Najafabadi A, Nejati-Moheimani M, Siadat SD, Shahcheraghi F, et al. In silico design, cloning and high level expression of L7/L12-TOmp31 fusion protein of Brucella antigens. ResPharm Sci. 2015;10:436–445. [PMC free article] [PubMed] [Google Scholar]
- 13.Jahanian-Najafabadi A, Bouzari S, Oloomi M, Habibi Roudkenar M, Shokrgozar M. Assessment of selective toxicity of insect cell expressed recombinant A1-GMCSF protein toward GMCSF receptor bearing tumor cells. Res Pharm Sci. 2012;7:133–140. [PMC free article] [PubMed] [Google Scholar]
- 14.Bazaral M, Goscienski P, Hamburger R. Characteristics of human antibody to diphtheria toxin. Infec Immun. 1973;7:130–136. doi: 10.1128/iai.7.2.130-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perera V, Corbel M. Human antibody response to fragments A and B of diphtheria toxin and a synthetic peptide of amino acid residues 141-157 of fragment A. EpidemiolInfec. 1990;105:457–468. doi: 10.1017/s095026880004807x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolf JM, Eidels L. Structure-function analyses of diphtheria toxin by use of monoclonal antibodies. Infec Immun. 1993;61:994–1003. doi: 10.1128/iai.61.3.994-1003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Audibert F, Jolivet M, Chedid L, Alouf J, Boquet P, Rivaille P, et al. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. 1981:593–594. doi: 10.1038/289593a0. [DOI] [PubMed] [Google Scholar]
- 18.Sesardic D, Khan V, Corbel MJ. Targeting of specific domains of diphtheria toxin by site-directed antibodies. Microbiol. 1992;138:2197–2203. doi: 10.1099/00221287-138-10-2197. [DOI] [PubMed] [Google Scholar]
- 19.Lobeck K, Drevet P, Léonetti M, Fromen-Romano C, Ducancel F, Lajeunesse E, et al. Towards a recombinant vaccine against diphtheria toxin. Infect Immun. 1998;66:418–423. doi: 10.1128/iai.66.2.418-423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CW, Lee SF, Halperin SA. Expression and immunogenicity of a recombinant diphtheria toxin fragment A in Streptococcus gordonii. Appl Environ Microbiol. 2004;70:4569–4574. doi: 10.1128/AEM.70.8.4569-4574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savar NS, Jahanian-Najafabadi A, Mahdavi M, Shokrgozar MA, Jafari A, Bouzari S. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J Biotechnol. 2014;175:31–37. doi: 10.1016/j.jbiotec.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang J WJ-W, Hong T. Expression, purification and cytotoxicity of diphtheria toxin fragment (DT-386) China Biotechnol. 2004;24:6. [PubMed] [Google Scholar]