Abstract
Epidermal growth factor receptor (EGFR) is usually expressed in squamous cell anal carcinoma (SCAC) and anti-EGFR agents could represent a valid treatment strategy, also considering that KRAS and BRAF mutations are rare events in this type of cancer. However, no data are available on NRAS status in SCAC. In this study we analyzed NRAS status (exons 2–4) by Pyrosequencing in a case series of 50 SCAC patients previously characterized in our laboratory for KRAS, BRAF, PIK3CA mutations and HPV and HIV infections. We found no mutation in NRAS gene. These results confirm that since the principal anti-EGFR resistance mechanisms are almost absent in SCAC, anti-EGFR agents should be considered for the treatment of this type of cancer.
Anal cancer accounts for about 1–5% of all gastrointestinal malignancies, and infection of human papilloma virus (HPV) represents the main etiologic factor1. The standard treatment for locoregional disease is combined chemoradiation, which is associated with a 3-year disease-free survival of about 70%. Of those patients who relapse following chemoradiotherapy (CRT), around 40% undergo salvage therapy with abdominoperineal resection2. Locally advanced or metastatic disease is still incurable, with only limited responses reported for palliative chemotherapy3. New therapeutic approaches are required for these patients.
As EGFR expression in anal carcinoma is observed in a high number of patients (about 80–90% of cases)4,5,6, anti-EGFR drugs have been studied for the treatment of SCAC, showing anti-tumoral activity7,8,9,10. The use of anti-EGFR monoclonal antibodies (mAb anti-EGFRs) in colorectal cancer has shown that KRAS and NRAS represent the main resistance mechanisms to this type of treatment and are thus used in clinical practice to select the patients who should not be treated with anti-EGFR therapy11. BRAF and PIK3CA could also have a prognostic role in determining those patients with a worse outcome12,13,14. KRAS mutation seems to be a rare event in SCAC. Some studies, including a study from our group, have shown that no KRAS mutation is present in SCAC4,5,6,15,16, whereas others have reported rates of KRAS mutation of 1–5%17,18. A low frequency of BRAF mutations has also been observed in SCAC, with a rate of 0–5%15,17,18. A higher rate of PIK3CA mutation between 16% and 22%, has been observed15,18. However, no correlation with patient prognosis was observed for these mutations, as well as for HPV infection. To date, no data are available on the frequency of NRAS mutation in SCAC. In this study we analyzed the status of NRAS in a case series of SCAC previously characterized for HPV, KRAS, BRAF and PIK3CA.
Materials and Methods
Case Series
We analyzed a case series of 50 consecutive SCAC patients treated with chemotherapy and radiotherapy at Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS in Meldola and at the Medical Oncology Units of Faenza, Ravenna and Macerata Hospitals from March 2001 to August 2012, for whom histological material was available. Forty-eight patients received the Nigro scheme with standard dosages19, while the remaining 2 patients underwent treatment with cisplatin plus 5-fluorouracil. This case series was previously characterized by our group for HPV infection, KRAS (exons 2–4), BRAF (exons 11 and 15) and PIK3CA (exons 9 and 20) status15.
The study protocol was reviewed and approved by the Medical Scientific Committee of IRST IRCCS, and written informed consent was obtained from patients or from their next of kin for the use of biological samples for research purposes. In addition, the experiments in this study were conducted in accordance with approved guidelines and regulations.
Mutation analysis
Genomic DNA was extracted from Formalin-fixed paraffin-embedded (FFPE) tumor blocks, as previously described15. NRAS (exons 2, 3 and 4) status was analyzed by Pyrosequencing using anti-EGFR MoAb response (NRAS status) (Diatech, Jesi, Ancona, Italy), according to the manufacturer’s instructions. Reactions were run on a PyroMark Q96 ID (Qiagen, Hilden, Germany).
Statistical analyses
Descriptive statistics were reported as frequencies and percentages for categorical variables and median and range for continuous variables. Progression-free survival (PFS) was calculated from the first day of treatment to the date of first observation of disease progression or death resulting from any cause, whichever occurred first, or the last follow-up for patients who were still alive and had not progressed. Overall survival (OS) was calculated from the first day of treatment to the date of death from any cause or the last follow-up. PFS, OS and their 95% confidence intervals (95% CI) were estimated using the Kaplan-Meier life-table method and survival curves were compared by the logrank test. Statistical significance was assumed for P < 0.05. Statistical analyses were carried out with SAS Statistical software (version 9.4, SAS Institute, Cary, NC, USA).
Results
Clinical-pathological characteristics of SCAC patients included in this study are reported in Table 1. Fifty patients (19 males, 31 females) were analyzed. Median age was 62 years (range, 37–87 years). Twenty-nine (58%) patients had early stage disease (stage I and II), 19 (38%) had regional nodal involvement (stage III), and 2 (4%) had distant metastasis (stage IV). All patients were KRAS (exons 2–4) and BRAF (exons 11 and 15) wild-type (wt), whereas 11 showed a PIK3CA mutation (8 at exon 9 and 3 at exon 20). All patients had HPV infection (45 HPV type 16 and 5 other HPV types), 6 of which had also HIV infection. All 50 patients resulted wt for codons 12–13 (exon 2), 58-59-61 (exon 3) and 117–146 (exon 4) of NRAS gene.
Table 1. Clinical-pathological characteristics of patients.
| n (%) | |
|---|---|
| Gender | |
| Female | 31 (62) |
| Male | 19 (38) |
| Age, years | |
| <50 | 7 (14) |
| ≥50 and <70 | 29 (58) |
| ≥70 | 14 (28) |
| Grade | |
| G1 | 2 (4) |
| G2 | 32 (64) |
| G3 | 16 (32) |
| TNM (T) | |
| t1-2 | 27 (54) |
| t3-4 | 23 (46) |
| Stage | |
| I | 8 (16) |
| II | 21 (42) |
| III | 19 (38) |
| IV | 2 (4) |
| HIV infection | |
| Yes | 6 (12) |
| No | 44 (88) |
| HPV infection | |
| HPV-16 | 45 (90) |
| Other HPV | 5 (10) |
| PIK3CA status | |
| wt | 39 (78) |
| mutated | 11 (22) |
| KRAS status | |
| wt | 50 (100) |
| mutated | — |
| BRAF status | |
| wt | 50 (100) |
| mutated | — |
HIV, human immunodeficiency virus; HPV, human papillomavirus.
wt, wild type.
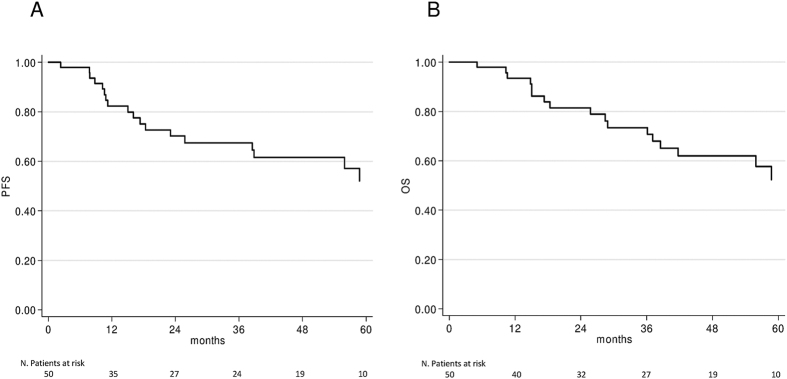
At a median follow-up of 50 months (range 2 to 178 months), 11 patients had progressed (7 with local and 4 with distant relapse) and 10 had died from causes other than SCAC. PFS was 82% (95% CI 71–93) at one year, 67% (95% CI 53–82) at 3 years, and 52% (95% CI 34–70) at 5 years. OS was 93% (95% CI 86–100) at 1 year, 73% (95% CI 60–87) at 3 years, and 52% (95% CI 34–70) at 5 years. (Fig. 1 and Table 2). PFS and OS in relation to clinical-pathological characteristics of patients and in accordance to PIK3CA status, HIV and HPV infections are reported in Table 2. Tumor grade and size, lymph node involvement and stage were significantly associated with PFS and OS. Presence of HIV infection, type of HPV infection and PIK3CA status were not correlated with survival.
Figure 1.
Kaplan-Meier survival curves of (A) progression-free survival (PFS) and (B) overall survival (OS).
Table 2. PFS and OS in relation to clinical-pathological characteristics of patients.
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n patients | % 1 year (95% CI) | % 3 years (95% CI) | % 5 years (95% CI) | P | % 1 year (95% CI) | % 3 years (95% CI) | % 5 years (95% CI) | P | |
| Overall | 50 | 82 (71–93) | 67 (53–82) | 52 (34–70) | — | 93 (86–100) | 73 (60–87) | 52 (34–70) | — |
| Grade | |||||||||
| 1 + 2 | 34 | 87 (75–99) | 76 (60–92) | 55 (31–79) | 93 (85–100) | 78 (63–94) | 59 (36–82) | ||
| 3 | 16 | 73 (51–96) | 49 (22–76) | 39 (11–67) | 0.048 | 93 (81–100) | 63 (37–89) | 36 (8–63) | 0.040 |
| T | |||||||||
| 1–2 | 27 | 92 (81–100) | 87 (73–100) | 58 (32–84) | 96 (88–100) | 86 (72–100) | 62 (35–88) | ||
| 3–4 | 23 | 76 (55–97) | 46 (19–73) | 46 (19–73) | 0.020 | 88 (73–100) | 53 (26–79) | 44 (17–71) | 0.014 |
| Lymph node status | |||||||||
| Negative | 31 | 86 (73–99) | 78 (63–94) | 57 (35–79) | 100 | 85 (71–99) | 59 (37–81) | ||
| Positive | 19 | 76 (56–97) | 44 (17–72) | 44 (17–42) | 0.037 | 82 (63–100) | 51 (24–79) | 41 (13–69) | 0.013 |
| Stage | |||||||||
| 1 | 8 | 100 | 100 | 83 (54–100) | 100 | 100 | 83 (54–100) | ||
| 2 | 21 | 85 (70–100) | 80 (63–98) | 56 (30–83) | 100 | 78 (59–97) | 62 (36–87) | ||
| 3 | 19 | 76 (55–96) | 43 (15–71) | 43 (15–71) | 87 (71–100) | 65 (39–90) | 37 (9–65) | ||
| 4 | 2 | 50 (0–100) | 0 | 0 | 0.0003 | 50 (0–100) | 0 | 0 | <0.0001 |
| HIV status | |||||||||
| Negative | 44 | 80 (68–92) | 69 (55–84) | 53 (35–72) | 93 (85–100) | 74 (60–88) | 55 (37–73) | ||
| Positive | 6 | 100 | 33 (0–87) | 33 (0–87) | 0.521 | 100 | 67 (13–100) | 0 | 0.324 |
| PIK3CA status | |||||||||
| wt | 39 | 83 (71–96) | 68 (52–84) | 54 (34–73) | 92 (83–100) | 73 (57–88) | 56 (37–75) | ||
| mutated | 11 | 78 (51–100) | 65 (32–97) | 43 (2–84) | 0.883 | 100 | 75 (45–100) | 30 (0–75) | 0.860 |
| HPV | |||||||||
| 16 | 45 | 80 (68–92) | 66 (51–81) | 51 (32–71) | 93 (85–100) | 73 (58–87) | 48 (28–68) | ||
| #16 | 5 | 100 | 80 (45–100) | 53 (5–100) | 0.904 | 100 | 80 (45–100) | 80 (45–100) | 0.854 |
PFS, progression-free survival; OS, overall survival; HIV, human immunodeficiency virus; HPV, human papillomavirus; wt, wild type.
Discussion
The aim of our study was to analyze the status of NRAS gene in a case series of SCAC patients previously characterized in our laboratory for KRAS, BRAF and PIK3CA genes. To our knowledge, this is the first study to analyze NRAS status in SCAC. Given that mutations occurring on KRAS exon 2–4 in colorectal cancer – in addition to those occurring on NRAS exon 2–4 – may play a role in determining resistance to moAb-EGFRs11, these molecular characterizations could also be investigated in SCAC in view of the potential usefulness of these antibodies in this type of cancer.
Other studies have analyzed the frequency of KRAS, BRAF and PIK3CA mutations in SCAC, showing a very low rate of KRAS and BRAF mutations15,16,17,18, and a slightly higher frequency of PIK3CA mutation15,18. No correlation between PIK3CA mutation and patients prognosis was reported in this study with an updated follow up, confirming what previously stated15.
We demonstrated that no NRAS mutation is present in SCAC and confirmed the absence of potential resistance mechanisms to moAb-EGFR, such as cetuximab or panitumumab. This data, together with previous evidence on KRAS and BRAF, suggest that cetuximab could be a valid treatment strategy in SCAC.
We confirmed that PIK3CA mutations have no prognostic role in this type of cancer, whereas tumor grade and size, lymph node involvement and stage are significantly associated with PFS and OS. Moreover, infection of HPV 16 was significantly associated with a longer PFS but not with OS.
A recent phase I study has demonstrated that the adding of cetuximab to a chemo-radiotherapy with 5-fluorouracil (5-FU) and mitomycin-C in SCAC induced a complete remission rate of 73% with a relative high toxicity10. High toxicity was observed also in other clinical trials20,21, suggesting that the relation between toxicity, chemotherapy dosage and radiation techniques should be better clarified to optimize this promising combination treatment strategy for SCAC. In addition to this data, a recent case report has shown a dramatic response to cetuximab in combination with cisplatin and 5-FU in a patient with metastatic anal cancer9.
Moreover, there are no therapies available after progression to cisplatin and 5-FU that can improve survival. A case report showed a complete response to cetuximab in monochemotherapy in a patient with refractory metastatic anal carcinoma suggesting a possible use in this setting22.
There are numerous similarities between SCAC and head and neck squamous cell carcinoma (HNSCC), both in terms of etiology and pathology. In particular, as observed in SCAC, high EGFR expression and a low percentage of RAS mutations have also been reported in HNSCC23,24,25, and this was the basic starting point for research into mAb anti-EGFRs in this type of cancer. The approval of cetuximab for the treatment of advanced and metastatic HNSCC26,27 further reinforces the potential effectiveness of anti-EGFR treatment in SCAC.
A limitation of the study consists in the fact that, due to its retrospective nature, not all information on the chemoradiotherapy administered was available, in particular that pertaining to the different radiotherapy dosages delivered. Moreover, despite the known disadvantages of a composite endpoint, we decided to use such an endpoint because of the potential underreporting of disease recurrences caused by the non-homogeneous availability of follow-up data from the different centers involved in the study.
Our results demonstrated that NRAS gene is wt in SCAC patients. These data, together with those reported on KRAS status, seem to confirm the potential usefulness of anti-EGFR drugs in the treatment of SCAC. Results of ongoing clinical trials will clarify the tolerability and the efficacy of these agents in combination with conventional CRT.
Additional Information
How to cite this article: Capelli, L. et al. No evidence of NRAS mutation in squamous cell anal carcinoma (SCAC). Sci. Rep. 6, 37621; doi: 10.1038/srep37621 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors thank Veronica Zanoni for editorial assistance.
Footnotes
Author Contributions A.C.G. and P.U. designed the study, A.C.G., G.L.F., M.S., M.G., S.T., J.C. collected and analyzed patients clinical information, L.C. conduced the experiments, L.S. and M.P. were the pathologists that selected tumor tissues, E.S. performed the statistical analyses, L.C., A.C.G., P.U. wrote the manuscript. All authors reviewed the manuscript.
References
- Skamperle M., Kocjan B. J., Maver P. J., Seme K. & Poljak M. Human papillomavirus (HPV) prevalence and HPV type distribution in cervical, vulvar, and anal cancers in central and eastern Europe. Acta Dermatovenerol. Alp. Pannonica Adriat. 22, 1–5 (2013). [PubMed] [Google Scholar]
- Schiller D. E. et al. Outcomes of salvage surgery for squamous cell carcinoma of the anal canal. Ann. Surg. Oncol. 14, 2780–2789 (2007). [DOI] [PubMed] [Google Scholar]
- Faivre C. et al. 5-Fluorouracile and Cisplatinum Combination Chemotherapy for Metastatic Squamous-Cell Anal Cancer. Bull. Cancer 86, 861–865 (1999). [PubMed] [Google Scholar]
- Paliga A. et al. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors? Br. J. Cancer 107, 1864–1868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampino M. G., Magni E., Sonzogni A. & Renne G. K-ras status in squamous cell anal carcinoma (SCC): it’s time for target-oriented treatment? Cancer Chemother. Pharmacol. 65, 197–199 (2009). [DOI] [PubMed] [Google Scholar]
- Van Damme N. et al. Epidermal growth factor receptor and K-RAS status in two cohorts of squamous cell carcinomas. BMC Cancer 10, 189-2407-10-189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmettler H., Komminoth P., Schmid M. & Duerr D. Efficacy of Cetuximab in Combination with FOLFIRI in a Patient with KRAS Wild-Type Metastatic Anal Cancer. Case Rep. Oncol. 5, 428–433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukan N. et al. Cetuximab-based treatment of metastatic anal cancer: correlation of response with KRAS mutational status. Oncology 77, 293–299 (2009). [DOI] [PubMed] [Google Scholar]
- Rogers J. E., Silva N. N. & Eng C. Cetuximab in combination with cisplatin and 5-Fluorouracil induces dramatic response in metastatic refractory squamous cell carcinoma of the anal canal. J. Gastrointest. Oncol. 6, E82–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon O., Guren M. G., Radu C., Gunnlaugsson A. & Johnsson A. Phase I study of cetuximab in combination with 5-fluorouracil, mitomycin C and radiotherapy in patients with locally advanced anal cancer. Eur. J. Cancer 51, 2740–2746 (2015). [DOI] [PubMed] [Google Scholar]
- Heinemann V. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1065–1075 (2014). [DOI] [PubMed] [Google Scholar]
- Ulivi P. et al. Predictive role of multiple gene alterations in response to cetuximab in metastatic colorectal cancer: a single center study. J. Transl. Med. 10, 87-5876-10-87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E. et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 29, 2011–2019 (2011). [DOI] [PubMed] [Google Scholar]
- De Roock W. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010). [DOI] [PubMed] [Google Scholar]
- Casadei Gardini A. et al. KRAS, BRAF and PIK3CA status in squamous cell anal carcinoma (SCAC). PLoS One 9, e92071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge E. S. et al. No evidence of oncogenic KRAS mutations in squamous cell carcinomas of the anogenital tract and head and neck region independent of human papillomavirus and p16(INK4a) status. Hum. Pathol. 45, 2347–2354 (2014). [DOI] [PubMed] [Google Scholar]
- Serup-Hansen E., Linnemann D., Hogdall E., Geertsen P. F. & Havsteen H. KRAS and BRAF mutations in anal carcinoma. APMIS 123, 53–59 (2015). [DOI] [PubMed] [Google Scholar]
- Martin V. et al. EGFR, KRAS, BRAF, and PIK3CA characterization in squamous cell anal cancer. Histol. Histopathol. 29, 513–521 (2014). [DOI] [PubMed] [Google Scholar]
- Nigro N. D., Vaitkevicius V. K. & Considine B. Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis. Colon Rectum 17, 354–356 (1974). [DOI] [PubMed] [Google Scholar]
- Deutsch E. et al. Unexpected toxicity of cetuximab combined with conventional chemoradiotherapy in patients with locally advanced anal cancer: results of the UNICANCER ACCORD 16 phase II trial. Ann. Oncol. 24, 2834–2838 (2013). [DOI] [PubMed] [Google Scholar]
- Olivatto L. O. et al. Phase 1 study of cetuximab in combination with 5-fluorouracil, cisplatin, and radiotherapy in patients with locally advanced anal canal carcinoma. Cancer 119, 2973–2980 (2013). [DOI] [PubMed] [Google Scholar]
- Silva N. N. & Eng C. Management of refractory metastatic anal squamous cell carcinoma following disease progression on traditional chemoradiation therapy. J. Adv. Pract. Oncol. 3, 161–169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckx C. et al. Mutation analysis of genes in the EGFR pathway in Head and Neck cancer patients: implications for anti-EGFR treatment response. BMC Res. Notes 7, 337-0500-7-337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland P. et al. Human papillomavirus and gene mutations in head and neck squamous carcinomas. ANZ J. Surg. 82, 362–366 (2012). [DOI] [PubMed] [Google Scholar]
- Sheikh Ali M. A. et al. Expression and mutation analysis of epidermal growth factor receptor in head and neck squamous cell carcinoma. Cancer. Sci. 99, 1589–1594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. A. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2010). [DOI] [PubMed] [Google Scholar]
- Vermorken J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008). [DOI] [PubMed] [Google Scholar]