Abstract
Background
Decreased estrogen levels in postmenopausal women may cause an increase in oral symptoms including dry mouth, burning sensation of the mouth, and taste alterations. Management of salivary gland hypofunction by various modalities had been tried with variable results and associated side effects or discomfort.
Aim
To evaluate the effects of transcutaneous electric nerve stimulation (TENS) on whole salivary flow rate in postmenopausal females with and without oral dryness.
Methods
Fifty postmenopausal women, based on their response to Xerostomia Inventory, were divided into 2 groups of 25 each; group 1 were postmenopausal women with oral dryness (PMD + OD) and group 2 were postmenopausal women without oral dryness (PMD − OD). Unstimulated whole saliva collection was done by low forced spitting method. External salivary stimulation of parotid gland by electrodes of TENS unit was done and sialometry was repeated. The salivary flow rates were compared within both groups before and after stimulation and between the two groups.
Results
The mean salivary flow rates at baseline were statistically significantly lower in the PMD + OD group than the PMD − OD group. There was a mean increase of 0.33 ml and 0.46 ml with TENS stimulation in PMD + OD and PMD − OD groups, respectively.
Conclusion
Postmenopausal women with perception of oral dryness had lower salivary flow rates. 90% of the subjects, irrespective of oral dryness status, responded to TENS therapy. TENS stimulation resulted in a statistically significant increase in the quantity of whole saliva flow rate in postmenopausal women with or without oral dryness.
Keywords: Postmenopausal women, Salivary stimulation, TENS, Xerostomia
1. Introduction
Menopause is a physiological process, which typically occurs in the fifth decade of life in women and is defined as the time at which cyclic ovarian function as manifested by menstruation, ceases.1, 2 Various studies have suggested that menopause initiates a host of physiologic changes that include endocrinological alterations and atrophy of tissues lining the vagina and in the urinary tract. Decreased estrogen levels may cause hot flushes, sweating, osteoporosis, cardiovascular disease, cognitive disease, urogenital infections, and skin changes in menopausal and postmenopausal women.2 Apart from these, they may also experience an increase in oral symptoms that may result from endocrine disturbances (reduced estrogen), calcium and vitamin deficiencies, and various psychologic factors during menopausal years.1 The oral symptoms may include dry mouth, burning sensation of the mouth and taste alterations.
The etiology of oral discomfort in menopausal women had been related to alterations in the quantity or the quality of saliva. Saliva is a critical fluid in maintaining oral health. Alterations in salivary function may lead to impairment of oral tissues and have large impact on the patient's quality of life. A higher incidence of dental caries, oral mucositis, dysphagia, oral infections, and altered taste has been reported in individuals with reduced salivary flow.3 Management of salivary gland hypofunction had been tried using palliative measures, medications, psychological counseling, acupuncture with variable results, and associated side effects or discomfort.4
Transcutaneous electric nerve stimulation (TENS) is a well-known physical therapy, which is predominantly used for the management of chronic pain.5 Apart from application in pain, use of TENS for noninvasive electronic stimulation of reflex salivation in xerostomic patients has been encouraging. The advantages of noninvasiveness, safety, ease of technique and good acceptance rates by patients make it an attractive treatment modality for xerostomia as an alternative to the existing regimens.4 Though few studies have demonstrated salivary stimulation in healthy individuals or patients with xerostomia, so far no study had been conducted to assess the efficacy of TENS for salivary stimulation in postmenopausal women. Hence, the present study was planned to assess the unstimulated whole salivary flow rates of postmenopausal women, and the efficacy of TENS on salivary stimulation in this cohort. The objectives of the study were to assess the perception of oral dryness by Xerostomia Inventory (XI) in postmenopausal women and evaluate the effectiveness of TENS stimulation on whole salivary flow rates.
2. Materials and methods
2.1. Ethical clearance
The study protocol was approved by the institutional ethical committee. Informed consent was obtained from all subjects. The ethical standards followed were in accordance with Helinski Declaration of 1975.
2.2. Study design
A randomized unblinded interventional study was conducted in postmenopausal women cohort over a period of 6 months.
2.3. Selection and description of participants
Fifty postmenopausal females who attained menopause at least 1 year back, aged 45 years or more, attending the department of Oral Medicine & Radiology, Swami Devi Dyal Dental College, Panchkula, were recruited for the study. Subjects who underwent hysterectomy, were under hormone replacement therapy and had comorbid diseases of salivary glands were excluded from the study.
2.4. Technical information
After obtaining informed consent from postmenopausal women, they was asked to fill Xerostomia inventory (XI, shown in Fig. 1),6 and the severity of XI was estimated. Affirmative answers to at least 3 questions from the XI were considered as being positive for xerostomia, and such patients were considered to be suffering from oral dryness. Though initially 58 patients filled the questionnaire (33 did not have oral dryness, while 25 patients had oral dryness), only 50 patients were enrolled in the study, 25 each in the two groups to facilitate statistical evaluation. The study population was then arranged into 2 groups, based on response to XI, as postmenopausal women with oral dryness (PMD + OD, group 1) and without oral dryness (PMD − OD, group 2).
Fig. 1.

Xerostomia Inventory.
Detailed clinical examination, including an assessment of oral health by the use of Decayed, Missing, Filled Teeth (DMFT) Index and Oral Hygiene Index – Simplified (OHI-S), was done for both the groups.7, 8 Assessment of salivary hypofunction was carried out by checking lip, oral mucosal dryness, tongue blade test and sialometry. The salivary collection was planned to be performed the next day between 9 and 11 am, to avoid any possible salivary stimulation from food intake and erroneous results. Patients were instructed to refrain from eating, drinking and smoking 90 min prior to salivary collection in the next appointment. Sialometry was conducted the next day to collect unstimulated whole saliva of the postmenopausal women. Unstimulated saliva was collected with ‘low forced spitting’ method in a graduated test tube for 5 min, at 1-min intervals, with the patient in an upright sitting posture with forward head position so as to allow drooling of the saliva passively9 (Fig. 2).
Fig. 2.
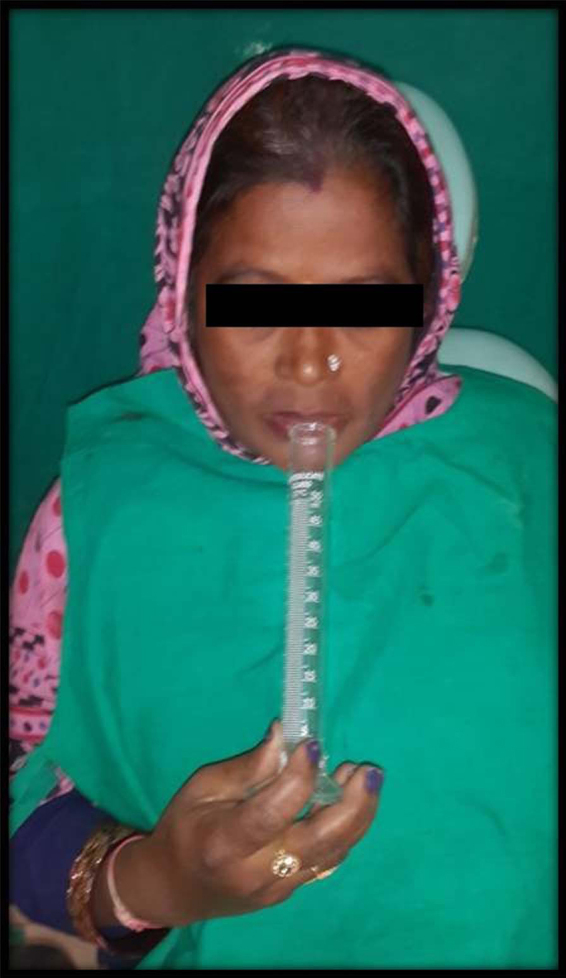
Sialometry in resting state.
The TENS unit used was Ultrasonic TENS (marketed by DR.Glow, Mumbai, India). The technical specifications of the TENS unit were 220 V, A/C 50 Hz, 0–100 mA at 1 k load, biphasic wave form, available in pulsed/continuous form, and 2 intensities I and II. The electrode of TENS unit (size 4 × 2 inches) was then placed vertically, externally on skin overlying the parotid gland, in the preauricular area bilaterally, 1 cm in front of the tragus area, with TENS unit in off position. Saliva collection was repeated with the TENS unit in the off position to assess the placebo effect of using TENS. This was repeated in all patients to judge whether any patient would have an increase in salivary output just on placing the TENS unit without activation. The TENS unit was then activated in the continuous mode and intensity control switch was increased to tolerable level of patient for 15 min. All the patients were subjected initially to intensity I, and intensity was increased to II, if there was no twitching or any other discomfort experienced by the patient. Optimal intensity was defined as the maximum intensity the patient perceived to be comfortable. Forty-eight patients out of 50 patients tolerated intensity II. At this optimal intensity, stimulated saliva was collected for 5 min with the same method in a separate graduated test tube and flow rates were compared (Fig. 3).
Fig. 3.
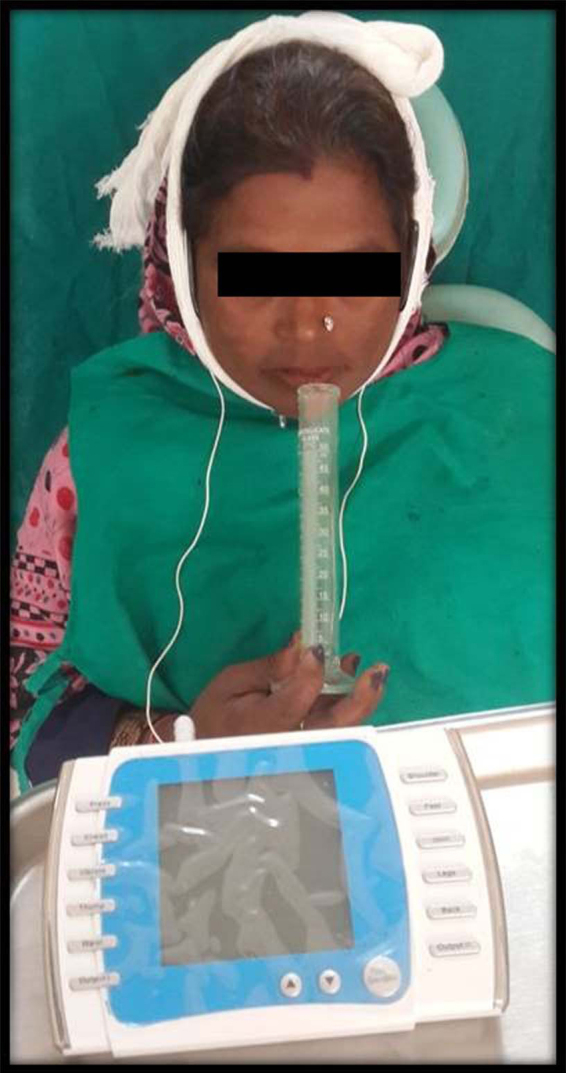
Sialometry after TENS stimulation.
Statistical analysis was done by independent samples T test and Mann–Whitney U test to compare the baseline parameters, unstimulated salivary flow rates and the salivary stimulation by TENS between the groups.
3. Results
Twenty-five patients, who answered at least 3 questions in the XI with an affirmative answer, thus were categorized as PMD + OD. The rest of the 25 patients did not have symptoms of xerostomia and were categorized as PMD − OD. The mean age and duration of menopause of PMD + OD group was slightly higher than the PMD − OD group, but was not statistically significant, as shown in Table 1A, Table 1B. The mean scores of XI in PMD + OD group were significantly higher than PMD − OD group. However, the groups did not differ significantly for DMFT scores as well as OHI-S scores (Table 2A, Table 2B, Table 2C).
Table 1A.
Mean age and duration of menopause of the two groups.
| Groups | Mean age (SD) (years) | Range (years) | Mean duration of menopause (SD) (years) | Range (years) |
|---|---|---|---|---|
| PM + OD | 61.16 ± 10.36 | 45–82 | 15.88 ± 9.79 | 3–37 |
| PM − OD | 58.48 ± 7.86 | 46–76 | 11.36 ± 7.04 | 2–27 |
Table 1B.
Comparison between baseline parameters between groups by independent samples test.
|
t-Test for equality of means |
|||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (2-tailed) | Mean difference | Std. error difference | 95% Confidence interval of the difference |
||
| Lower | Upper | ||||||
| Age (years) | 1.030 | 48 | 0.308 | 2.680 | 2.602 | −2.553 | 7.913 |
| Menopause (years) | 1.873 | 48 | 0.067 | 4.520 | 2.413 | −0.332 | 9.372 |
Table 2A.
Clinical examination findings of both groups.
| Group | Parameter | N | Mean | Std. deviation | Minimum | Maximum | Percentiles |
||
|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | |||||||
| PMD + OD | XI (mean score) | 25 | 7.76 | 2.29 | 4 | 11 | 6.00 | 8.00 | 10.00 |
| Tongue blade | 25 | 1.00 | 0.00 | 1 | 1 | 1.00 | 1.00 | 1.00 | |
| Decayed | 25 | 4.00 | 2.56 | 2 | 10 | 3.00 | 3.00 | 4.50 | |
| Missing | 25 | 9.67 | 6.88 | 4 | 22 | 4.00 | 6.50 | 15.75 | |
| Filled | 25 | 2.00 | 1.41 | 2 | 2 | 0.75 | 2.00 | 3.25 | |
| OHI-S | 25 | 2.76 | 0.38 | 2 | 3 | 2.49 | 2.49 | 3.17 | |
| PMD − OD | XI (mean score) | 25 | 0.96 | 0.79 | 0 | 2 | 0.00 | 1.00 | 2.00 |
| Tongue blade | 25 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0.00 | |
| Decayed | 25 | 4.53 | 1.88 | 1 | 7 | 3.00 | 4.00 | 6.00 | |
| Missing | 25 | 9.89 | 8.50 | 2 | 26 | 3.00 | 9.00 | 16.00 | |
| Filled | 25 | 2.00 | 1.41 | 1 | 3 | 0.75 | 2.00 | 3.25 | |
| OHI-S | 25 | 2.63 | 0.39 | 2 | 3 | 2.49 | 2.75 | 2.87 | |
Table 2B.
Comparison of clinical parameters between the groups by Mann–Whitney test.
| Mann–Whitney test | ||||
|---|---|---|---|---|
| Ranks | ||||
| Group | N | Mean rank | Sum of ranks | |
| XI | PMD + OD | 25 | 38.00 | 950.00 |
| PMD − OD | 25 | 13.00 | 325.00 | |
| Tongue blade | PMD + OD | 25 | 38.00 | 950.00 |
| PMD − OD | 25 | 13.00 | 325.00 | |
| Decayed | PMD + OD | 25 | 9.75 | 78.00 |
| PMD − OD | 25 | 13.20 | 198.00 | |
| Missing | PMD + OD | 25 | 11.25 | 135.00 |
| PMD − OD | 25 | 10.67 | 96.00 | |
| Filled | PMD + OD | 25 | 2.00 | 2.00 |
| PMD − OD | 25 | 2.00 | 4.00 | |
| OHI-S | PMD + OD | 25 | 8.20 | 41.00 |
| PMD − OD | 25 | 7.90 | 79.00 | |
Table 2C.
Statistical comparison of clinical parameters between the groups.
| Test statistics | ||||||
|---|---|---|---|---|---|---|
| XI (mean score >3/11) | Tongue blade | Decayed | Missing | Filled | OHI-S | |
| Mann–Whitney U | 0.000 | 0.000 | 42.000 | 51.000 | 1.000 | 24.000 |
| Wilcoxon W | 325.000 | 325.000 | 78.000 | 96.000 | 4.000 | 79.000 |
| Z | −6.120 | −7.000 | −1.216 | −0.219 | 0.000 | −0.127 |
| Asymp. Sig. (2-tailed) | <0.001b | <0.001b | 0.224 | 0.826 | 1.000 | 0.899 |
| Exact Sig. [2*(1-tailed Sig.)] | 0.265a | 0.862a | 1.000a | 0.953a | ||
Not corrected for ties.
Highly significant.
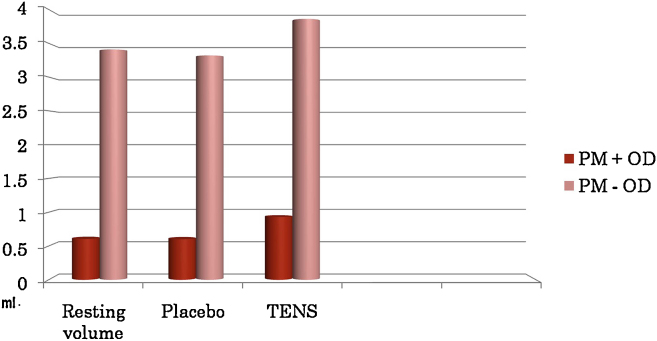
The mean salivary flow rates at baseline were statistically significantly lower in the PMD + OD group than the PMD − OD group. Clinical signs of salivary hypofunction were significantly positive in PMD + OD group (Table 3A, Table 3B, Table 3C). The baseline salivary flow and salivary flow with TENS machine in the OFF position did not vary significantly in the two groups. All the patients tolerated TENS stimulation well without any discomfort or pain. However, forty-eight out of 50 subjects tolerated intensity II without discomfort, while the other two subjects were comfortable with intensity I. 90% (23 subjects of PMD + OD, and 22 subjects of PMD − OD) of the subjects of both groups responded to TENS therapy. There was a mean increase of 0.33 ml and 0.46 ml with TENS stimulation in PMD + OD and PMD − OD groups, respectively. The difference in mean increase was statistically significant (P = 0.031) as shown in Table 4 and Fig. 4.
Table 3A.
Sialometry before and after TENS stimulation of both groups.
| Group | Parameter | N | Mean | Std. deviation | Minimum | Maximum | Percentiles |
||
|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th (median) | 75th | |||||||
| PMD + OD | Resting volume | 25 | 0.60 | 0.61 | 0.10 | 2 | 0.2000 | 0.3000 | 0.7500 |
| Placebo | 25 | 0.60 | 0.61 | 0 | 2 | 0.20 | 0.30 | 0.75 | |
| TENS | 25 | 0.93 | 0.89 | 0 | 3 | 0.40 | 0.50 | 1.00 | |
| PMD − OD | Resting volume | 25 | 3.40 | 1.06 | 0.30 | 5 | 3.0000 | 3.5000 | 4.0000 |
| Placebo | 25 | 3.32 | 1.07 | 0 | 5 | 3.00 | 3.50 | 4.00 | |
| TENS | 25 | 3.86 | 1.15 | 0 | 5 | 3.50 | 4.00 | 4.50 | |
Table 3B.
Comparison of sialometry between the groups.
| Mann–Whitney test | ||||
|---|---|---|---|---|
| Ranks | ||||
| Group | N | Mean rank | Sum of ranks | |
| Resting volume | PMD + OD | 25 | 14.00 | 350.00 |
| PMD − OD | 25 | 37.00 | 925.00 | |
| Total | 50 | |||
| Placebo | PMD + OD | 25 | 14.22 | 355.50 |
| PMD − OD | 25 | 36.78 | 919.50 | |
| Total | 50 | |||
| TENS | PMD + OD | 25 | 14.26 | 356.50 |
| PMD − OD | 25 | 36.74 | 918.50 | |
| Total | 50 | |||
Table 3C.
Statistical comparison of sialometry between the groups.
| Resting volume | Placebo | TENS | |
|---|---|---|---|
| Mann–Whitney U | 25.000 | 30.500 | 31.500 |
| Wilcoxon W | 350.000 | 355.500 | 356.500 |
| Z | −5.616 | −5.512 | −5.485 |
| Asymp. Sig. (2-tailed) | <0.001** | <0.001** | <0.001** |
Highly significant.
Table 4.
The mean increase in both the groups.
| Groups | Resting volume (ml) | Placebo effect (ml) | TENS stimulation (ml) | Mean increase (ml) |
|---|---|---|---|---|
| PM + OD | 0.60 ± 0.61 | 0.60 ± 0.61 | 0.93 ± 0.89 | 0.33 ± 0.27 |
| PM − OD | 3.40 ± 1.06 | 3.32 ± 1.07 | 3.86 ± 1.15 | 0.46 ± 0.1 |
| P value | <0.001 | <0.001 | <0.001 | 0.031 |
Fig. 4.
Graph showing comparison of salivary stimulation between groups.
4. Discussion
The oral cavity is a moist environment wherein saliva constantly coats its inner surfaces and occupies the space between lining oral mucosa and the teeth. Saliva is a complex fluid, the important role of which is to maintain the well being of oral cavity.5 Saliva maintains neutral pH and is essential for maintaining enamel mineralization. The fluid not only lubricates the mouth and upper pharynx but also modulates oral flora, aids in digestion of food, and facilitates speech and swallowing. It also plays a role in oral immunology and possesses a number of antibacterial enzymes, such as lysozyme, peroxidase, histatins, and lactoferrin.4
At rest, saliva secretion ranges from 0.25 to 0.35 ml/min, mainly contributed by the submandibular and sublingual glands.10 Salivary glands are innervated, either directly or indirectly, by the parasympathetic and sympathetic arms of the autonomic nervous system. “Parasympathetic” innervation to the salivary glands is carried via cranial nerves. The parotid gland receives its parasympathetic input from the glossopharyngeal nerve (CN IX) via the otic ganglion.10 Sensory, electrical, or mechanical stimuli can raise the secretion rate to 1.5 ml/min.4
Xerostomia, a subjective feeling of oral dryness, is a common clinical phenomenon and is present in about 40% of adults over the age of 50 years. Xerostomia and salivary gland hypofunction are associated with local and systemic conditions, advancing age, postmenopausal status, some medical disorders, head and neck radiation, smoking, and recreational drug usage. Apart from medication, psychological stress, depression, and anxiety may also contribute to oral dryness among the elderly and significantly affect their quality of life.11
Treatment of xerostomia is a challenge, since traditional methods like increased water intake and chewing nonsugar candies might be cumbersome. Chewing gum bases may need to be avoided in those with temporomandibular disorders, which may be frequently coexisting in these patients. Artificial saliva preparations are often objectionable. Systemic agents like pilocarpine and cevimeline stimulate salivary flow but often have unfavorable side effects, such as profuse sweating, rhinitis, dyspepsia, etc. and cannot be used in patients with comorbid diseases.11 Hence, search for alternative methods, which have better actions with minimal side effects, is in vogue.
TENS is a well-known physical therapy, which is useful for the relief of pain. Electrical stimulation is directed to chronic pain areas via surface electrodes, and current passed through these areas reduces or eliminates pain. It is a non-invasive, safe, easy to master, low-cost procedure that is generally well accepted by the patients.5 The advantage of the device is that it is an extraoral device that can be used to those intolerant to intraoral stimulation devices or products, even during meal times.10 The role of electrostimulation of salivary glands with TENS as a treatment for xerostomia has been studied in the past and had shown promise.5 However, effect of TENS on salivary stimulation in postmenopausal women had not been studied so far. This curiosity had led the investigators to conduct the study in postmenopausal women.
In the present study, in the fifty postmenopausal women that were recruited, 50% who perceived oral dryness, as was deciphered from their responses to XI, had a greater duration of menopause, were older in age, and had statistically significant lower baseline salivary flow rates. The PMD + OD and PMD − OD groups did not differ with respect to the OHI and DMFT scores. This suggests that postmenopausal women did not differ in oral health status, despite their perception of oral dryness. Our results could not be compared with earlier studies, as these indices had not been evaluated among postmenopausal women with or without oral dryness earlier. Dural et al.1 reported that DMFT scores and OHI scores were significantly higher in postmenopausal women when compared with premenopausal women.
All the study participants tolerated TENS therapy well and did not show any side effects to the therapy. Both the groups (PMD + OD and PMD − OD) responded to TENS therapy by increased salivation, though PMD − OD group had higher stimulated flow rates. Though similar studies had not been conducted in postmenopausal women to permit adequate comparison, Vijayan et al.12 reported a statistically significant improvement in salivary flow in postradiation patients following TENS therapy, similar to our study. None of the study participants in our study showed an increase in salivation with the TENS machine in the off position, thus negating the role of any placebo effect.
TENS facilitated salivation could be attributed to the following reasons:10
-
1.
Direct stimulation: It is postulated that during stimulation with TENS, there is direct stimulation of the auriculotemporal nerve that supplies the secretomotor fibers to the parotid gland. Direct “sympathetic” innervation of the salivary glands takes place via preganglionic nerves in the thoracic segments T1–T3, which synapse in the superior cervical ganglion with postganglionic neurons that release norepinephrine, which is then received by adrenergic receptors on the acinar and ductal cells of the salivary glands, leading to an increase in cyclic adenosine monophosphate (cAMP) levels and the corresponding increase of saliva secretion.
-
2.
Reflex facilitation: It is hypothesized that peripheral stimulation of the gland results in a reflex facilitation of central output from the salivary nucleus from the medulla. The early investigators suggest that normal physiologic salivary reflexes are augmented.10
4.1. Conclusion
Postmenopausal women with perception of oral dryness had lower salivary flow rates. 90% of the subjects, irrespective of oral dryness status, responded to TENS therapy. TENS of parotid gland resulted in a statistically significant increase in the quantity of whole saliva flow rate in postmenopausal women with or without oral dryness.
The limitations of the study include a small sample size and measurement of salivary stimulation rates only on a single occasion. The effects of TENS stimulation must be studied for longer periods, on a larger sample size, to confirm the results of this preliminary report.
Conflicts of interest
The authors have none to declare.
Contribution details
Aravinda Konidena and Dheeraj Sharma conceptualized and designed the study, acquired data, and analyzed and interpreted the data. They also drafted the article and revised it critically for important intellectual content, and finally approved the version to be published. Gagan Puri, Avani Dixit, Deepa Jatti, and Rajesh Gupta analyzed and interpreted the data, and revised it critically for important intellectual content, and final approval of the version to be published.
References
- 1.Dural S., Gungor M., Berna L. Evaluation of the effect of menopause on saliva and dental health. Hacet Dişhekimliği Fak Derg. 2006;30(3):15–18. [Google Scholar]
- 2.Santosh P., Nidhi S., Sumita K., Farzan R., Bharati D., Ashok K.P. Oral findings in postmenopausal women attending dental hospital in Western Part of India. J Clin Exp Dent. 2013;5(1):e8–e12. doi: 10.4317/jced.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat S., Hegde S., Bharthi, Sujatha D., Ganapathy A study on evaluation of the effect of menopause on saliva and dental health. J Adv Dent Res. 2010;1(1):33–35. [Google Scholar]
- 4.Nimma V.B., Ramesh T., Reddy R.S., Reddy L.R., Swapna L.A. Effect of TENS on whole saliva in healthy adult Indians: evaluation of influence of protocol on quantity of saliva measured. Cumhur Dent J. 2012;15(3):235–240. [Google Scholar]
- 5.Kumud M., Vaishali K., Shekhar K. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on salivary flow in patients with xerostomia. Ann Dent Res. 2012;2(2):44–50. [Google Scholar]
- 6.Thomas W.M., Chalmers J.M., Spencer A.J., Williams S.M. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16(1):12–17. [PubMed] [Google Scholar]
- 7.World Health Organization . 4th ed. 1997. Oral health surveys: basic methods. Geneva. [Google Scholar]
- 8.Greene J.C., Vermilion J.R. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 9.Navazesh M., Kumar S.K.S. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139(S):35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 10.Dabholkar T.Y., Yardi S. A Study on effect of Conventional TENS on Salivation. Indian J Physiother Occup Ther. 2012;6(4):296–300. [Google Scholar]
- 11.Bowers L.M., Fox P.C., Brennan M.T. Salivary gland diseases. In: Glick M., editor. Burket's oral medicine. 12th ed. People's Medical Publishing House; USA: 2015. pp. 219–262. [Google Scholar]
- 12.Vijayan A., Asha M.L., Babu S., Chakraborty S. Prospective phase II study of the efficacy of transcutaneous electrical nerve stimulation in post-radiation patients. Clin Oncol. 2014;26(12):743–747. doi: 10.1016/j.clon.2014.09.004. [DOI] [PubMed] [Google Scholar]