ABSTRACT
A 28-year-old man presented with severe left visual loss and normal right visual acuity. The left fundus examination showed temporal pallor and complete absence of the nerve fibre layer (NFL) of papillomacular bundle. Right fundus examination showed focal loss of inferotemporal NFL. Magnetic resonance and serum aquaporin-4 antibody were negative. After 14 months of the initial visual involvement, the patient suffered subacute visual loss in contralateral eye. Genetic study revealed the 11778 point mitochondrial DNA (mtDNA) mutation associated with Leber hereditary optic neuropathy (LHON). Although very rare, interval of involvement of second eye greater than 12 months can occurs in LHON. Detailed optic nerve examination and careful interpretation of optical coherence tomography (OCT) printout support the diagnosis.
KEYWORDS: Fellow eye involvement, Leber hereditary optic neuropathy, optical coherence tomography
Introduction
Leber hereditary optic neuropathy (LHON) is a mitochondrial inheritance disease characterised by central, painless, subacute, and bilateral (simultaneous or sequential) visual loss.1 Simultaneous involvement has already been shown in several case reports, whereas most cases have non-simultaneous involvement of the second eye in an interval shorter than 12 months.2,3 Rarely, visual loss of the eye contralateral occurs in an interval longer than a year.4–6
In this report, we presented a patient with LHON and involvement of the second eye after an interval of 14 months.
Case report
A 28-year-old man with complaint of central and subacute visual loss in the left eye occurred 1 year ago. No complaints in the right eye (RE). He denied ocular trauma, ocular surgery, systemic diseases, smoking, or alcoholism. He had no similar cases in the family. He reported weight loss of 90 kg in the last year and made use of appetite suppressant.
At the initial examination, visual acuity was 20/25 in right eye and 20/200 in left eye associated with relative afferent pupillary defect. Left optic disc showed temporal pallor and complete absence of the nerve fibre layer (NFL) of papillomacular bundle (Figure 1). The right eye showed focal defect of the inferior temporal NFL and juxtapapillary telangiectasia (Figure 1). Visual field showed cecocentral scotoma in left eye and mild superior parafoveal defect (Figure 2). Optical coherence tomography (OCT) analysis showed a significant reduction in the temporal NFL thickness in left eye, but normal NFL thickness in right eye in Garway-Heath map (Figure 2). However, in TSNIT (temporal-superior-nasal-inferior-temporal). graph, NFL thickness line showed deflection out of the normal range in inferior temporal sector of the right eye (Figure 2). There was no macular change in both eyes in OCT analysis. Magnetic resonance imaging (MRI) scanning showed no signal increase or enhancement in left optic nerve. Neurologic examination was otherwise normal, included cerebrospinal fluid and anti-aquaporin-4 antibody.
Figure 1.
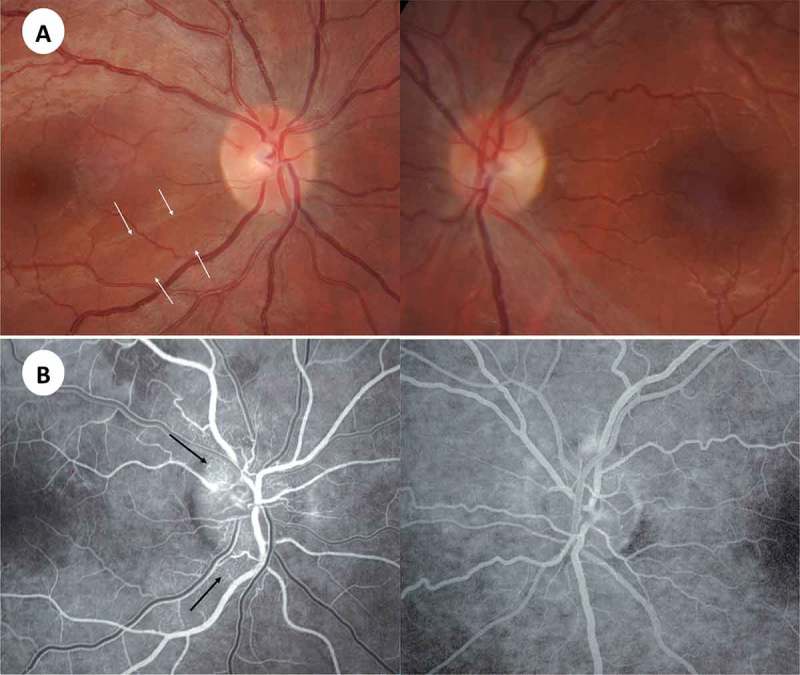
(A) Presymptomatic stage 1 year before symptoms started in the right eye. Note focal defect in inferotemporal nerve fibre layer (arrows). Atrophic stage showing loss of papillomacular bundle in the left eye. (B) Fluorescein angiography during acute stage of right optic nerve showing microangiopathy (arrows).
Figure 2.
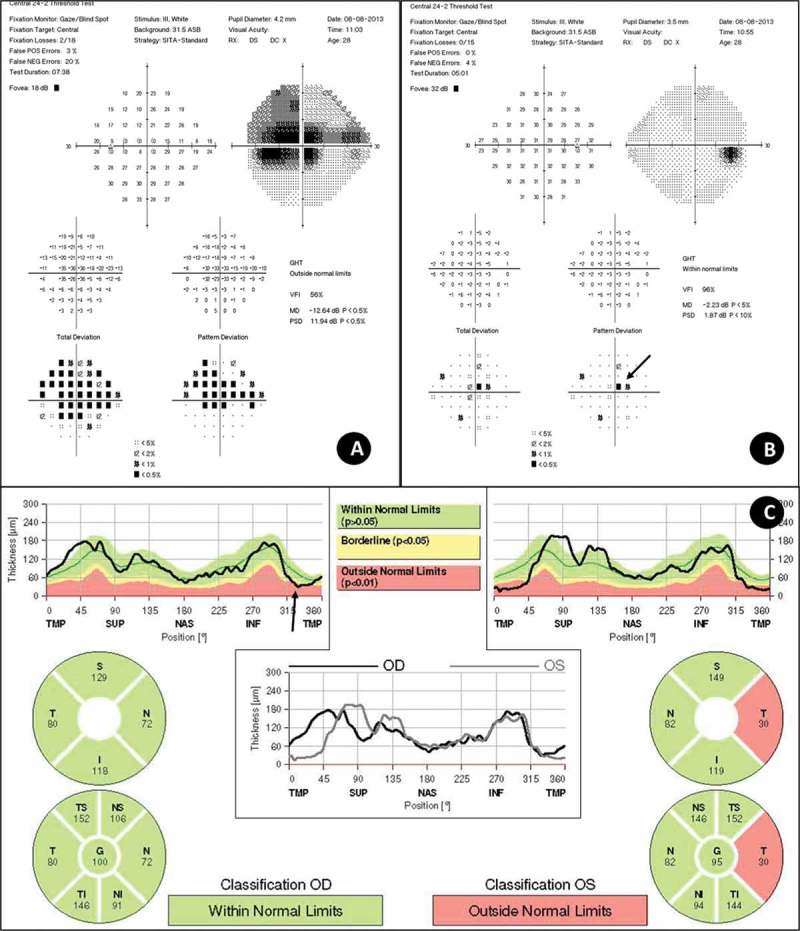
(A and B) Analysis of visual fields, performed on the Humphrey 24-2 Sita-Standard, show (A) severe cecocentral scotoma in left eye and (B) mild cecocentral scotoma, predominantly in the superior side (arrow). (C) Peripapillary retinal nerve fibre layer (RNFL) measured by OCT of the both eyes. Note loss of temporal RNFL in the left eye in both Garway-Heath map and TSNIT graph. In the right eye, Garway-Heath map was normal whereas TSNIT graph showed a downward slope in inferotemporal region (arrow).
Given the suspicion of LHON, genetic study was performed and revealed the 11778 point mitochondrial DNA (mtDNA) mutation associated with LHON. After 14 months of the initial visual involvement, the patient suffered subacute visual loss in contralateral eye. Visual acuity of right eye was 20/200 and counting fingers in left eye. Fluorescein angiography showed juxtapapillary telangiectasia in the right eye and no extravasation compatible with pseudo-swelling of the optic nerve of both eyes (Figure 1).
Discussion
LHON is a mitochondrial genetic disorder that affects young men more frequently, occurring usually from 15 to 30 years of age. Principal manifestations of the disease are bilateral, painless, and acute or subacute visual loss, usually ranging from 20/200 to count fingers, associated dyschromatopsia, central or cecocentral scotoma, and relative preservation of peripheral vision.
The pathogenesis of LHON remains unknown. It is believed that the phenotypic expression of the disease occurs by the association of genetic disorder and exposure to situations that provide oxidative stress, such as vitamin B12 deficiency, smoking, and alcohol use, resulting in death of retinal ganglion cells and optic atrophy. The diagnosis is performed by molecular studies for the verification of mutations in mtDNA. Most of LHON (i.e., 90–95% of patients) results from one of three primary mutations. The 11778 point mutation is the most common and associated with poor visual prognosis. The 3460 mutation is the second most common, but 22% of patients may have some recovery of vision. Already the mutation 14484 shows better prognosis, since about 40% of patients recover vision partially.1
Usually, the involvement of second eye occurs in a period of weeks to months in most patients with LHON. Rarely, this interval is greater than 1 year, which could be mistaken for unilateral optic neuropathy or maculopathy. Our patient presented with visual loss in the second eye with an interval of 14 months. Nikoskelainen et al.4 observed only three patients (of 53 individuals with LHON) with an interval greater than 6 months and one of them presented visual loss in the second eye after follow-up of 16 years. Brenner et al.5 described the case of a male patient who presented visual loss in the right eye at 2 years old and left eye involvement after 12 years. Riordan-eva et al.3 described a patient with unilateral visual loss, who maintained good vision in the contralateral eye for 4 years before its involvement. Ohden et al.6 reported a patient who had involvement of the first eye at 5 years old and contralateral eye after 18 years. The analysis of the clinical characteristics of these four patients3–6 showed that all had 11778 mutation, and three patients were men and aged 10 years or less.3,5,6 Mitochondrial DNA mutation and gender were typical for LHON in these patients; however, the preferential involvement in the first decade differs from most patients, which typically occurs between ages of 15 and 35 years.1
The cause of interval of involvement of second eye greater than 1 year remains unknown. It is believed that heteroplasmy (i.e., mixture of mutant and wild-type mtDNAs) could be involved in the expression of mitochondrial diseases, including LHON, whereas the level of heteroplasmy correlates with the severity of clinical phenotype.7 It already has been showed that mothers with less than 80% mutant mtDNA in the blood have less clinically affected sons than with 100% mutant mtDNA in LHON.8 Furthermore, the level of heteroplasmy can vary between cells in the same tissue or organ, from organ to organ within the same person, and between individuals in the same family.8 The patient related here could have different levels of heteroplasmy between his optic nerves, which would lead to vision loss in separate episodes, as already discussed by Ohden et al.6
Detailed optic nerve examination of our patient allowed the identification of focal NFL loss (confirmed by OCT analysis). Early diagnosis of LHON in patients with unilateral involvement allows beginning preventive measures so as to avoid exposure to substances with potential toxicity to the optic nerve, such as cigarette and alcohol, or to treat with idebenone, which could prevent or slow the contralateral visual loss.9,10
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- [1].Yen MY, Wang AG, Wei YH.. Leber’s hereditary optic neuropathy: a multifactorial disease. Prog Retin Eye Res 2006;25:381–396. [DOI] [PubMed] [Google Scholar]
- [2].Newman NJ, Lott MT, Wallace DC.. The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol 1991;111:750–762. [DOI] [PubMed] [Google Scholar]
- [3].Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE.. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain 1995;118(Pt 2):319–337. [DOI] [PubMed] [Google Scholar]
- [4].Nikoskelainen EK, Huoponen K, Juvonen V, Lamminen T, Nummelin K, Savontaus ML.. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmology 1996;103:504–514. [DOI] [PubMed] [Google Scholar]
- [5].Brenner L, Bynke G, Bynke H.. Leber’s hereditary optic neuropathy: a report of two unusual cases. Neuro-ophthalmology 1999;22:239–244. [Google Scholar]
- [6].Ohden KL, Tang PH, Lilley CC, Lee MS.. Atypical Leber hereditary optic neuropathy: 18 year interval between eyes. J Neuro-ophthalmol 2016;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [7].Stewart JB, Chinnery PF.. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet 2015;16:530–542. [DOI] [PubMed] [Google Scholar]
- [8].Chinnery PF, Andrews RM, Turnbull DM, Howell NN.. Leber hereditary optic neuropathy: does heteroplasmy influence the inheritance and expression of the G11778A mitochondrial DNA mutation? Am J Med Genet 2001;98:235–243. [DOI] [PubMed] [Google Scholar]
- [9].Klopstock T, Metz G, Yu-Wai-Man P, Buchner B, Gallenmuller C, Bailie M, Nwali N, Griffiths PG, von Livonius B, Reznicek L, Rouleau J, Coppard N, Meier T, Chinnery PF.. Persistence of the treatment effect of idebenone in Leber’s hereditary optic neuropathy. Brain 2013;136(Pt 2):e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sadun AA. Are we there yet? Is neuro-ophthalmology at the cusp of a paradigm shift? Lessons from leber hereditary optic neuropathy. J Neuro-ophthalmol 2013;33:189–197. [DOI] [PubMed] [Google Scholar]


