Abstract
The purpose of this study was to evaluate multifocal visual evoked potential (mfVEP) and pattern-reversal visual evoked potential (PVEP) changes in patients with pathology at various levels of the visual pathway determined by other methods. Six patients with different visual pathway disorders, including vascular ischaemic events and compressive optic neuropathy, were reviewed. All patients were tested with both mfVEP and full-field and half-field PVEPs. Results were assessed in relation to other diagnostic tests such as magnetic resonance imaging, Humphrey visual field test, and optical coherence topography. The cases in this study demonstrate a potential higher sensitivity of mfVEP compared with conventional PVEPs in detecting lesions affecting the peripheral field, horizontal hemifields, and lesions of the post-chiasmal pathway. The limitation of the PVEP in this setting is probably due to phase cancellation and overrepresentation of the macular region. mfVEP provides a more accurate assessment of visual defects when compared with conventional PVEP. The independent assessment of different areas of the visual field improves the detection and localization of lesions and provides an objective topographical map that can be used in clinical practice as an adjunct to other diagnostic tests and to assess disease progression.
Keywords: Multifocal VEP, visual evoked potential, visual pathway
INTRODUCTION
Visual pathway disorders can be diagnosed by clinical evaluation and imaging. However, if subjective tests are inconclusive or not explained by clinical findings, objective investigations such as visual evoked potentials may be of use.
The visual evoked potential (VEP) has been used in the diagnosis of various neuro-ophthalmological diseases for many years. The conventional full-field pattern-reversal VEP (PVEP) provides a summed response of all neuronal elements stimulated and is prone to cancellation and distortion.1,2 Furthermore, the full-field PVEP response is greatly dependent on the function of the central macula region3–5 and, as a result, a lesion localised in the periphery of the visual field could easily be missed.
Multifocal VEP (mfVEP), on the other hand, enables simultaneous recording from multiple regions of the visual field, allowing assessment of a much larger cross-sectional area of the optic nerve and, therefore, more accurate functional evaluation of the visual pathway.1 Such an objective visual field topographic map may have useful applications in clinical practice.
Currently, the mfVEP has been predominantly used in the assessment of patients with glaucoma and optic neuritis.6,7 However, relatively few studies have targeted other visual pathway disorders.8–11 Furthermore, although there have been some studies comparing mfVEP with conventional VEP, predominantly in the setting of optic neuritis and glaucoma,12,13 there are no studies comparing the utility of these techniques in the setting of confirmed chiasmal and retro-chiasmal pathology, particularly using selective half and central field in addition to full-field stimulation.
In this study, we assess the relative utility of mfVEP compared with PVEP in several cases with pathology at various levels of the visual pathway that have been confirmed by other objective techniques.
METHODS
Subjects
Six patients with different visual pathway pathology diagnosed by a neurology/neuro-ophthalmology consultant were selected from a large neurology/neuro-ophthalmology service. The selected cases included vascular ischaemic events, compressive optic neuropathy, and inflammatory demyelination. The tenets of the Declaration of Helsinki were followed and informed consent was obtained from all patients.
PVEP Testing and Analysis
Full-field, right and left half-field VEPs were tested using a Medelec Synergy version 15.0 (Oxford Instruments, Oxford, UK). Pattern-reversal stimulation was performed using a Dell CRT monitor with a 20-inch screen, alternating black and white checkerboard stimulation (32-minute checks) reversed at a rate of 2/s. Monocular testing was performed. Gold cup disc electrodes (10 mm diameter) were used. In accordance with the international 10/20 system,14 the active electrode was placed on the scalp over the visual cortex at Oz with the reference electrode placed at Fz and the ground electrode was placed on the forehead. The distance between the patient and the stimulus was 70 cm. VEPs were recorded in two trials for each eye, averaging at least 128 responses. Cut-offs for normal values were <112 ms for P100-peak latencies and ≥2 µV for amplitudes, the inter-eye right to left half-field latency asymmetry was 7 ms, and the inter-eye left to right half-field amplitude ratio was 3:1. These cut-off limits were previously established laboratory normal measurements and represent values beyond 2 standard deviations from the mean.
mfVEP Recording and Analysis
mfVEP testing was performed using Accumap (ObjectiVision, Sydney, Australia), described elsewhere.15 Opera (ObjectiVision) software was used to correlate the pattern-reversal sequence with the electrical signals recorded to create a combined topographic map and compare it with a built-in database to create amplitude and latency deviation plots. A visual defect for mfVEPs was defined as a cluster of at least 3 abnormal points on the amplitude deviation plot with 2 segments p < 0.02 and at least 1 segment p < 0.01 or a cluster of 3 or more abnormal segments on inter-eye asymmetry deviation plot with p < 0.01 or 2 or more zones with p < 0.005.16
Optical Coherence Tomography (OCT) Recording and Analysis
OCT was performed using a Spectralis scanner (Heidelberg Engineering, Heidelberg, Germany). The retinal nerve fibre layer (RNFL) protocol was used to evaluate RNFL thickness. The pupils were not dilated. Scan quality was considered acceptable if the quality scores were more than 25 and the scan was well centred on the optic nerve.
Visual Field Testing
Monocular visual fields were tested using Humphrey visual field (HVF) analyser (Carl Zeiss Meditec, Dublin, CA, USA). SITA (Swedish interactive thresholding algorithm) standard 24-2 protocol was used.
Brain Magnetic Resonance Imaging (MRI) Testing
Brain MRI was performed using 3.0 Tesla GE MR750 scanners (GE Healthcare, Little Chalfont, UK).
RESULTS
PVEP and mfVEP Changes in Branch Retinal Artery Occlusion
Case 1
An 83-year-old woman presented with a 5-week history of painless right eye visual impairment. There were no other visual or systemic symptoms. She had a past history of polymyalgia rheumatica and cataract surgery in her left eye.
On examination, Snellen visual acuity was 6/12 in the right eye and 6/9 in the left. Fundus examination of the right eye showed an ischaemic pale retina inferiorly with a cholesterol embolus in the inferior retinal artery (Figure 1A). Inferotemporal branch retinal artery occlusion was diagnosed.
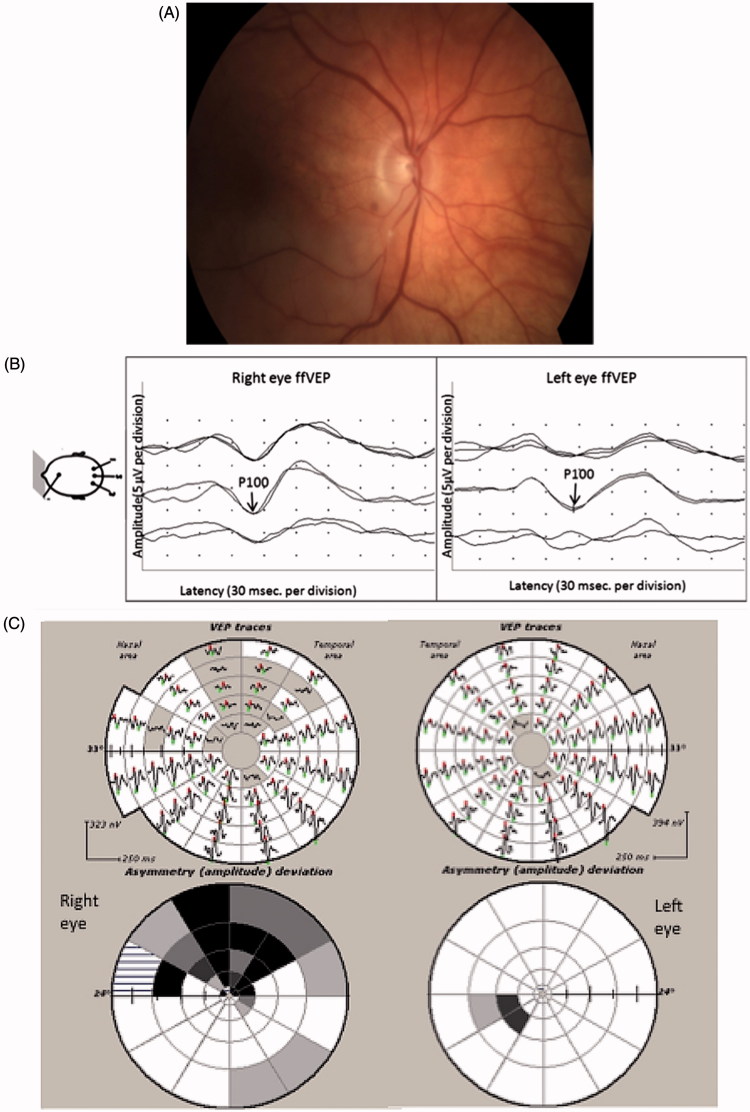
FIGURE 1.
(A) Fundus photography of the right eye showing an embolic inferotemporal branch retinal artery occlusion. (B) Full-field PVEP of the right and the left eyes showing normal amplitude and latency. The right eye PVEP has higher amplitude and better-defined waveform. (C) mfVEP showing reduction of amplitude in the upper field of the right eye. (D) OCT of the right eye showing an inferior RNFL thickness reduction.
Full-field PVEP amplitude and latency were normal (Figure 1B); however, mfVEP of the right eye showed significant reduction in amplitude in the upper field (Figure 1C). This correlated well with the reduction of RNFL thickness of the right eye inferiorly (Figure 1D).
Comments: Case 1
In this case, full-field PVEPs in the affected eye were normal. Moreover, the waveform had higher amplitude and was better defined than in the unaffected eye. This contradiction is likely caused by the anatomy of the visual cortex. It is known that the upper retina (lower visual field) projects to the upper bank of the sulcus calcarinus (cuneus gyrus), whereas the lower retina (upper visual field) projects to lower bank of the sulcus calcarinus (lingual gyrus). Since both banks are facing each other, the polarity of the cortical dipoles from the lower and upper hemifields is almost opposite. This results in a cancellation effect of amplitude in the non-affected eye. In the affected eye, however, since the response from the upper hemifield is almost extinguished, there is no cancellation and the average full-field signal looks larger, although it is mostly generated by the lower hemifield. The findings suggest a greater sensitivity of mfVEP compared with full-field PVEP in the setting of pathology selectively affecting one horizontal hemifield.
PVEP and mfVEP Changes in Compressive Optic Neuropathy
Case 2 (Pituitary Adenoma)
A 75-year-old woman presented with a history of progressive visual loss of her left eye during the previous 6 months. She had a history of pituitary adenoma that had been operated on in 1998. Her Visual acuity in the left eye was hand movement and in the right eye was 6/9. An afferent pupillary defect was present. MRI scans showed residual and possibly recurrent pituitary macro-adenoma affecting the left optic nerve and chiasm (Figure 2A). Visual field perimetry showed almost total visual field loss in the left eye and an upper temporal scotoma in the right eye (Figure 2B).
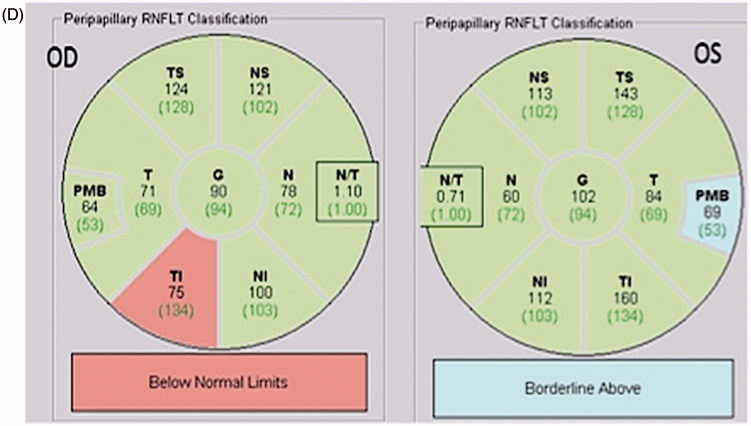
FIGURE 2.
(A) Axial T1-weighted MRI with contrast showing a large mass (white arrow) occupying pituitary fossa and extending into the suprasellar cistern and right cavernous sinus consistent with a pituitary tumour. (B) Humphrey 24-2 visual fields showing total visual field loss in the left eye and upper temporal scotoma in the right eye. (C) Full-field PVEP of both eyes showing no consistent response in the left eye and normal amplitude and latency in the right eye. The half-field PVEP of the right eye shows smaller amplitude in the right half with normal latency in both half-fields. (D) mfVEP showing total reduction of amplitude in the left eye and temporal hemianopia in the right eye.
Full-field PVEP showed no consistent response from the left eye. The right eye full-field latency and amplitude were normal. The right half-field amplitude of right eye was small (1.2 µV), whereas the response from the left half-field demonstrated normal amplitude (2.3 µV) (Figure 2C). PVEP of both half-fields of the right eye showed P100 latency within normal limits. mfVEP showed a clear right eye temporal hemianopia with normal amplitude of the left hemifield (Figure 2D). This example highlights the importance of mfVEP in accurate detection of vertical hemifield pathology.
Case 3 (Pituitary Tumour in Multiple Sclerosis Patient)
A 51-year-old woman presented with deterioration of vision in her right eye. She described it as an area of obscuration in the temporal field. There was no associated pain or change in the colour vision. The patient had a long history of multiple sclerosis (MS).
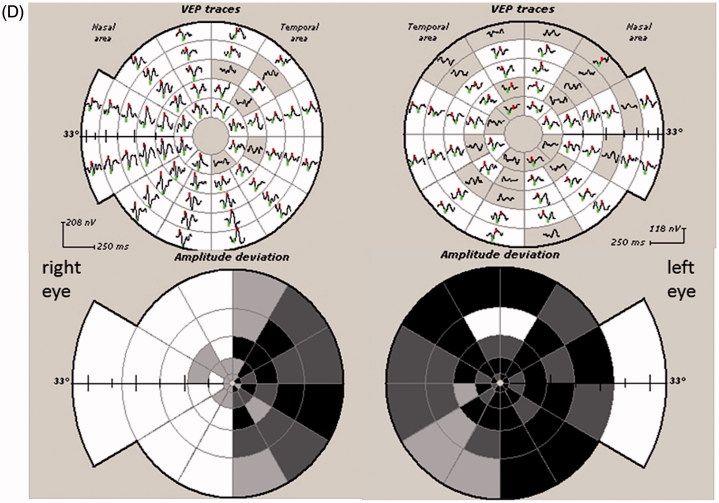
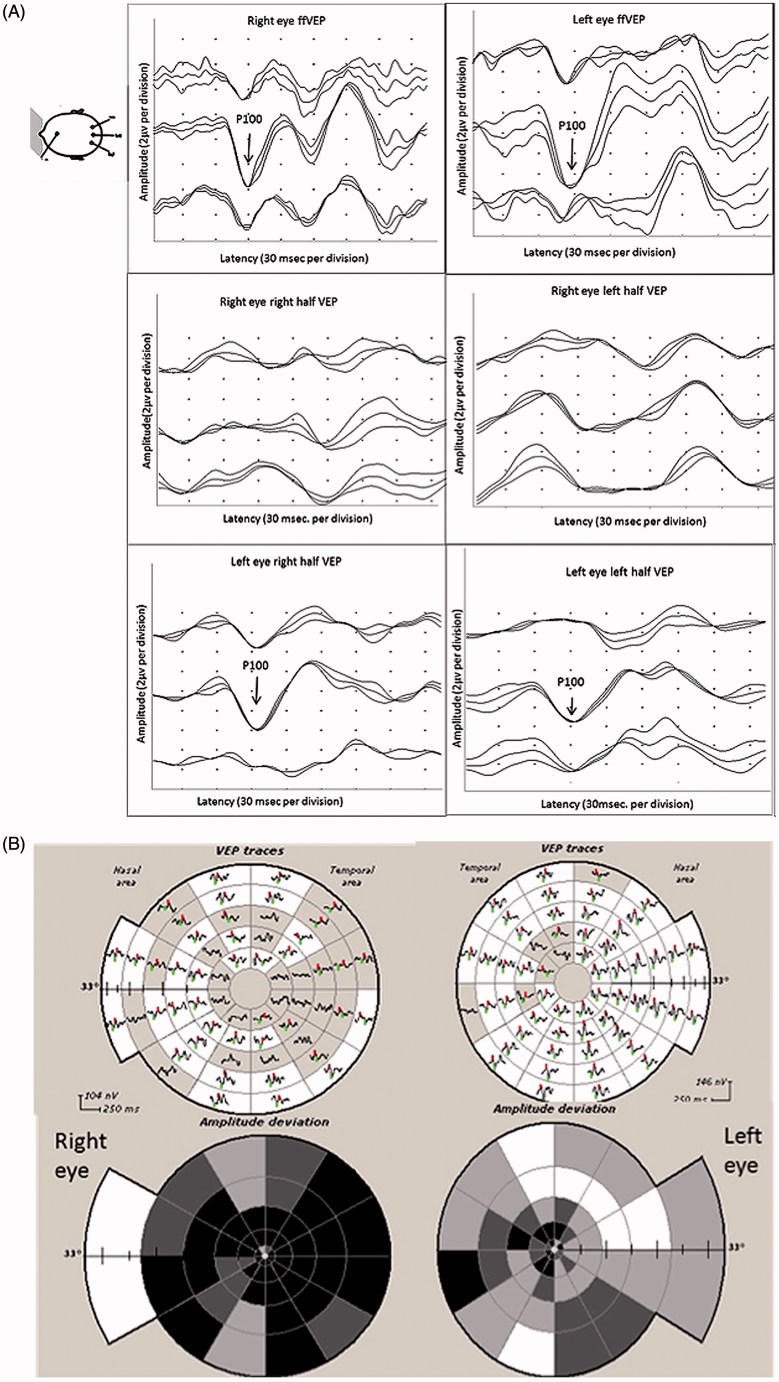
On examination, her visual acuity was 6/18 in the right eye and 6/9 in the left eye. The optic disc appeared normal. Full-field PVEP of right eye showed delayed P100. The amplitude was smaller than the left eye, but still within normal limits (5.5 µv). Half-field PVEP waves in the right eye were poorly formed on both sides. Left eye full-field and half-field PVEPs were within normal limits (Figure 3A). In view of her MS, history optic neuritis was considered. mfVEP, however, demonstrated a dramatic loss of amplitude in the right eye and significant involvement of the left eye, predominantly on the temporal side (Figure 3B).
FIGURE 3.
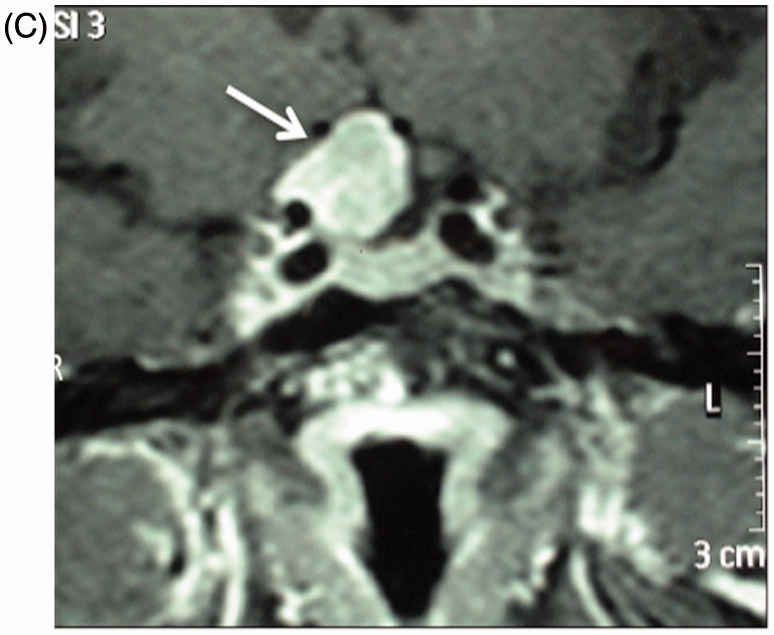
(A) Full-field PVEP of both eyes showing normal amplitude and latency. Half-field PVEP of the right eye showing poorly formed waves with reduction of amplitude and latency delay. Both half-fields of the left eye were within normal limits. (B) mfVEP showing severe reduction of amplitude in the right eye, with apparent left eye involvement. (C) T1-weighted axial MRI showing suprasellar mass extending toward the right optic nerve consistent with pituitary adenoma.
An MRI scan of the brain confirmed extensive demyelination in keeping with her MS, but in addition there was a suprasellar mass extending toward the right optic nerve (Figure 3C).
Comments: Cases 2 and 3
Patients with a pituitary adenoma can present to the neuro-ophthalmology clinic with visual symptoms secondary to the tumour. Up to 80% of non-functional pituitary adenomas and around 20% of patients with hormone-secreting tumours will present with visual disturbance as their primary complaint.17 The classic visual field defect is a bitemporal hemianopia due to the compression of the crossed nasal fibres in the chiasm. Many other variants of the typical visual field defect have been reported in the literature.17 Around 16% of patients will present to the neuro-ophthalmologist as in case 2 with one eye blind and a temporal visual field loss in the other eye. Conventional PVEP abnormalities in this setting are well described18; however, in case 2 the full-field PVEP was normal in the right eye and normal in both eyes in case 3. The extent of the defect was more obvious in the mfVEP compared with half-field PVEP, which showed only a mild asymmetry in case 2. Previous studies that have compared mfVEP with HVF in patients with compressive optic neuropathies showed a higher sensitivity of mfVEP,9,19 but there are no studies comparing VEP techniques. The findings in these cases support the usefulness of the mfVEP in this pathological condition.
PVEP and mfVEP Changes in Central Visual Pathway Lesions
Case 4 (Anterior Choroidal Artery Infarction)
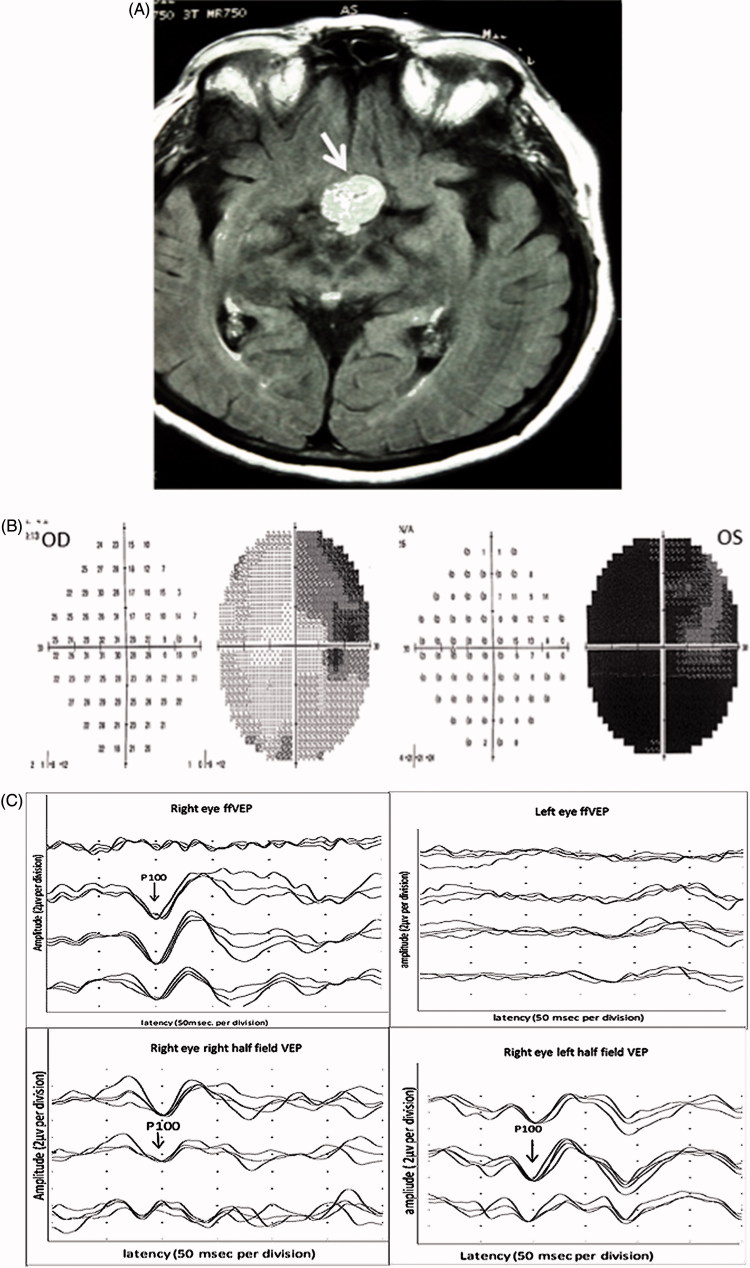
A 42-year-old woman presented with a history of acute onset headache, nausea, and vomiting. This was associated with visual disturbance as well as facial paraesthesia and heaviness in the left arm and leg. The symptoms improved over the next 24 hours except for the visual disturbance. Her MRI scan showed features consistent with an anterior choroidal artery infarction involving the right hippocampus, medial temporal lobe, and posterior thalamus (Figure 4A). Visual field testing revealed left relative hemianopia (Figure 4B). Full-field PVEP for both eyes was within normal limits. Left-field PVEP showed lower amplitude and prolonged latency for both eyes, worse in the left eye (Figure 4C). mfVEP confirmed the presence of left superior homonymous quadrantanopia (Figure 4D).
FIGURE 4.
(A) Humphrey 24-2 visual fields showing left congruous relative hemianopia. (B) Full-field PVEP of both eyes showing normal amplitude and latency. Half-field PVEP shows lower amplitude and prolonged latency of the left field in both eyes, worse in the left eye. (C) Flair and T2-weighted images showing signs of infarction (white arrow) involving the right hippocampus, medial temporal lobe, and posterior thalamus, including a region of the lateral geniculate body. (D) mfVEP showing left superior homonymous quadrantanopia.
Case 5 (Sub-acute Infarcts Due to Septic Emboli)
A 73-year-old woman presented with a 1-week history of fever after a trip to Europe and Hong Kong. She was diagnosed with endocarditis and required mitral valve replacement. Her condition was complicated by septic emboli with sub-acute infarcts in the right occipital, left cerebellar, right parietal, and right frontal lobes (Figure 5A).
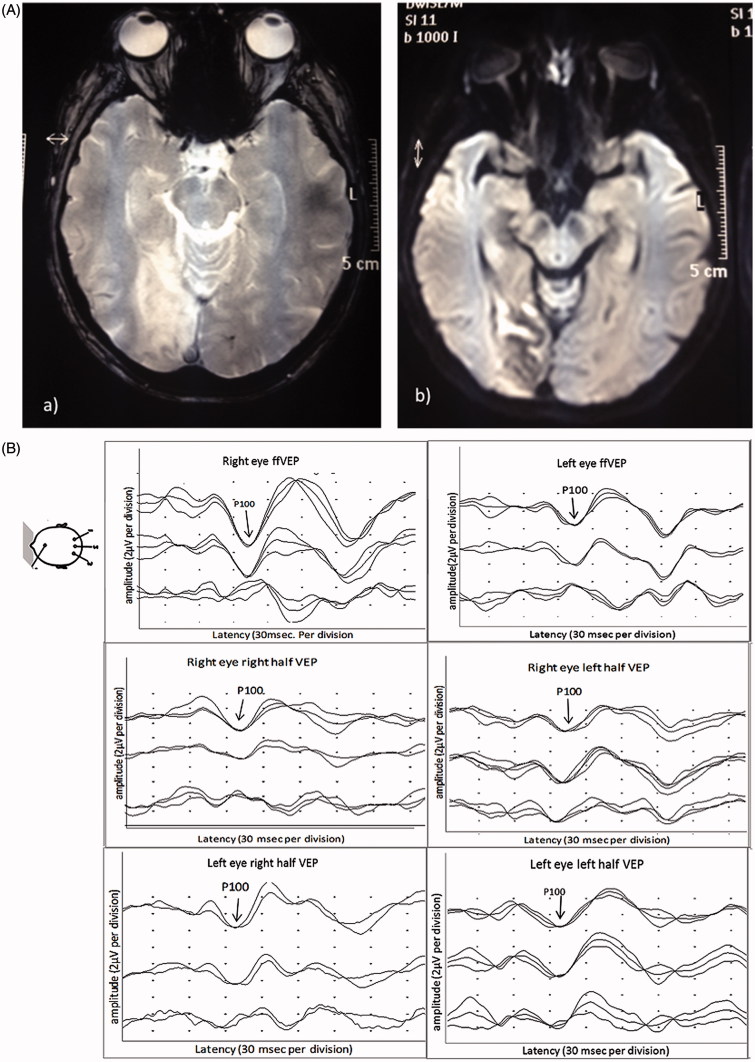
FIGURE 5.
(A) (a) Fast field echo (FFE) MRI images and (b) diffusion-weighted images (DWI) images showing sub-acute infarcts in the right occipital lobe. (B) Full-field PVEP of both eyes showing amplitude asymmetry not exceeding the cut-off for normal values. Half-field PVEP of both eyes is within normal limits. (C) mfVEP showing an incongruous left homonymous hemianopia.
She was left with a residual field defect, but otherwise her neurological function returned to normal. The P100 latency and amplitude of full-field PVEP of both eyes were within normal limits. Although the half-field PVEPs of the right eye were within normal limits, the amplitude was asymmetrical (right half-field 3.1 µV, left half-field 2 µV). Similar findings were seen in the left eye. The latency was within normal limits for both sides (Figure 5B). Although full-field PVEP of the left eye amplitude was relatively reduced compared with the right eye, half-field PVEP was inconclusive. mfVEP, on the other hand, clearly demonstrated an incongruous left homonymous hemianopia, which corresponded to the lesion seen on the MRI (Figure 5C).
Comments: Cases 4 and 5
Several studies have reported the ability of conventional VEP to detect field loss due to lesions involving the visual pathway and higher visual centres.20–24 Although Bradnam et al.20 reported that PVEP quadrantic field testing has high sensitivity and specificity in detecting visual field defects, others disagree. Maitland and associates24 concluded that PVEP analysis is of limited value in assessing patients with homonymous or bitemporal hemianopias.
mfVEP, as demonstrated by cases 4 and 5, has the capacity to detect abnormalities in the posterior visual pathways that may be missed by conventional pattern-reversal studies. We have previously reported a good correlation between HVF defects and mfVEP in patients with retro-chiasmatic visual pathway lesions.8 It should be noted, however, that in the setting of lesions in higher visual centres (e.g. V2/V3), the mfVEP may be normal as it arises mostly from V1.10
PVEP and mfVEP Changes in Ischaemic Optic Neuropathy
Case 6
A 50-year-old man presented with a history of painless visual loss in his left eye. On examination, his visual acuity was 6/6 in both eyes but the left optic disc was swollen.
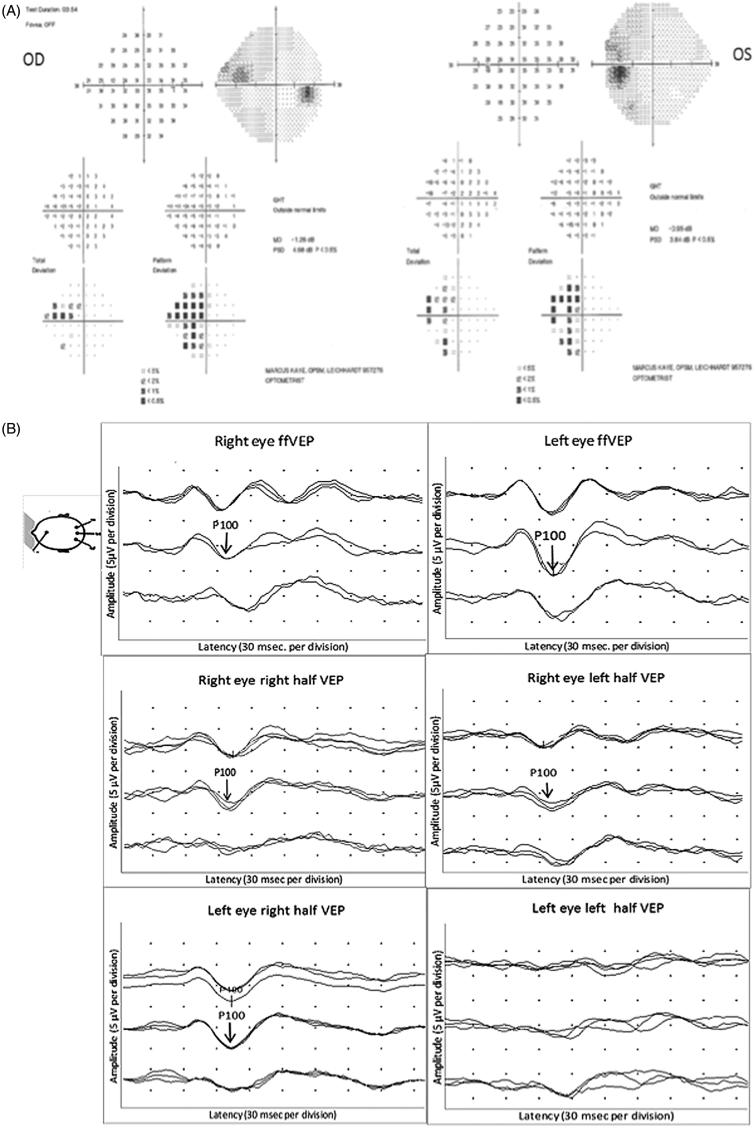
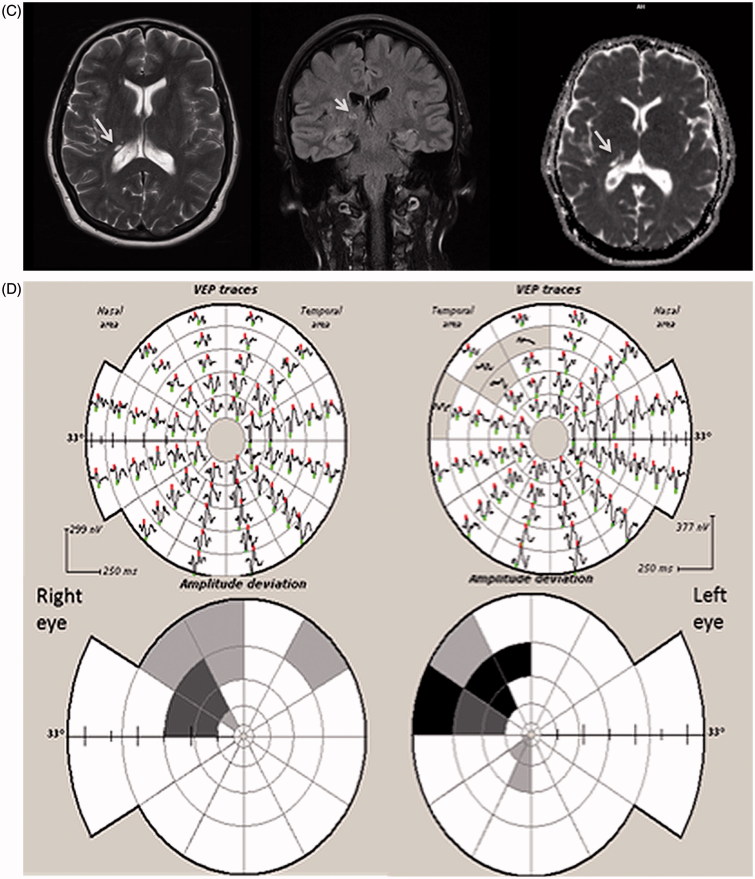
An MRI scan of his brain and optic nerves was normal. Lumbar puncture was also unremarkable. Full-field and half-field VEPs were normal in both eyes (Figure 6A). HVF testing and mfVEP, however, demonstrated a peripheral visual field defect in his left eye, which was accompanied by a corresponding reduction in RNFL thickness (Figure 6B). This could only be revealed on full-field PVEP through annular stimulation after macular masking. The patient was treated for an anterior ischaemic optic neuropathy.
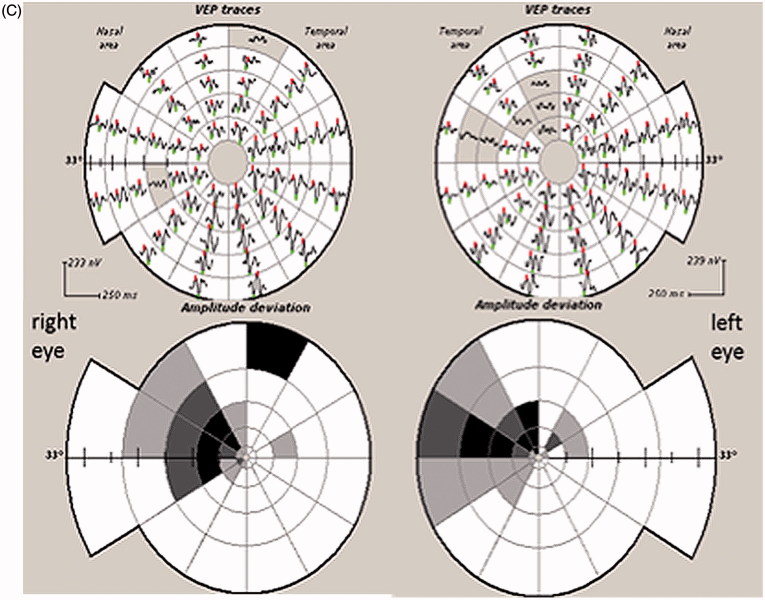
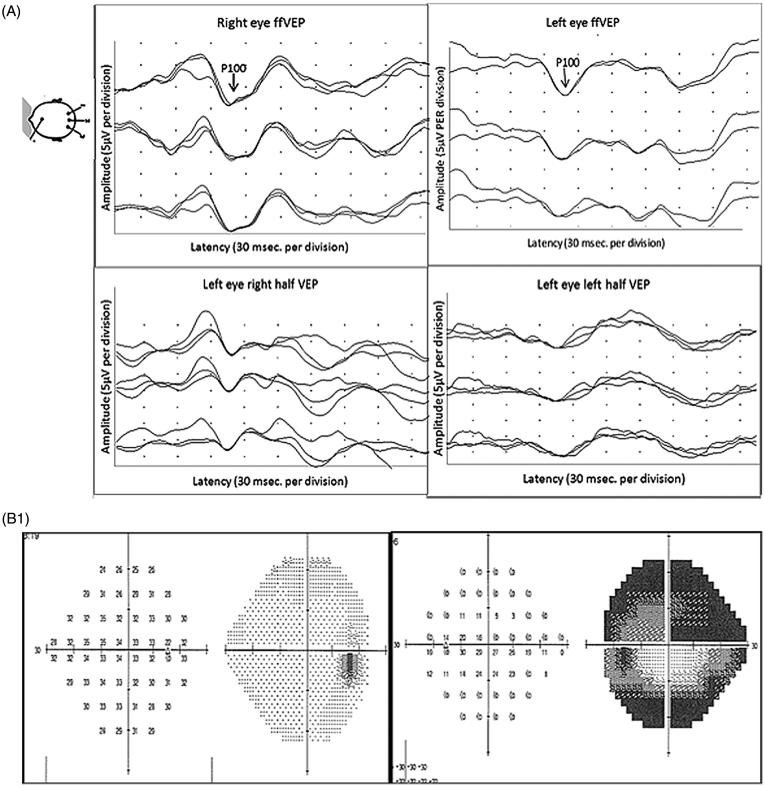
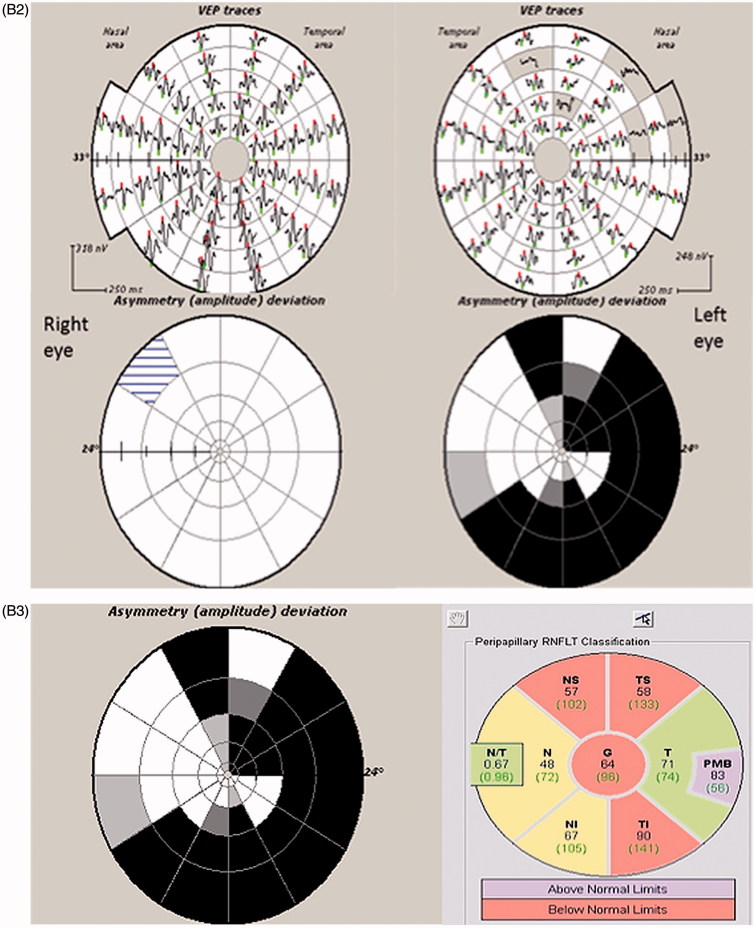
FIGURE 6.
(A) Full-field PVEP of both eyes and half-field PVEP of the left eye were within normal limits. (B1) Humphrey 24-2 visual fields of the left eye showing a peripheral visual field defect. (B2) mfVEP traces and amplitude asymmetry maps of the left eye showing peripheral reduction of amplitude. (B3) The area of amplitude reduction on mfVEP correlates well with RNFL thinning on OCT.
Comments: Case 6
Full-field and half-field VEPs were normal in this patient because the majority of PVEP is driven by the central 4° of the visual field,5 which was intact. Therefore, despite his extensive peripheral visual field defects, he had a normal full-field PVEP and his central visual acuity was unaffected. mfVEP, on the other hand, was able to detect pathology affecting peripheral fibres with relatively preserved central vision.
DISCUSSION
There is limited number of studies comparing conventional PVEP and mfVEP changes in cases of confirmed visual pathway pathology.12,13 This case series demonstrates that mfVEP, as an objective test for visual fields, is potentially more sensitive than PVEP in detecting focal visual pathway pathology. The findings in our cases of a normal PVEP response when the central vision was preserved even if there was a significant peripheral visual field defect, as in cases 1 and 6, can be explained by the fact that a large proportion of the PVEP is generated by macular fibres, since the macula has higher density of cones and ganglion cells in comparison with the peripheral retina. At the same time, larger numbers of cortical neurons are involved in the processing of visual stimuli from the central visual field. It has been estimated that around 50–60% of the visual cortex is devoted to macular representation.25 In addition, the check size used routinely for pattern VEP stimulation is selected to obtain an optimal response from central and para-central visual fields26–29 and, as a consequence, is sub-optimal for the peripheral retina.
mfVEP, on the other hand, uses a dartboard pattern of 58 segments that contains 4 × 4 checks cortically scaled to increase in size from the centre to the periphery in order to optimise the response from different parts of the visual field.30 It is therefore capable of greater resolution of visual pathway function including fibres from the peripheral visual field. Moreover, mfVEP techniques allow independent assessment of fibres sub-serving different regions of the visual field, minimising the susceptibility to phase cancellation and distortion, which may be evident in PVEP as a result of potential summation as in cases 1 and 2.
As any other test, mfVEP has some limitations, such as inter-subject variability. The main cause of variability in response between individuals is their cortical anatomy and conductivity of underlying tissue.31 Since the cortex is folded differently in every individual, the position of the primary visual area and its relation to the location of recording electrodes can result in noticeably different mfVEP responses. mfVEP responses from eyes of the same individual are usually very similar due to the fact that they project to the same cortical region.32 Electroencephalography (EEG)-based normalization has significantly improved the quality of recordings and decreased inter-subject variability allowing a gender- and sex-normative database to be used by mfVEP software packages to create a grading scale with probability maps.33 Caution should be practiced when comparing results from different machines because they may have different stimulus and recording conditions. Algorithms used for amplitude and latency analysis may vary as well. Technical limitations such as eyelid position, refractive errors, and poor fixation may all influence the results of recordings and increase noise levels, and should be taken into account and minimised if possible when recording mfVEP.34 Patients with significant nystagmus or tremor cannot perform the test. It takes about 20 minutes to test both eyes, and studies have shown that mfVEP testing is well tolerated by patients.35,36
In summary, mfVEP may provide a more accurate assessment of visual defects when compared with PVEP. The independent assessment of different areas in the visual field improves the detection and localization of lesions and provides an objective topographical map that can be used in clinical practice. Although the observational nature of the study limits the application of the findings to a larger population of patients, these findings support the need for larger studies to evaluate the relative utility of mfVEP in this clinical setting compared with the conventional pattern-reversal technique.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Klistorner AI, Graham SL, Grigg JR, Billson FA. Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 1998;39:937–950 [PubMed] [Google Scholar]
- 2.Blumenhardt L, Halliday A. Hemisphere contributions to the composition of the pattern-evoked potential waveform. Exp Brain Res 1979;36:53–69 [DOI] [PubMed] [Google Scholar]
- 3.Celesia GG. Evoked potential techniques in the evaluation of visual function. J Clin Neurophysiol 1984;1:55–76 [DOI] [PubMed] [Google Scholar]
- 4.Celesia GG, Polcyn RD, Holden JE, Nickles RJ, Gatley JS, Koeppe RA. Visual evoked potentials and positron emission tomographic mapping of regional cerebral blood flow and cerebral metabolism: can the neuronal potential generators be visualized? Electroencephalogr Clin Neurophysiol 1982;54:243–256 [DOI] [PubMed] [Google Scholar]
- 5.MacKay DM, Jeffreys DA. Visually evoked potentials and visual perception in man. In: R Jung, editor. Visual Centers in the Brain. New York: Springer; 1973:647–678 [Google Scholar]
- 6.Grover LK, Hood DC, Ghadiali Q, Grippo TM, Wenick AS, Greenstein VC, Behrens MM, Odel JG. A comparison of multifocal and conventional visual evoked potential techniques in patients with optic neuritis/multiple sclerosis. Doc Ophthalmol 2008;117:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klistorner A, Graham SL, Martins A, Grigg JR, Arvind H, Kumar RS, James AC, Billson FA. Multifocal blue-on-yellow visual evoked potentials in early glaucoma. Ophthalmology 2007;114:1613–1621 [DOI] [PubMed] [Google Scholar]
- 8.Klistorner A, Graham S, Grigg J, Balachandran C. Objective perimetry using the multifocal visual evoked potential in central visual pathway lesions. Br J Ophthalmol 2005;89:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danesh-Meyer HV, Carroll SC, Gaskin BJ, Gao A, Gamble GD. Correlation of the multifocal visual evoked potential and standard automated perimetry in compressive optic neuropathies. Invest Ophthalmol Vis Sci 2006;47:1458–1463 [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Shinoda K, Kimura I, Mashima Y, Oguchi Y, Ohde H. Discordance between subjective perimetric visual fields and objective multifocal visual evoked potential-determined visual fields in patients with hemianopsia. Am J Ophthalmol 2007;143:295–304 [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman M, Ambika S, Gandhi RA, Bassi SR, Ravi P, Sen P. Multifocal visual evoked potential recordings in compressive optic neuropathy secondary to pituitary adenoma. Doc Ophthalmol 2010;121:197–204 [DOI] [PubMed] [Google Scholar]
- 12.Grippo TM, Hood DC, Kanadani FN, Ezon I, Greenstein VC, Liebmann JM, Ritch R. A comparison between multifocal and conventional VEP latency changes secondary to glaucomatous damage. Invest Ophthalmol Vis Sci 2006;47:5331–5336 [DOI] [PubMed] [Google Scholar]
- 13.Klistorner A, Fraser C, Garrick R, Graham S, Arvind H. Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol 2008;116:19–27 [DOI] [PubMed] [Google Scholar]
- 14.Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP. ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 2010;120:111–119 [DOI] [PubMed] [Google Scholar]
- 15.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, O’Day J, Yiannikas C. Multifocal VEP and OCT in optic neuritis: a topographical study of the structure–function relationship. Doc Ophthalmol 2009;118:129–137 [DOI] [PubMed] [Google Scholar]
- 16.Arvind H, Graham S, Leaney J, Grigg J, Goldberg I, Billson F, Klistorner A. Identifying preperimetric functional loss in glaucoma: a blue-on-yellow multifocal visual evoked potentials study. Ophthalmology 2009;116:1134–1141 [DOI] [PubMed] [Google Scholar]
- 17.Minniti G, Esposito V, Piccirilli M, Fratticci A, Santoro A, Jaffrain-Rea ML. Diagnosis and management of pituitary tumours in the elderly: a review based on personal experience and evidence of literature. Eur J Endocrinol 2005;153:723–735 [DOI] [PubMed] [Google Scholar]
- 18.Holder GE, Bullock PR. Visual evoked potentials in the assessment of patients with non-functioning chromophobe adenomas. J Neurol Neurosurg Psychiatry 1989;52:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semela L, Yang EB, Hedges TR, Vuong L, Odel JG, Hood DC. Multifocal visual-evoked potential in unilateral compressive optic neuropathy. Br J Ophthalmol 2007;91:445–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradnam MS, Montgomery D, Evans AL, Keating D, McClure EA, Damato BE, McFadzean R. Objective detection of hemifield and quadrantic field defects by visual evoked cortical potentials. Br J Ophthalmol 1996;80:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biersdorf WR, Bell RA, Beck RW. Pattern flash visual evoked potentials in patients with homonymous hemianopia. Doc Ophthalmol 1992;80:51–61 [DOI] [PubMed] [Google Scholar]
- 22.Haimovic IC, Pedley TA. Hemi-field pattern reversal visual evoked potentials. II. Lesions of the chiasm and posterior visual pathways. Electroencephalogr Clin Neurophysiol 1982;54:121–131 [DOI] [PubMed] [Google Scholar]
- 23.Blumhardt L, Barrett G, Kiss A, Halliday A. The pattern-evoked potential in lesions of the posterior visual pathways. Ann N Y Acad Sci 2006;338:264–289 [DOI] [PubMed] [Google Scholar]
- 24.Maitland CG, Aminoff MJ, Kennard C, Hoyt WF. Evoked potentials in the evaluation of visual field defects due to chiasmal or retrochiasmal lesions. Neurology 1982;32:986–986 [DOI] [PubMed] [Google Scholar]
- 25.McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol 1994;78:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurita-Tashima S, Tobimatsu S, Nakayama-Hiromatsu M, Kato M. Effect of check size on the pattern reversal visual evoked potential. Electroencephalogr Clin Neurophysiol 1991;80:161–166 [DOI] [PubMed] [Google Scholar]
- 27.Beck RW, Cleary PA. Optic Neuritis Treatment Trial: one-year follow-up results. Arch Ophthalmol 1993;111:773–775 [DOI] [PubMed] [Google Scholar]
- 28.Yiannikas C, Walsh J. The variation of the pattern shift visual evoked response with the size of the stimulus field. Electroencephalogr Clin Neurophysiol 1983;55:427–435 [DOI] [PubMed] [Google Scholar]
- 29.Ristanović D, Hajduković R. Effects of spatially structured stimulus fields on pattern reversal visual evoked potentials. Electroencephalogr Clin Neurophysiol 1981;51:599–610 [DOI] [PubMed] [Google Scholar]
- 30.Balachandran C, Klistorner AI, Graham SL. Effect of stimulus check size on multifocal visual evoked potentials. Doc Ophthalmol 2003;106:183–188 [DOI] [PubMed] [Google Scholar]
- 31.Brindley G. The variability of the human striate cortex. J Physiol 1972;225:1P. [PMC free article] [PubMed] [Google Scholar]
- 32.Hood DC, Zhang X, Greenstein VC, Kangovi S, Odel JG, Liebmann JM, Ritch R. An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 2000;41:1580–1587 [PubMed] [Google Scholar]
- 33.Klistorner AI, Graham SL. Electroencephalogram-based scaling of multifocal visual evoked potentials: effect on intersubject amplitude variability. Invest Ophthalmol Vis Sci 2001;42:2145–2152 [PubMed] [Google Scholar]
- 34.Hood DC, Odel JG, Winn BJ. The multifocal visual evoked potential. J Neuroophthalmol 2003;23:279–289 [DOI] [PubMed] [Google Scholar]
- 35.Bjerre A, Grigg JR, Parry NR, Henson DB. Test–retest variability of multifocal visual evoked potential and SITA standard perimetry in glaucoma. Invest Ophthalmol Vis Sci 2004;45:4035–4040 [DOI] [PubMed] [Google Scholar]
- 36.Gardiner SK, Demirel S. Assessment of patient opinions of different clinical tests used in the management of glaucoma. Ophthalmology 2008;115:2127–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]