Abstract
We compared monocular and binocular absolute thresholds of dark adaptation in two separate study populations. Eighteen healthy individuals (Group A) and 13 patients with chronic respiratory insufficiency (Group B) were examined three times each by computerised dark adaptometry with simultaneous but separate recordings from each eye and binocularly. The respiratory patients received oxygen supplement at visits 1 and 3. In Group A, at all three visits, binocular dark adaptation was significantly more sensitive (40.5%) than monocular dark adaptation with either eye. In Group B, at visits 1 and 3, binocular dark adaptation was also significantly more sensitive than monocular dark adaptation (40.5% higher than the right and 47% higher than the left eye). However, in Group B, at visit 2 without oxygen treatment, no significant differences were observed between monocular and binocular sensitivities. Binocular dark vision was superior to monocular dark vision in healthy individuals and in patients with respiratory insufficiency that were provided oxygen supplementation. Furthermore, deficit in oxygen seems to affect binocular summation, perhaps by impaired enhancement in the central nervous system.
Keywords: Binocular summation, dark adaptation, monocular vision, neural summation, probability summation
INTRODUCTION
Binocular summation has previously been described for different aspects of vision, including acuity, hyperacuity, contrast sensitivity, grating detection, and pattern recognition.1–8 However, several studies have reported conflicting results on the existence of binocular enhancement of dark vision.3,4 In a thorough review by Blake and Fox,3 an examination of different aspects of method and theory showed that the majority of studies pointed towards binocular summation at absolute threshold. Thorn and Boynton4 listed a number of studies that concluded that binocular summation exists, but also listed almost as many studies with the opposite conclusion. Studies that did not confirm the existence of binocular summation at absolute threshold had often not observed the importance of certain factors such as the size of the stimuli and that the same corresponding retinal loci should be exposed and ideally at the same time.3,9 Furthermore, there had been large interindividual differences in several studies and the authors concluded that binocular summation occurred in some subjects but not in others. The heterogeneity of previous studies and their different conclusions raises questions about the phenomenon of binocular summation at absolute threshold, its magnitude, and its dependence on experimental conditions. This study aimed to answer some of these questions.
In the literature, binocular summation is explained by two main models. The first, termed "probability summation”, was presented by Pirenne10 as merely the increase in the probability of seeing with two eyes compared with one when the eyes are regarded as independent receptors. Legge5,11 has presented a theoretical description on the subject. He proposed, to predict binocular summation for contrast, the quadratic summation model: √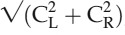 , where CL and CR are the contrast sensitivities for the left and right eyes, respectively. If both eyes are equal, the summation value equals √2, that is, 1.414, or an enhancement of 41.4%. The second model to explain binocular summation is referred to as “neural summation”2–4,12 and is the assumption that binocular summation is a signal enhancement that takes place in the central nervous system. The literature on binocular summation will be discussed further in Discussion.
, where CL and CR are the contrast sensitivities for the left and right eyes, respectively. If both eyes are equal, the summation value equals √2, that is, 1.414, or an enhancement of 41.4%. The second model to explain binocular summation is referred to as “neural summation”2–4,12 and is the assumption that binocular summation is a signal enhancement that takes place in the central nervous system. The literature on binocular summation will be discussed further in Discussion.
A computerised dark adaptometer (JUTA 1001), giving separate but simultaneous recordings from each eye and binocularly, has enabled us to study binocular summation at absolute threshold with a new degree of precision and validity. We have used JUTA 1001 in two separate studies. The first, originally designed to evaluate the new apparatus, was done on healthy young subjects. JUTA 1001 had 50% reduction of examination time and showed good reproducibility of results.13 Merely as an incidental observation of this study the subjects showed higher light sensitivity when both eyes were used than with only the right or the left eye.
The second study with JUTA 1001 was on patients having chronic respiratory insufficiency. The motivation for doing this study was twofold. Earlier studies have demonstrated that patients with carotid artery disease14,15 and patients with polycytemia16 have reduced dark adaptation that improves after treatment. Therefore, we wanted to study if dark adaptation was also affected in patients with respiratory insufficiency and a reduced oxygen level in the blood. The patients, in contrast to healthy individuals, were found to have normal dark adaptation in spite of hypoxia. This finding was explained by the circulatory effects of the blood gases and the fact that the retina is supplied by two separate vascular systems with different properties. The inner retina is vascularised by the central retinal artery and its branches and its blood flow is autoregulated by the arterial partial pressures of carbon dioxide (Paco2) and oxygen (Pao2). Increased Paco2 leads to vasodilation and increased blood flow, whereas reduced Paco2 has the opposite effect. Increased Pao2 induces vasoconstriction and diminished blood flow, whereas a decreased Pao2 results in vasodilation and increased blood flow. The outer retina is supplied with oxygen, mainly by diffusion from the choroidal circulation, which may not be autoregulated by oxygen, but it seems that increased Paco2 results in an increase of the choroidal blood flow. Healthy subjects at high altitudes have hypoxia and, due to hyperventilation, also hypocapnia. The hypocapnia can be expected to reduce the blood flow at least in the inner retina and together with hypoxia results in impaired dark adaptation. In contrast, hypoxic patients with respiratory failure are normocapnic or hypercapnic which would produce vasodilation of both inner and outer retinal circulation. The resulting increase in blood flow would, at least in theory, counteract the hypoxia-induced reduction of dark adaptation, which will instead be maintained.17
Since there is strong evidence of impaired neuropsychological functions in patients with respiratory insufficiency, a condition that at least in part is reversible with oxygen therapy,18–22 in our second study we also wanted to study if there was an effect on binocular summation of dark vision, since this in part may be explained by neural summation in the central nervous system.
The present article deals solely with the matter of binocular enhancement. As it is based on findings from the two studies mentioned above13,17 some data from these studies are shortly presented below, regarding subjects, dark adaptometry procedure, and blood gases. In this paper, we report our results and discuss the findings on binocular summation in healthy young subjects and older patients with respiratory insufficiency. It should be noted that the two groups represent completely different populations and that the healthy individuals do not represent a “control group” for the patients. Our purpose is to present the results from each group separately in order to illustrate the phenomenon of binocular summation.
MATERIALS AND METHODS
Subjects
We examined two groups of individuals (Groups A and B). The inclusion criterion for both groups was a corrected visual acuity of at least 0.5 according to the Snellen decimal chart. Exclusion criteria were ophthalmological diseases (including amblyopia, glaucoma, or vascular occlusion), neurological disease, or malignancies.
Group A consisted of 18 healthy individuals (9 men and 9 women; age 25–44, mean 34.4 years) that were examined by computerised dark adaptometry.13 All subjects were non-smokers and none were taking medication. All subjects in Group A completed three dark adaptometries.
Group B consisted of 13 patients with respiratory failure (6 men and 7 women; age 57–84, mean 68.7 years) that were examined by dark adaptometry.17 All patients were non-smokers or had quit smoking prior to the prescription of long-term oxygen treatment (LTOT). In Group B, 2 subjects did not understand the instructions given on the first visit. Thus, for Group B, there were results from 11 subjects at visit 1 and 13 subjects at visits 2 and 3.
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Lund University, Sweden. Written informed consent was obtained from each healthy individual and each patient.
Examination Schedule
All individuals in Groups A and B were tested by dark adaptometry (see below) at each of three visits on separate days.
Group B had the following schedule: At visits 1 and 3, all patients received their normal oxygen dosage from the Oxygenator (Oxygen Concentrator Zefir 5; Air Liquide Santé International, Paris, France). At visit 2, the patients did not receive oxygen therapy for at least 30 min and up to 8 h (mean 4 h). At each visit, an analysis of arterial blood gases from the radial artery was immediately performed to determine oxygen saturation, partial pressure of O2 (Pao2), partial pressure of CO2 (Paco2), pH, base excess (BE), and standard bicarbonate (HCO3).
Dark Adaptometry
Dark adaptometry records the changes in retinal sensitivity in darkness over time.23–25 We used a computerised dark adaptometer (JUTA 1001) for the examinations. The method, which allows separate but simultaneous, i.e. interleaved, recordings of the right eye, left eye, and both eyes, has been previously described in detail.13
The intensity of light at dark adaptometry was measured in steps. At step 1, the basic level with the strongest light, the test light intensity was 0.031 cd/m2 after having been filtered through the liquid crystal display (LCD) shutters (see below). The intensity of the test light was decreased in a stepwise fashion with a relation between consecutive steps of 1/√2. Step 2 had an intensity of 0.031/√2 cd/m2, step 3 had an intensity of 0.031/√22 cd/m2, and so on. Thus, each step is 0.15 log units. In this report, we use the step number to describe the performance level of the subject. For calculations of the percentage increase of binocular versus monocular dark adaptation, we used the values of light intensity, given in cd/m2, as follows: [100 − binocular light detected (cd/m2)/monocular light detected (cd/m2) × 100]%.
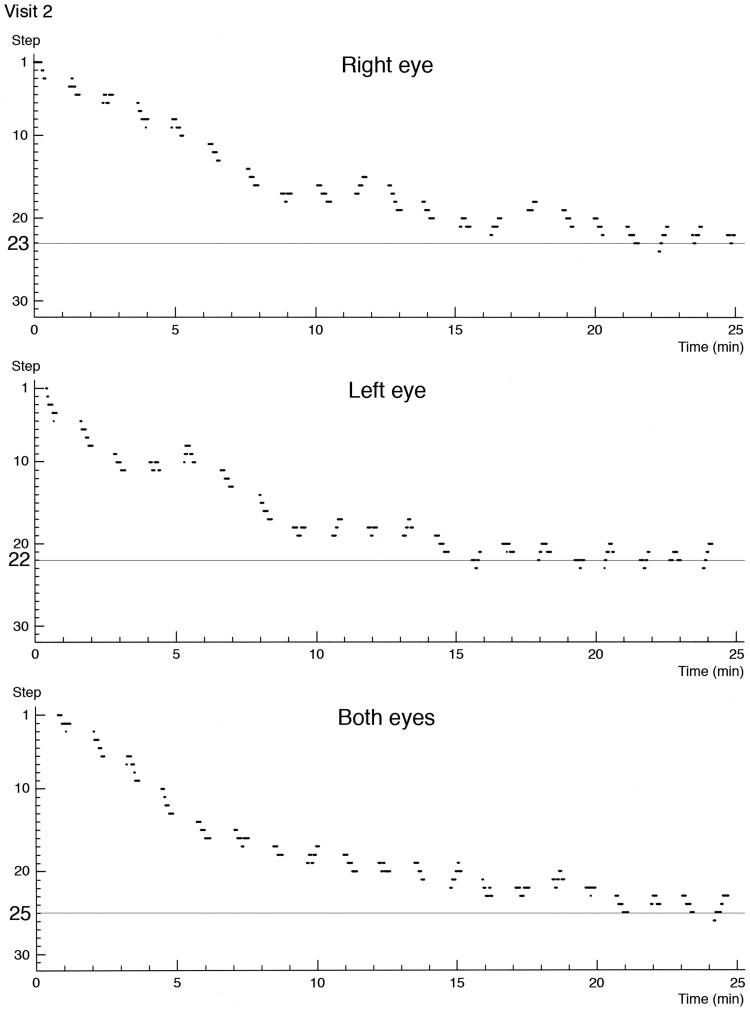
The subject was placed in front of a white hemispheric bowl. Preadaptation with 5 min of exposure of both eyes to white light (1000 cd/m2) was followed by 25 min of dark adaptometry, during which the subject was focusing a red fixation light placed 6 degrees above the yellow (585 nm) test light, with a diameter of 14 mm. The test light is 35 cm in front of the subject who is exposed to either light or no light. The subject was asked to press a button when a light was seen and not to press the button when no light was recognised. The test light, being lit at random, had a maximum duration of 4.0 s when not interrupted by the subject pressing the button. When three correct responses were given to three consecutive stimuli, the current light intensity was decreased by one step. The three consecutive stimuli were randomly selected, but randomness was reduced by prohibiting the outcome of three consecutive “no-light” events. Consequently, a subject could never pass a sequence and decrease light intensity by only being passive. When a single erroneous response was given, the light intensity was immediately increased by one step, i.e. the procedure was a 3 down 1 up staircase. As a measure of performance, we accepted the lowest step value obtained in which the subject delivered three correct answers consecutively and was not able to progress further down the scale. The lowest step (highest number) represented the lowest test light luminance level the subject could see, and thus the highest light sensitivity of the subject. The increase of retinal sensitivity with time in darkness was recorded separately for the right eye, the left eye, and both eyes as dark adaptometry curves (Figure 1).
FIGURE 1.
Dark adaptometry curves of a 47-year-old healthy man for the right eye, left eye, and both eyes obtained simultaneously from the same test session (visit 2). The step number on the y-axis represents the test light luminance level. One step corresponds to 0.15 log units. The further down along the scale with increasing step number, the lower the test light luminance level is. The lowest perceived level is marked by a horizontal line indicating the step number on the y-axis.
Two LCD shutters, one in front of each eye, controlled if the right eye, the left eye, or both eyes were exposed to the test light. Each of these alternatives was tested for 20 s in the sequence: right eye, left eye, both eyes. The sequence was then repeated. All examinations were performed with the pupil in its natural state.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Paired t-tests were used to compare the lowest level of dark adaptation for each eye in the monocular condition, with the lowest level for both eyes in the binocular condition. This comparison was made for each visit separately, for the mean results of all visits (Group A), and for the mean results of visits 1 and 3 (Group B). Paired t-tests were also used for Group B to compare the values for pH, Pao2, Paco2, BE, HCO3, and oxygen saturation between visits 1 and 2, 2 and 3, and 1 and 3.
RESULTS
Group A
All 18 individuals in Group A were ophthalmologically healthy with normal visual acuity (0.9–1.0 on each eye). The refractions were between −6.50 and +2.25. The degrees of astigmatism were between 0 and −1.0. Intraocular pressures ranged from 11 to 19, with a mean of 14.4 mm Hg for both right and left eyes. The visual fields ad modum Donders were normal. The pupillary reactions and undilated fundus examinations were also normal.
The results of dark adaptometry for Group A are presented in Table 1. The binocular sensitivity was significantly higher than the monocular sensitivity at each visit, and for the mean results of all three visits. The binocular summation ranged from 32.8% to 47.5% in a single visit. The mean binocular summations of all three visits were 40.5% for the right eye and 40.5% for the left eye.
TABLE 1.
Lowest achieved dark adaptation levels and luminance with corresponding levels and intervals in 18 healthy subjects at three separate visits, and mean results from all three visits: (1 + 2 + 3)/3.
| Visit | Condition | Dark adaptation level [steps; mean ± SEM] | p | Luminance [cd/m2 (×10−5); mean (interval)] | Binocular summation [mean%] |
|---|---|---|---|---|---|
| 1 | Both eyes | 24.8 ± 0.45 | 0.81 (0.49–0.95) | ||
| Right eye | 23.3 ± 0.48 | <0.0001 | 1.37 (1.16–1.61) | 40.8 | |
| Left eye | 23.4 ± 0.44 | <0.0001 | 1.32 (1.13–1.54) | 38.6 | |
| 2 | Both eyes | 24.7 ± 0.46 | 0.84 (0.72–0.98) | ||
| Right eye | 23.6 ± 0.60 | <0.05 | 1.25 (0.99–1.51) | 32.8 | |
| Left eye | 22.8 ± 0.83 | <0.01 | 1.60 (1.22–2.16) | 47.5 | |
| 3 | Both eyes | 25.4 ± 0.51 | 0.66 (0.55–0.79) | ||
| Right eye | 23.7 ± 0.72 | <0.0001 | 1.18 (0.65–1.52) | 44.1 | |
| Left eye | 24.2 ± 0.54 | <0.0001 | 1.01 (0.83–1.20) | 34.8 | |
| (1 + 2 + 3)/3 | Both eyes | 25.0 ± 0.27 | 0.76 (0.69–0.83) | ||
| Right eye | 23.5 ± 0.34 | <0.0001 | 1.27 (1.13–1.43) | 40.5 | |
| Left eye | 23.5 ± 0.36 | <0.0001 | 1.27 (1.12–1.44) | 40.5 |
Comparisons of dark adaptation between monocular values of each eye and binocular values showed that binocular values were consistently significantly higher (p values). One dark adaptation step corresponds to 0.15 log units. Binocular summation was calculated from the luminance values in cd/m2 in the following equation: [100 − binocular light detected (cd/m2)/monocular light detected (cd/m2) × 100]%.
Group B
All 13 patients in Group B had been on LTOT for at least 4 months and up to 12 years. The causes of chronic respiratory failure included 10 patients with obstructive and 3 patients with restrictive pulmonary disorders. All patients were treated with domiciliary oxygen for ≥16 h per day with flow rates ranging from 1 to 4.5 L/min (mean 2.0 L/min).
The best corrected visual acuity ranged from 0.6 to 1.0 (mean 0.82) in the right eyes and from 0.6 to 1.0 (mean 0.78) in the left eyes. The refractions ranged between −1.75 and +3.25. The degrees of astigmatism ranged between 0 and −3.5. Intraocular pressures in the right eyes ranged from 12 to 20 mm Hg (mean 16.5) and in the left eyes from 12 to 19 mm Hg (mean 16.4). Pupillary reactions and undilated fundus examinations were normal in all patients. Three of the patients had undergone operations for cataracts with intraocular lens implantations in both eyes. Four of the patients had a minor cataract in one eye.
Between visits 1 and 2, there were significant differences in the Pao2 (mean difference [Δ] 2.15 kPa; p < 0.001) and the arterial oxygen saturation (mean Δ 6.3%; p < 0.005). Almost the same significant differences were observed between visits 2 and 3 in the Pao2 (mean Δ 2.08 kPa; p < 0.001) and the arterial oxygen saturation (mean Δ 5.8%; p < 0.005). No significant differences were found in the Paco2, BE, HCO3, or pH between visits 1 and 2 or between visits 2 and 3. For visits 1 and 3, oxygen supplementation was provided during testing, and no significant differences were found for any of the parameters.
The results of dark adaptometry for Group B are presented in Table 2. Generally, the patients had lower luminance sensitivities than the healthy subjects. The binocular sensitivity of the patients was significantly higher than the monocular sensitivity at visits 1 and 3, when the patients were allowed to continue oxygen treatment during the test. Similar significant differences were observed in the mean results of visits 1 and 3. At visit 2, when the patients were without oxygen treatment, no significant differences were found between the binocular and monocular sensitivities. The binocular summation from visits 1 and 3 ranged from 34.7% to 58.7%, with a mean of 40.5% for the right eye and 47% for the left eye. At visit 2, the binocular summation was 32.9% for the right eye and 23.2% for the left eye.
TABLE 2.
Lowest achieved dark adaptation levels and luminance with corresponding levels and intervals in 13 patients with respiratory failure at three separate visits, and mean results from visits 1 and 3: (1 + 3)/2.
| Visit | Condition | Dark adaptation level [steps; mean ± SEM] | p | Luminance [cd/m2 (×10−5); mean (interval)] | Binocular summation [mean%] |
|---|---|---|---|---|---|
| 1 | Both eyes | 20.9 ± 0.68 | 3.12 (2.47–3.95) | ||
| Right eye | 19.5 ± 0.86 | <0.005 | 5.18 (3.85–6.98) | 39.7 | |
| Left eye | 18.4 ± 1.31 | <0.05 | 7.56 (4.80–11.9) | 58.7 | |
| 2 | Both eyes | 20.4 ± 0.85 | 3.75 (2.80–5.04) | ||
| Right eye | 19.2 ± 1.08 | NS (0.15) | 5.59 (3.85–8.13) | 32.9 | |
| Left eye | 19.6 ± 0.76 | NS (0.28) | 4.88 (3.75–6.36) | 23.2 | |
| 3 | Both eyes | 20.8 ± 0.76 | 3.28 (2.52–4.27) | ||
| Right eye | 19.2 ± 0.79 | <0.05 | 5.59 (4.25–7.35) | 41.4 | |
| Left eye | 19.5 ± 0.97 | <0.05 | 5.02 (3.59–7.03) | 34.7 | |
| (1 + 3)/2 | Both eyes | 20.7 ± 0.44 | 3.21 (2.69–3.83) | ||
| Right eye | 19.3 ± 0.52 | <0.0005 | 5.40 (4.43–6.58) | 40.5 | |
| Left eye | 19.2 ± 0.57 | <0.005 | 6.05 (4.60–7.96) | 47.0 |
At visits 1 and 3, the patients received oxygen treatment, but no oxygen was provided at visit 2. Comparisons of dark adaptation between monocular values of each eye and binocular values showed that binocular values were significantly higher (p values) when the patients received oxygen. No statistical difference was found between monocular and binocular values when no supplemental oxygen was provided. One dark adaptation step corresponds to 0.15 log units. Binocular summation was calculated from the luminance values in cd/m2 in the following equation: [100 − binocular light detected (cd/m2)/monocular light detected (cd/m2) × 100]%.
DISCUSSION
In a single session, our computerised dark adaptometer tested each eye separately and both eyes together in short cycles of 20 s repeatedly during the 25 min of dark adaptation. These short cycles allowed us to avoid a fatigue bias that can typically obscure results in repeated exams when the three different conditions are tested separately. The LCD shutters are not visible in the completely dark testing environment, and the subject is not aware when testing switches between monocular and binocular conditions. The red fixation point is visible during the entire dark adaptation, as it is placed above the visual field that is occluded by the LCD shutters. This has a number of advantages. First, there is no problem with heterophoria, i.e. latent strabismus, as the fixation point is continuous. Second, when binocularly tested, the eyes are synchronously exposed to the test light without any lag between the eyes. Third, throughout the dark adaptation, the same corresponding retinal loci are exposed 6 degrees parafoveally. Fourth, the pupil size is not affected as the binocular viewing of the fixation light is maintained. Also, the computerised dark adaptometry does not require the involvement of an examiner; this ensures that each test condition is identical. The element of guessing is minimised by the test algorithm, as three correct consecutive responses are required to pass to the next “level” with lower luminance. One erroneous response causes a return to the previous level with a higher luminance. We believe that these factors combine to provide highly valid results.
There are conflicting reports on the existence of binocular summation of dark vision at absolute threshold.3,4 A number of variables that may affect binocular summation are summarised by Blake and Fox,3 including individual differences, pupil size, fixation, method of stimulus presentation, and other personal factors such as motivation. Thus, an obvious difficulty in assessing previous studies is the vast heterogeneity of their methods. Most studies on binocular summation at absolute threshold were performed in the first half of the 20th century and were reviewed by Blake and Fox3 and by Thorn and Boynton.4 The combination of different methods for testing dark adaptation and typically small study populations probably accounts for a large part of the conflicting results in this field. Small study populations are common in the research of binocular summation, regardless of which visual parameter is chosen; for example Frisén and Lindblom6 studied 8 subjects, Legge5 studied 6 subjects, Meese et al.8 studied 3 subjects, Thorn and Boynton4 studied 3 subjects, and Zlatkova et al.7 studied 3 subjects. Thus, in comparison, our two study populations are quite large, with a total of 31 subjects and 91 test runs with dark adaptometry.
Most previous studies that reported binocular summation found summation values between 1 and about 2, i.e. 0% and 100%, largely depending on which visual task was studied.6 For sine-wave gratings, the summation value generally corresponds to √2. This √2 value has also been observed in studies on increment detection, flicker sensitivity, and spatial resolution.3 For other tasks, such as visual acuity, the summation value has been reported to be 11%1 and 8%.26 Frisén and Lindblom6 proposed that the binocular summation depends on the complexity of the task, with higher values for simple tasks and lower values for more complex tasks. For dark adaptation, varying degrees of binocular summation has been reported, ranging from none27,28 to 70–80%.4 In the two groups that we studied with dark adaptometry, there was significant and consistent evidence of binocular summation. In young healthy individuals, we found a binocular summation of 40.5% compared with the right eye and 40.5% compared with the left eye. The corresponding values for the patients with respiratory insufficiency with oxygen treatment were 40.5% and 47%. Lythgoe and Phillips29 reported binocular summation with a factor of 1.4, which corresponds well to our data.
Our patients with respiratory disease (Group B) generally had higher absolute thresholds, i.e. luminance values, than the healthy subjects (Group A). At the same time, the patients were considerably older (mean age 68.7 years) than the healthy individuals (mean age 34.4 years). It is well known that the dark vision of elderly individuals is poorer than that of the young. There is a progressive deterioration with advancing age that can be explained by several factors, such as pupillary miosis, diminished transparency of the ocular media (especially cataract), delayed rhodopsin regeneration, and carotid artery disease.14,15,30–32 We believe that some of these facts may explain the difference in final threshold levels between Group A and Group B. Interestingly, the effect of cataract on dark vision is dependent on the wavelength of the test light. Longer wavelengths, e.g. yellow, will decrease dark vision much less than shorter wavelengths, e.g. violet.31 We used yellow test light in JUTA 1001. Consequently, the effect of lens aging on final threshold levels among our patients should be very small.
Theoretically, the two essential components of binocular summation are probability summation and neural summation in the central nervous system. Probability summation as a concept was presented by Pirenne10 in 1943 and is the increased probability of seeing with two eyes as compared with one. Essential to the theory behind probability summation is that the two eyes are regarded as completely different receptors. Pirenne10 made the comparison that the same result would be obtained by “two eyes belonging to different persons, the flash being seen when at least one of the two persons has seen it.” However, Eriksen33 pointed out that the theorem by Pirenne10 in many visual tests does not take into account the guessing factor. Due to this, it tends to overpredict the binocular summation and should not be used when guessing is a part of the detection performance. More modern models of predicting binocular summation are the multistate model,33 which takes into account the guessing factor, the integration model,12 and the quadratic summation model by Legge.5,11
Neural summation is assumed to exist when the summation exceeds that of probability summation and its existence is supported by several investigators.3 Matin2 in a study using spatial separation of flashes found that probability of detection was dependent on the interval separating the two flashes. At intervals less than 100 ms, the binocular summation was greater than the calculated value from the probability summation theory, whereas when the intervals exceeded 100 ms it was equivalent. The author’s conclusion was that neural summation exists and that “a common sensory path for the two eyes exists central to the optic chiasm.” Kristofferson34 using the integration model of signal detection has also showed summation that is greater than probability. Several investigators have found that binocular summation increases when corresponding retinal areas are stimulated as compared with stimulation of non-corresponding areas; this also implies the existence of neural summation.4,9,35 Furthermore, the existence of neural enhancement is supported by the finding that some visual cortical neurons are binocularly activated,36,37 which is further evidence that the two eyes are not completely independent receptors.
Legge,5,11 who proposed the quadratic summation rule to describe binocular summation for contrast, also found summation in excess of what the probability summation model would predict. He also noted that the summation for high-contrast discrimination is close to 1, which indicates no summation at all. This was theoretically explained by the internal noise in the separate channels becoming correlated at supra-threshold contrast and thus decreasing the binocular summation. Paradoxically, the implication would be that “two cooperating persons, each looking with one eye, would likely do a better job at discriminating contrasts than one individual looking with two eyes.” This could be understood by realizing that “a relaxation of the independence assumption of probability summation need not result in improved binocular summation.”5 This also implies that the assumption of two completely independent receptors is incorrect, which is in direct conflict with Pirennes probability summation theory. Consequently, probability summation does not impose a lower bound on binocular summation. It is reasonable to accept that probability summation exists but the exact value is not possible to calculate given the weaknesses in the theory mentioned above.
In a review article by Hynninen et al.38 including 81 studies, the psychological characteristics of patients with respiratory insufficiency are discussed. The authors concluded that there is an effect on neuropsychological functioning such as impairment on memory functions and higher cognitive functions. According to Grant et al.,39 the degree of hypoxemia may contribute to the test performance impairment. In a report by Stuss et al.,40 severely hypoxemic patients obtained lower scores than mildly hypoxemic patients on tests of complex attention, information processing, and memory tasks. Krop et al.18 found improvement in visual memory, verbal memory, and motor speed in patients with respiratory insufficiency after receiving oxygen therapy for 6 months. Other studies on patients with respiratory insufficiency have found at least in part reversible impairment of neuropsychological functions with oxygen therapy.19–22 Obviously, the level of oxygen in the blood is important for optimal cognitive functions. Is likewise a part of dark visions binocular summation a sensitive central nervous function that requires normal oxygen levels in the blood to perform optimally?
In the patients with respiratory insufficiency, we found no significant binocular summation at visit 2, when they were not provided with supplemental oxygen. Interestingly, the decrease in oxygen saturation did not significantly affect their dark adaptometries, i.e. the lowest test light luminance levels detected were similar in all three visits.17 The reduced oxygen saturation only appeared to affect the difference in sensitivity between one eye and both eyes. The binocular summation was 32.9% for the right eye and 23.2% for the left eye. Obviously, these numbers must be interpreted with caution; however, the binocular summation appears to be reduced by approximately 8% to 17%, compared with when the patients were provided with supplemental oxygen. As probability summation should be the same regardless of oxygen treatment, this result may be an indication of reduced neural summation. Perhaps in the central nervous system, cells essential for neural summation were impaired by the lack of oxygen.
In summary, previous studies on the existence of binocular summation at absolute threshold in dark adaptometry have reported conflicting results. This has probably been due to small study populations and methodological differences. In our present investigation, we have shown binocular summation at absolute threshold in dark adaptometry in 31 individuals, consisting of both healthy subjects and patients with respiratory insufficiency. Our method was simple with high validity, and the number of examined individuals was relatively large. Thus, we confirm that binocular summation exists at absolute threshold. Moreover, our results indicated that hypoxemia impaired the binocular summation, as patients with respiratory insufficiency did not have significant binocular enhancement in the absence of supplemental oxygen. This result implies the existence of neural summation.
Acknowledgements
This study was supported by grants from Region Skåne, the Herman Järnhardt Foundation, and the Frederico Hecht Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Bárány E. A theory of binocular visual acuity and an analysis of the variability of visual acuity. Acta Ophthal 1946;24:63–92 [Google Scholar]
- 2.Matin L. Binocular summation at the absolute threshold of peripheral vision. J Opt Soc Am 1962;52:1276–1286 [DOI] [PubMed] [Google Scholar]
- 3.Blake R, Fox R. The psychophysical inquiry into binocular summation. Percept Psychophys 1973;14:161–185 [Google Scholar]
- 4.Thorn F, Boynton R. Human binocular summation at absolute threshold. Vis Res 1974;14:445–458 [DOI] [PubMed] [Google Scholar]
- 5.Legge GE. Binocular contrast summation—I. Detection and discrimination. Vis Res 1984;24:373–383 [DOI] [PubMed] [Google Scholar]
- 6.Frisén L, Lindblom B. Binocular summation in humans: evidence for a hierarchic model. J Physiol 1988;402:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlatkova MB, Anderson RS, Ennis FA. Binocular summation for grating detection and resolution in foveal and peripheral vision. Vis Res 2001;41:3093–3100 [DOI] [PubMed] [Google Scholar]
- 8.Meese TS, Georgeson MA, Baker DH. Binocular contrast vision at and above threshold. J Vis 2006;6:1224–1243 [DOI] [PubMed] [Google Scholar]
- 9.Schaad D. Binocular summation in scotopic vision. J Exp Physiol 1935;18:391–413 [Google Scholar]
- 10.Pirenne MH. Binocular and uniocular threshold of vision. Nature 1943;152:698–699 [Google Scholar]
- 11.Legge GE. Binocular contrast summation—II. Quadratic summation. Vis Res 1984;24:385–394 [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Swets JA. Signal detection theory and psychophysics. In: Green D M, Swets JA, editors. Signal Detection Theory and Psychophysics. New York: Wiley; 1966:243–248 [Google Scholar]
- 13.Havelius U, Thylefors J, Lundgren A, Krakau T. Reduced examination time by monocular and bilateral examination in the same session with a new computerized dark adaptometer. Tech Ophthal. 2005;3:37–42 [Google Scholar]
- 14.Havelius U, Bergqvist D, Falke P, Hindfel B, Krakau CET. I. Impaired dark adaptation in symptomatic carotid artery disease. Neurology 1997;49:1353–1359 [DOI] [PubMed] [Google Scholar]
- 15.Havelius U, Bergqvist D, Hindfelt B, Krakau CET. II. Improved dark adaptation after carotid endarterectomy. Evidence of a longterm ischemic penumbra? Neurology 1997;49:1360–1364 [DOI] [PubMed] [Google Scholar]
- 16.Havelius U, Berglund S, Falke P, Hindfelt B, Krakau T. Impaired dark adaptation in polycythemia: improvement after treatment. Acta Ophthal Scand 2000;78:53–57 [DOI] [PubMed] [Google Scholar]
- 17.Thylefors J, Piitulainen E, Havelius U. Dark adaptation during systemic hypoxia induced by chronic respiratory insufficiency. Invest Ophthal Vis Sci 2009;50:1307–1312 [DOI] [PubMed] [Google Scholar]
- 18.Krop HD, Block AJ, Cohen E. Neuropsychologic effects of continuous oxygen therapy in COPD. Chest 1973;64:317–322 [DOI] [PubMed] [Google Scholar]
- 19.Heaton RK, Grant I, McSweeney AJ, Adams KM, Petty TL. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1983;143:1941–1947 [PubMed] [Google Scholar]
- 20.Wilson DK, Kaplan RM, Timms RM, Dawson RA. Acute effects of oxygen treatment upon information processing in hypoxemic COPD patients. Chest 1985;64:317–322 [DOI] [PubMed] [Google Scholar]
- 21.Borak J, Sliwinski P, Tobiasz M, Gorecka D, Zielinski J. Psychological status of COPD patients before and after one year of long–term oxygen therapy. Monaldi Arch Chest Dis 1996;51:7–11 [PubMed] [Google Scholar]
- 22.Hjalmarsen A, Waterloo K, Dahl A, Jorde R, Viitanen M. Effect of long-term oxygen therapy on cognitive and neurological dysfunction in chronic obstructive pulmonary disease. Eur Neurol 1999;42:27–35 [DOI] [PubMed] [Google Scholar]
- 23.Duke-Elder S, Weale RA. The sensation of light. In: Duke-Elder S, editor. System of Ophthalmology. Vol. 4 London: Henry Kimpton Publisher; 1968:558–561 [Google Scholar]
- 24.Duke-Elder S, Smith RJH. Examination of the visual functions. In: Duke-Elder S, editor. System of Ophthalmology. Vol. 7 London: Henry Kimpton Publishers; 1975:388–390 [Google Scholar]
- 25.Hart WM., Jr Visual adaptation. In: Hart WM, Jr, editor. Adler’s Physiology of the Eye Clinical Application. St. Louis: Mosby-Year Book; 1992:510–512 [Google Scholar]
- 26.Horowitz MW. An analysis of the superiority of binocular over monocular visual acuity. J Exp Psychol 1949;39:581–596 [DOI] [PubMed] [Google Scholar]
- 27.Bartlett NR, Gagné RM. On binocular summation at threshold. J Exp Psychol 1939;25:91–99 [Google Scholar]
- 28.Forbes LM, Mote FA. A comparison of the variability of binocular and monocular threshold measurements during dark adaptation in the human eye. J Comp Physiol Psychol 1956;49:431–436 [DOI] [PubMed] [Google Scholar]
- 29.Lythgoe RJ, Phillips LR. Binocular summation during dark adaptation. J Physiol 1938;91:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson GW, Yudkin J. Effect of age upon dark adaptation. J Physiol 1944;103:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunkel RD, Gouras P. Changes in scotopic visibility thresholds with age. Arch Ophthal 1963;69:4–9 [DOI] [PubMed] [Google Scholar]
- 32.Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vis Res 1999;39:3975–3982 [DOI] [PubMed] [Google Scholar]
- 33.Eriksen CW. Independence of succesive inputs and uncorrelated error in visual form perception. J Exp Psychol 1966;72:26–35 [DOI] [PubMed] [Google Scholar]
- 34.Kristofferson AB. Monocular and Binocular Detection Thresholds for Targets Varying in Size and Retinal Position. Ann Arbor, MI: University of Michigan. Vision Research Laboratories Technical Report 1958; No. 2144-290-T
- 35.Battersby WS, Defabaugh GL. Neural limitations of visual excitability: after-effects of subliminal stimulation. Vis Res 1969;9:757–768 [DOI] [PubMed] [Google Scholar]
- 36.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol 1968;195:215–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the Macaque monkey. J Comp. Neurol 1972;146:421–450 [DOI] [PubMed] [Google Scholar]
- 38.Hynninen KM, Breite MH, Wiborg AB, Pallesen S, Nordhus IH. Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res 2005;59:429–443 [DOI] [PubMed] [Google Scholar]
- 39.Grant I, Prigatano GP, Heaton RK, McSweeney AJ, Wright EC, Adams KM. Progressive neuropsychologic impairment and hypoxemia. Relationship in chronic obstructive pulmonary disease. Arch Gen Psychiatry 1987;44:999–1006 [DOI] [PubMed] [Google Scholar]
- 40.Stuss DT, Peterkin I, Guzman DA, Guzman C, Troyer AK. Chronic obstructive pulmonary disease: effects of hypoxia on neurological and neuropsychological measures. J Clin Exp Neuropsychol 1997;19:515–524 [DOI] [PubMed] [Google Scholar]