Abstract
Background
A wide variety of kidney diseases ultimately lead to tubulointerstitial damage. The initial site of injury is usually the renal tubules, with activation of fibroblasts occurring later. Self-limited disease is characterized by transient cellular activation with timed deactivation and ultimately a return to normal functioning, whereas sustained responses characterize chronic disease and the development of irreversible fibrosis. The underlying molecular and cellular mechanisms of this cascade of events remain an area of active research. Current data overwhelmingly support a role for crosstalk between the tubular epithelium and the interstitial fibroblast that mediates both repair/regeneration and progressive disease. This epithelial-mesenchymal communication (EMC) is regulated by a variety of soluble ligands binding to cell surface receptors to induce intracellular signaling events.
Summary
EMC is an important mechanism whereby tubular epithelium and fibroblasts/mesenchymal cells crosstalk to affect renal physiology and pathology. Numerous soluble factors such as sonic hedgehog, Wnt ligands, transforming growth factor-β, hepatocyte growth factor, connective tissue growth factor, and angiotensin II all participate in bidirectional EMC. Recent studies have also identified exosomes as a vehicle to mediate EMC during kidney injury. In general, while the short-term activity of EMC factors is renoprotective, prolonged activation of these factors leads to chronic disease and fibrosis.
Key Messages
The discovery of a complex and intricate system of communication between tubular cells and fibroblasts is a new paradigm in our understanding of renal fibrosis. An appreciation of both their regenerative and pathologic functions will inform the development and use of targeted therapeutic interventions.
Key Words: Acute kidney injury, Chronic kidney disease, Inflammation, Myofibroblast, Renal fibrosis
Introduction
Prevention of chronic kidney disease (CKD) and end-stage renal disease remains a major priority of health care worldwide. CKD is gaining in prevalence worldwide and has a strong negative effect on an individual's quality of life, morbidity, and mortality [1]. CKD is common, affecting 14% of the US population and 10.8% of the Chinese population. Beyond the health implications for an individual patient, end-stage renal disease also incurs an immense economic burden, accounting for over 30 billion USD in yearly expenditures in the USA alone [2,3].
Kidney injury on the molecular level involves complex interactions between all of the resident cell types as well as infiltrating cells from the circulation. While tubular cells are often the initial site of injury, fibroblasts are subsequently recruited and activated. Initial responses are likely an attempt at kidney repair and recapitulate embryologic developmental pathways. However, should repair processes persist, a profibrotic environment is promoted, leading to the replacement of normal kidney with scar tissue composed of a variety of extracellular matrix (ECM) components, including collagens and fibronectin [4]. Fibrosis is seen in virtually all types of progressive CKD and likely represents irreversible damage. As such, it is imperative to prevent fibrosis in order to improve patient outcomes.
The Key Players: Tubular Epithelial Cells and Fibroblasts
In the tubulointerstitium, it is the renal tubules that are the epicenter of early disease. While it is believed that these epithelial cells have excellent regenerative capacity after mild acute kidney injuries (AKI), data now indicate that severe or repeated injuries have lasting effects. Tubules can exhibit cell death, cell cycle arrest, mitochondrial dysfunction, and defects in fatty acid oxidation, to name a few, leading to proinflammatory and profibrotic phenotypic changes [5,6,7].
The development of fibrosis after epithelial injury is highly dependent on fibroblasts and myofibroblasts. Fibroblasts are mesenchymal cells that are normally present in the kidney interstitium, responsible for the generation of the scaffold required to maintain renal structures. In contrast, myofibroblasts are ‘activated’ cells associated with pathology and possessing a vastly increased capacity for ECM production and a contractile phenotype associated with α-smooth muscle actin (α-SMA) expression. Identification of the myofibroblast precursor has been an area of active research, with at least five possible source cells identified: resident interstitial fibroblasts, pericytes, circulating fibrocytes, endothelial cells, and the tubular epithelium. However, the concept of a complete epithelial-to-mesenchymal transition (EMT) during renal fibrosis has been difficult to prove definitively [4].
It may be more likely that epithelia undergo a ‘partial EMT’ in which epithelia acquire mesenchymal traits without a full EMT conversion. Recently, two studies demonstrated that tubular-specific expression of Snai1 or Twist1, previously implicated in EMT, led to partial EMT and the expression of profibrotic factors [8,9]. These factors are likely to have paracrine effects on fibroblasts to promote myofibroblast conversion and renal fibrosis, suggesting the importance of crosstalk between these cells and tubules.
Epithelial-Mesenchymal Communication
While EMT remains controversial, there is little doubt that some form of ‘epithelial-mesenchymal communication’ (EMC) occurs in damaged kidneys. EMC is mediated by soluble factors acting at short or long distances by binding to cell surface receptors. This leads to intracellular signaling activation and/or increased transcription of target genes. While many factors are implicated in renal injury, we will focus here on those supported by an abundance of experimental data as well as being specific for EMC.
Transforming Growth Factor-β
Transforming growth factor-β1 (TGF-β1) is the single most important soluble mediator of renal fibrosis. The most abundant of the three known mammalian TGF-β isoforms, active TGF-β1 binds to the type II TGF-β receptor, which in turn recruits the type I receptor. This complex leads to the phosphorylation and activation of Smad2 and Smad3, which translocate to the nucleus to upregulate target genes such as collagens. Interestingly, a latent form of TGF-β1 exists which can upregulate Smad7 to inhibit this pathway and may be antifibrotic [10].
Renal tubules produce TGF-β1 during disease. Both angiotensin II exposure and Snai1 overexpression induce TGF-β1 production in tubular cells [9,11]. After hypoxic injury, epithelial cells undergo cell cycle arrest, and during the prolonged time in the G2/M phase these cells produce large amounts of TGF-β1[6]. Increased tubular stretch, which may occur in obstructive renal injuries, is another inducer [12]. It should be noted that fibroblasts and inflammatory cells also produce TGF-β1[12].
Increased TGF-β1 expression leads to a variety of paracrine and autocrine effects on target cells. TGF-β1 is a potent mitogen for fibroblasts and causes myofibroblast transformation [10]. TGF-β1 also causes tubular cell apoptosis [13,14] and potently induces the EMT program, leading to the production of an increased matrix [13]. It also causes tubular cell hypertrophy, which may be closely linked to increased matrix production [15].
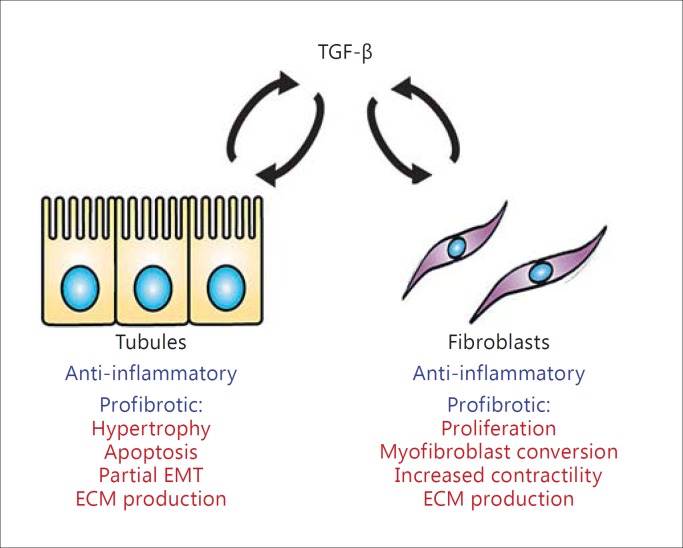
Overall, the potent effects of TGF-β1 make it one of the most profibrotic substances known. However, anti-TGF-β1 therapies have not been universally successful in preventing fibrosis. TGF-β1 has an anti-inflammatory role which may itself be antifibrotic, and blocking this may abrogate any positive effects [10]. Nonetheless, its role in progressive CKD is not to be underestimated, and it may yet be a target of successful therapeutic intervention in the future. The bidirectional effects of TGF-β1 on epithelia and fibroblasts are summarized in figure 1.
Fig. 1.
The role of TGF-β1 in EMC. Both tubules and fibroblasts can produce TGF-β1 under pathologic conditions, and this can act on either type of cell, leading to autocrine and paracrine effects. In the acute phase, TGF-β1 plays an anti-inflammatory role which may be protective. In the chronic phase, TGF-β1 is a strong inducer of fibrosis, leading to tubular cell apoptosis and hypertrophy as well as increased ECM generation and a partial EMT phenotype. In fibroblasts, TGF-β1 is a potent mitogen and stimulates myofibroblast conversion and the acquisition of a contractile phenotype along with greatly enhanced expression of ECM components.
Wnt/β-Catenin
An abundance of evidence supports the Wnt/β-catenin pathway in the development of renal fibrosis. Wnt/β-catenin is a critical developmental signaling pathway that is necessary for normal kidney organogenesis. Although quiescent in normal adult kidneys, this pathway is reactivated after a variety of injuries [16].
There are 19 different known Wnt, and all of them can bind any one of 10 frizzled (Fz) receptors at the cell surface. Low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) co-receptors are critical for activation of intracellular signaling. Wnt/Fz ligation leads to the activation of β-catenin in this so-called canonical pathway. β-Catenin normally resides in the cytoplasm as part of a multiprotein complex consisting of axin, glycogen synthase kinase 3β, dishevelled, and adenomatous polyposis coli. As part of this complex, β-catenin is phosphorylated and directed for proteasomal degradation. During active Wnt signaling, the complex dissociates via interactions with Fz and LRP5/6, leading to release of β-catenin, its escape from degradation, and subsequent cytoplasmic accumulation. This leads to nuclear translocation, where β-catenin fulfills one of its roles as a transcription factor regulator by recruiting T-cell factor and lymphoid enhancer-binding factor to upregulate target genes [16].
Wnt proteins can be expressed in both tubular cells and interstitial fibroblasts, and both cell types can respond to Wnt signaling, leading to paracrine and autocrine effects. However, at least some injury models lead to preferential upregulation of Wnt in activated myofibroblasts and not the epithelium, indicating predominantly unidirectional communication [17]. Supporting this, the majority of β-catenin activity appears to be induced in tubular cells during injury [18,19].
β-Catenin effects are numerous. Wnt/β-catenin signaling allows entry into the cell cycle via the upregulation of cyclin D1 and cyclin A [20]. This has important implications depending on whether the target cell is an epithelial cell attempting tubular repair, or a fibroblast promoting fibrosis. β-Catenin also enhances the expression of matrix metalloproteinase 7 (MMP-7), fibronectin, fibroblast-specific protein 1, plasminogen activator inhibitor 1, Snail1, and even the components of the renin-angiotensin system (RAS; see below) [16].
In the acute phase of kidney injury, Wnt/β-catenin is likely to be protective. A variety of nephrotoxic events lead to marked accumulation of β-catenin in renal tubules [20,21]. In both folic acid nephropathy and ischemia-reperfusion, tubule-specific ablation of β-catenin is harmful, leading to increased mortality, renal injury, and tubular cell apoptosis [18]. Ablation of either Fz or the LRP5/6 co-receptors leads to increased tubular injury after ischemia-reperfusion [22]. Via effects on cell cycle progression, Wnt/β-catenin is critical for tubular cell repopulation and repair [20]. It has also been suggested that a population of endogenous progenitor cells help to repopulate the tubular compartment after injury. These progenitor cells require activation of the Wnt/β-catenin pathway [23].
Interestingly, interstitial fibroblasts are activated and proliferate during AKI, with numbers dissipating after injury resolution [24]. The exact function of these fibroblasts during AKI is unknown, but it has been proposed that they are reparative. Wnt/β-catenin may help to regulate this response through the upregulation of tubular MMP-7 [25]. This protease is capable of upregulating Fas ligand on fibroblasts, which would lead to regulated removal of fibroblasts after AKI via apoptotic cell death [26]. In this instance, fibroblasts proliferate after injury to promote renal repair, but subsequent increases in Wnt lead to enhanced expression of tubule-derived MMP-7. This protease, in turn, acts to remove fibroblasts via apoptosis as the AKI resolves, allowing the kidney's microenvironment to return to normal. As such, Wnt/β-catenin forms a self-regulatory negative feedback loop to allow for the transient activation of fibroblasts to promote short-term repair after AKI.
In contrast to the beneficial transient activity in acute injuries, it appears that sustained Wnt/β-catenin activation is detrimental and drives the fibrotic response in chronic injuries. For instance, in mice with forced overexpression of Wnt1 in proximal tubules, robust interstitial fibrosis developed, associated with the activation of myofibroblasts and increased ECM generation [27]. In obstructive injury, Wnt4 potently induced myofibroblast differentiation [17]. The profibrotic cytokines TGF-β and connective tissue growth factor (CTGF) are also capable of directly upregulating Wnt/β-catenin activity [28,29].
The hypothesis that the duration of Wnt/β-catenin signaling is a key aspect to its role as either beneficial or pathologic has been tested experimentally. In moderate, self-resolving AKI due to ischemia-reperfusion, Wnt/β-catenin undergoes a transient upregulation associated with recovery and a ‘good’ renal outcome. Meanwhile, after inducing more severe AKI with a longer ischemic period, Wnt/β-catenin activity persists, and this leads to the development of renal fibrosis, an undeniably ‘bad’ outcome [30].
Many of these detrimental effects are the result of Wnt signaling on tubular epithelia. β-Catenin can upregulate nearly all components of the intrarenal RAS in the tubular compartment. RAS activation is a known profibrotic stimulus, and Wnt/β-catenin blockade in one study led to protection from activation as well as decreased myofibroblast activation and renal fibrosis [31]. In addition, the same MMP-7 which is protective in AKI appears to be detrimental in CKD, since MMP-7 can cleave E-cadherin in tubular cells, leading to disruption of epithelial cell layer integrity and acquisition of a more mesenchymal phenotype. The prolonged MMP-7 exposure also seemed to self-upregulate β-catenin activity in both renal epithelium and fibroblasts, forming a positive feedback loop leading to more injury (manuscript under review). The EMT inducer Snail1 is also a target of β-catenin, and pharmacologic blockade of β-catenin abrogates Snail activity and renal fibrosis [32].
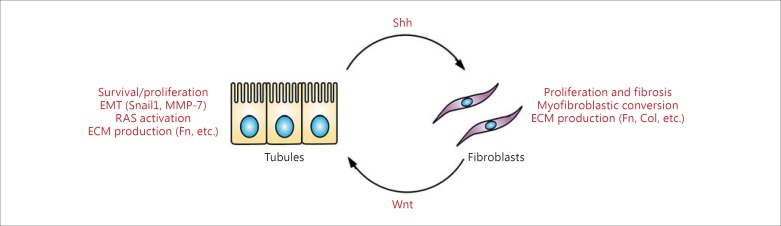
These data collectively suggest that Wnt/β-catenin mediates EMC between epithelial cells and fibroblasts in all stages of renal injury. In AKI, fibroblasts are activated and begin to secrete Wnt. These Wnt act predominantly on renal tubular cells, where there is strong activation of β-catenin after kidney injury, leading to upregulation of MMP-7. The MMP-7 in mild injury would negatively regulate fibroblasts through apoptosis and lead to repair and resolution. However, if the injury is severe enough to cause sustained fibroblast activation, Wnt secretion, and β-catenin activity, persistent MMP-7 expression induces EMT and increases β-catenin activity in both tubular cells (further promoting a more mesenchymal phenotype) and fibroblasts (inducing myofibroblast differentiation). RAS components would also be upregulated and promote fibrosis. Ultimately, this ‘feed-forward’ cycle promotes fibrosis and the progression of CKD. Therefore, Wnt/β-catenin is an active mediator of epithelial-fibroblast crosstalk, and increased understanding will help in the development of appropriately timed and targeted therapeutic interventions. These effects are summarized in figure 2.
Fig. 2.
Bidirectional EMC via Wnt/Shh signaling. In kidney injury, Wnt are predominantly expressed in activated proliferating fibroblasts and lead to increased β-catenin activity in tubular epithelial cells. This leads to increased cell survival as well as cell cycle progression to repair and regenerate injured tubules in the acute phase. Once the damage has been repaired, MMP-7 (itself a target of β-catenin) is secreted from tubules to induce apoptosis and return fibroblast numbers back to normal basal levels (negative feedback). However, during severe, repeated, and progressive injury, the tubules remain injured and secrete Shh, which causes profibrotic changes including further fibroblast proliferation, myofibroblast differentiation, and the increased generation of ECM. Meanwhile, these fibroblasts continue to produce Wnt, which activates β-catenin activity in tubular cells and causes EMT, ECM generation, Shh production, and upregulation of RAS (positive feedback). The resulting angiotensin II can further enhance the profibrotic changes in fibroblasts (not shown). Fn = Fibronectin; Col = collagen.
Hedgehog Signaling
Hedgehog (Hh) signaling, much like Wnt/β-catenin, is a key mammalian developmental pathway and regulates tissue patterning and organogenesis, among other functions. The three Hh ligands present in humans and mice are sonic Hh (Shh), desert Hh, and Indian Hh [33]. Of these three, the most is known about Shh.
Once Shh is secreted from cells, it binds to the cell surface receptor patched 1 (Ptch1). Binding results in de-repression of smoothened (Smo) and downstream activation of Gli transcription factors. Shh signaling leads to upregulation of Snail1, collagen, desmin, fibronectin, and α-SMA. Target genes also include members of the pathway itself, including upregulation of Ptch1 and Gli1, promoting further Shh signaling [34].
Several studies from independent laboratories have demonstrated that Hh ligands are predominantly produced by tubular cells, and that fibroblast or fibroblast-like cells are the targets. After ureteral obstruction, Shh and Indian Hh were upregulated in renal tubules. Using mice expressing LacZ under the control of the Gli1 or Gli2 promoter, it was found that interstitial fibroblasts, not tubules, were the cells that responded to these ligands [33,34]. Pericytes were also identified as target cells. These are mesenchymal cells surrounding the microvasculature and identified by positivity for platelet-derived growth factor receptor-β, and their proliferation was increased after Gli activation [33]. Shh also induces myofibroblast transformation, manifested as expression of α-SMA, fibronectin, collagen, and desmin. Blockade of Gli was capable of attenuating renal fibrosis in vivo and preventing fibroblast activation in vitro [34].
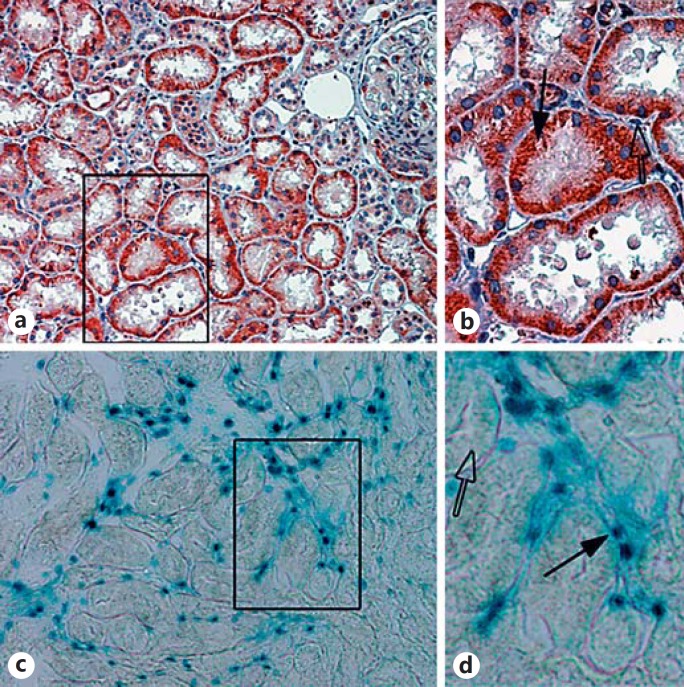
These findings were later extended to show that renal tubular cells upregulate Shh after a number of kidney injuries, including ischemia-reperfusion, 5/6 nephrectomy, and adriamycin exposure. Again, interstitial fibroblasts were identified as the responding cells (fig. 3). Fibroblasts were again shown to have enhanced proliferation in response to Shh. Furthermore, Shh overexpression hastened fibrotic development, while the Smo inhibitor cyclopamine reduced both fibroblast proliferation and renal fibrosis in vivo [35]. Specific blockade of Gli2 activation, rather than Gli1, appeared to be the key to preventing myofibroblast proliferation and renal fibrosis [36].
Fig. 3.
Shh is produced by renal tubules but acts on interstitial fibroblasts. a Immunohistochemistry for Shh after ischemia-reperfusion injury is shown with red indicating positive staining. Note the strong staining within injured tubules. b Inset showing an enlarged image of the boxed area in a. Shh staining is specific for renal tubules (black arrow) but not interstitial cells (open arrow). c Mice with the LacZ reporter under control of the Gli promoter show that only interstitial cells exhibit increased β-galactosidase activity (denoted as blue staining). d Inset showing an enlarged image of the boxed area in c. Gli reporter activity is specific for interstitial cells (black arrow) but is not present in tubular cells (open arrow).
Interestingly, although the Hh-responding cells have consistently been shown to be interstitial fibroblasts or pericytes, Hh signaling still had an indirect effect on tubular cells. By blocking Hh signals, macrophage infiltration after ureteral obstruction was decreased. As macrophages are a major source of Wnt, this action ablated the usual increase in β-catenin in renal tubular cells, and ultimately led to increased tubular apoptosis [37].
It is also possible that Shh/Gli activity in fibroblasts or pericytes would directly lead to the induction and release of Wnt from these cells. Hh signaling is capable of increasing Wnt in cancer cells [38], although validation of this phenomenon in renal fibroblasts is lacking. Conceptually at least, increased Wnt secretion would act on renal tubular cells, which in turn would be stimulated to secrete more Shh to activate fibroblasts, creating an injurious profibrotic signaling cycle (fig. 2). This type of EMC makes sense considering the evidence supporting a role for prolonged Wnt and Shh signaling in CKD. It also suggests that interruption of this cycle could be an effective treatment for chronic disease, a promising hypothesis that requires further investigation.
Angiotensin II
The RAS has long been known to be a pathologic mediator of chronic disease and fibrosis. Traditionally, it was understood as a multiorgan system involving multiple components, including angiotensinogen, renin, angiotensin-converting enzyme, and the two angiotensin receptors (AT1R and AT2R). Evidence now reveals that the diseased kidney can also express all of these components by itself. The overall effect is profibrotic and damaging and appears to be mediated by upregulation of TGF-β1[39]. The TGF-β1 cofactor CTGF is also a target of this pathway [40].
Several studies have shown the upregulation of key RAS components in both renal tubules and fibroblasts. As discussed above, it was shown that angiotensinogen, renin, angiotensin-converting enzyme inhibitor, and AT1R were upregulated in renal tubules during mouse kidney injury in a Wnt/β-catenin-dependent manner [41]. The AT1R is also expressed on renal fibroblasts, suggesting that angiotensin II mediates EMC. Indeed, in vitro studies have revealed that fibroblasts exposed to angiotensin II or III have increased matrix generation and proliferative capacity [39,42,43]. Signaling through AT1R also leads to increased fibrocyte infiltration in injured kidneys [43]. Finally, the identification and characterization of a prorenin receptor on distal tubular epithelia which can bind renin indicate autocrine signaling from the RAS as well. Interestingly, the RAS may interact with other pathways, as prorenin receptor activity enhances Wnt signaling [44].
Hepatocyte Growth Factor
Hepatocyte growth factor (HGF) is a renoprotective protein that also plays a role in the organogenesis of the liver, placenta, and skeletal muscles. In adult tissues, it is primarily produced in mesenchymal cells [45]. HGF binds to a single receptor, c-met, present on both tubular cells and fibroblasts, leading to receptor autophosphorylation and signal transduction. The downstream effects of this include cell survival, proliferation, differentiation, and migration [46].
Numerous studies show that HGF is protective against a variety of renal insults. In AKI, paracrine signaling occurs, as c-met is dramatically induced in renal tubules, and tubule-specific knockdown of this receptor leads to enhanced injury [47]. In chronic disease and fibrosis, HGF was capable of reducing proinflammatory cytokine release from tubular cells [48].
HGF also has autocrine effects on fibroblasts that are antifibrotic, primarily by effects on TGF-β signaling. HGF was capable of inducing fibroblast apoptosis, suppressing TGF-β1 and collagen expression in fibroblasts, and blocking Smad2/3 activity [49,50,51,52]. In addition, administration of HGF in vivo led to reduced renal fibrosis after obstruction, subtotal nephrectomy, angiotensin II infusion, and diabetes, and in some cases it could abrogate fibrosis even after it had started [49,53,54,55].
Connective Tissue Growth Factor
CTGF lacks a dedicated receptor but appears to interact with other proteins to promote a profibrotic environment. CTGF is a necessary cofactor for TGF-β signaling via direct interactions with the TGF-β receptor, downregulation of Smad7 activity, and inhibition of bone morphogenetic protein 7. CTGF can also bind LRP6, leading to β-catenin activity [29].
CTGF can be found in both tubular epithelial cells and fibroblasts in normal kidneys, but upregulation occurs after injury. In diseased histological tissue, CTGF largely colocalizes with α-SMA [56]. This suggests that fibroblasts/myofibroblasts are a major source, and this has been confirmed in vitro [57]. However, tubular epithelia can be stimulated to produce CTGF after exposure to injurious stimuli [6,58]. As CTGF is a cofactor for TGF-β, it is expected to be capable of promoting all of the TGF-β profibrotic effects in both fibroblasts and epithelial cells.
Exosomes
Exosomes are small, ∼40- to 100-nm vesicles contained within multivesicular bodies that are released from cells when these bodies fuse with the plasma membrane. These exosomes can then travel short or long distances and can be found in blood and urine, where they may be used as biomarkers. However, the exosomes can also be picked up by another cell with incorporation of its contents, typically consisting of the donor cell's cytoplasm, proteins, mRNA, and microRNAs. This transfer of information is another form of intercellular communication [59]. It is known that injured tubular cells pass exosomes containing TGF-β1 (and possibly other substances) to fibroblasts, which respond by proliferation, myofibroblast conversion, and matrix generation [60]. Exosomes could also enhance tubular cell (and animal) survival after AKI via downregulation of tubular apoptosis [61], and have been shown to mediate EMT [62].
Conclusions
It is now clear that there exists a complex EMC mediated by soluble factors in the diseased adult kidney. TGF-β, Wnt, Shh, angiotensin II, HGF, CTGF, and exosomes are all capable of inducing cellular changes in an autocrine and/or paracrine manner and can either promote repair or worsen injury. Their effects are summarized in table 1. The current evidence clearly supports a pivotal role for EMC in kidney disease.
Table 1.
Selected factors mediating EMC during kidney disease
| Factor | Capacity for production |
Cellular response to acute and chronic injury |
||
| tubule | Fibro/Mes | tubule | fibroblast/mesenchymal cell | |
| TGF-β1 | ++ | ++ |
Acute:
anti-inflammatory Chronic: profibrotic Apoptosis ECM generation Hypertrophy |
Acute:
anti-inflammatory Chronic: profibrotic Proliferation Myofibroblast conversion ECM generation |
| Wnt | + | ++ |
Acute:
repair/regeneration Cell survival/proliferation/progenitor recruitment Chronic: profibrotic Partial EMT (via Snail, Fn, MMP-7) ↑ RAS |
Acute:
injury resolution Apoptosis (via MMP-7) Chronic: profibrotic Myofibroblast conversion ECM generation |
| Shh | +++ | + | No direct effect via canonical pathway |
Chronic:
profibrotic Proliferation Myofibroblast conversion ECM generation |
| HGF | – | ++ |
Acute:
repair/regeneration Reducing tubular injury Chronic: repair/regeneration Tubular recovery Anti-inflammatory |
Acute: unknown Chronic: antifibrotic Antagonizing TGF-β1 Apoptosis ↓ ECM generation |
| CTGF | + | ++ | Same as TGF-β1 (cofactor) | Same as TGF-â1 (cofactor) |
| AngII | ++ | – |
Chronic:
profibrotic ↑ TGF-β1 |
Chronic:
profibrotic ↑ TGF-â1 Proliferation ↑ ECM generation |
PDGF = Platelet-derived growth factor; Fibro/Mes = fibroblast/mesenchymal cell; Fn = fibronectin; AngII = angiotensin II.
Additional themes emerge from this discussion. One is that the factors involved in EMC are also important in developmental pathways. This is intuitive in that injury would require a return to embryologic origins in order to regenerate and repair. This also reinforces the concept that understanding normal and aberrant renal development will provide key insights into kidney disease.
Another theme is the dual role that EMC plays in enabling repair as well as promoting injury. AKI leads to the elaboration of soluble factors that can lead to kidney repair. As part of this response, there are effective mechanisms in place to limit and ultimately turn off the production of these factors and prevent deleterious effects of overstimulation. However, if the injury is severe or prolonged, persistent signaling occurs and becomes maladaptive, leading to progressive CKD and fibrosis. This is an example of having ‘too much of a good thing’.
In conclusion, the continued dissection of these pathways, their interrelationships, and the way they influence different cell types will be crucial for understanding the molecular pathogenesis of renal disease. This knowledge will be pivotal in facilitating the development of appropriately timed and targeted therapies that can improve the care of renal disease in the future.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Acknowledgements
We regret that we were unable to include the excellent work of many investigators in this review due to space limitations. R.J.T. is supported by an American Heart Association Fellow-to-Faculty Award (16990086) and core facilities provided by National Institutes of Health (NIH) grant DK079307. This work was supported by NIH grants DK064005, DK091239, and DK106049, and by National Natural Science Foundation of China grants 81370839 and 81521003.
References
- 1.Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009;53(suppl 3):S4–S16. doi: 10.1053/j.ajkd.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZH. Nephrology in China. Nat Rev Nephrol. 2013;9:523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System . 2015 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 4.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21:998–1009. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 10.Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macconi D, Remuzzi G, Benigni A. Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int Suppl (2011) 2014;4:58–64. doi: 10.1038/kisup.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347:141–154. doi: 10.1007/s00441-011-1275-6. [DOI] [PubMed] [Google Scholar]
- 14.Gewin L, Vadivelu S, Neelisetty S, Srichai MB, Paueksakon P, Pozzi A, et al. Deleting the TGF-β receptor attenuates acute proximal tubule injury. J Am Soc Nephrol. 2012;23:2001–2011. doi: 10.1681/ASN.2012020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffler I, Wolf G. Transforming growth factor-β and the progression of renal disease. Nephrol Dial Transplant. 2014;29(suppl 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 16.Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Tan RJ, Fu H, Liu Y. Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest. 2016;96:156–167. doi: 10.1038/labinvest.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Iglesias DM, Corsini R, Chu L, Goodyer P. WNT/β-catenin signaling is required for integration of CD24+ renal progenitor cells into glycerol-damaged adult renal tubules. Stem Cells Int. 2015;2015:391043. doi: 10.1155/2015/391043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujigaki Y, Muranaka Y, Sun D, Goto T, Zhou H, Sakakima M, et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 2005;446:164–176. doi: 10.1007/s00428-004-1155-5. [DOI] [PubMed] [Google Scholar]
- 25.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou D, Tan RJ, Zhou L, Li Y, Liu Y. Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep. 2013;3:1878. doi: 10.1038/srep01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, et al. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol. 2016;27:781–790. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, et al. Interactions between β-catenin and transforming growth factor-β signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP) J Biol Chem. 2012;287:7026–7038. doi: 10.1074/jbc.M111.276311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol. 2014;10:700–711. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 30.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015040449. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipila P, West KA, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol. 2014;25:2187–2200. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramann R, Fleig SV, Schneider RK, Fabian SL, DiRocco DP, Maarouf O, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest. 2015;125:2935–2951. doi: 10.1172/JCI74929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauhauser AA, Ren C, Lu D, Li B, Zhu J, McEnery K, et al. Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am J Physiol Renal Physiol. 2015;309:F770–F778. doi: 10.1152/ajprenal.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qualtrough D, Rees P, Speight B, Williams AC, Paraskeva C. The hedgehog inhibitor cyclopamine reduces β-catenin-Tcf transcriptional activity, induces E-cadherin expression, and reduces invasion in colorectal cancer cells. Cancers (Basel) 2015;7:1885–1899. doi: 10.3390/cancers7030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-β pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 40.Iwanciw D, Rehm M, Porst M, Goppelt-Struebe M. Induction of connective tissue growth factor by angiotensin II: integration of signaling pathways. Arterioscler Thromb Vasc Biol. 2003;23:1782–1787. doi: 10.1161/01.ATV.0000092913.60428.E6. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schüttert JB, Liu MH, Gliem N, Fiedler GM, Zopf S, Mayer C, et al. Human renal fibroblasts derived from normal and fibrotic kidneys show differences in increase of extracellular matrix synthesis and cell proliferation upon angiotensin II exposure. Pflugers Arch. 2003;446:387–393. doi: 10.1007/s00424-003-1026-y. [DOI] [PubMed] [Google Scholar]
- 43.Sakai N, Wada T, Matsushima K, Bucala R, Iwai M, Horiuchi M, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008;26:780–790. doi: 10.1097/HJH.0b013e3282f3e9e6. [DOI] [PubMed] [Google Scholar]
- 44.Batenburg WW, Danser AH. (Pro)renin and its receptors: pathophysiological implications. Clin Sci (Lond) 2012;123:121–133. doi: 10.1042/CS20120042. [DOI] [PubMed] [Google Scholar]
- 45.Crestani B, Marchand-Adam S, Quesnel C, Plantier L, Borensztajn K, Marchal J, et al. Hepatocyte growth factor and lung fibrosis. Proc Am Thorac Soc. 2012;9:158–163. doi: 10.1513/pats.201202-018AW. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 47.Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol. 2004;15:2868–2881. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- 49.Iekushi K, Taniyama Y, Azuma J, Sanada F, Kusunoki H, Yokoi T, et al. Hepatocyte growth factor attenuates renal fibrosis through TGF-β1 suppression by apoptosis of myofibroblasts. J Hypertens. 2010;28:2454–2461. doi: 10.1097/HJH.0b013e32833e4149. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Spataro BC, Yang J, Dai C, Liu Y. 1,25-Dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68:1500–1510. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 51.Mou S, Wang Q, Shi B, Gu L, Ni Z. Hepatocyte growth factor suppresses transforming growth factor-beta-1 and type III collagen in human primary renal fibroblasts. Kaohsiung J Med Sci. 2009;25:577–587. doi: 10.1016/S1607-551X(09)70560-1. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai C, Yang J, Bastacky S, Xia J, Li Y, Liu Y. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- 54.Dworkin LD, Gong R, Tolbert E, Centracchio J, Yano N, Zanabli AR, et al. Hepatocyte growth factor ameliorates progression of interstitial fibrosis in rats with established renal injury. Kidney Int. 2004;65:409–419. doi: 10.1111/j.1523-1755.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Liu Y. Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2003;284:F349–F357. doi: 10.1152/ajprenal.00154.2002. [DOI] [PubMed] [Google Scholar]
- 56.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 57.Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J, et al. Connective tissue growth factor and IGF-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes. 2003;52:2975–2983. doi: 10.2337/diabetes.52.12.2975. [DOI] [PubMed] [Google Scholar]
- 58.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 59.van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011;80:1138–1145. doi: 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vella LJ. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]